ABSTRACT
In Japan, routine immunization for polio using the oral polio vaccine (OPV) was suspended in September 2012; subsequently, an immunization program with inactivated polio vaccines (IPVs), the conventional IPV (cIPV) derived from virulent strains, and IPV derived from Sabin strains (sIPV), was introduced. However, the immunity induced by sIPV is not well characterized. This study assessed and compared neutralizing antibodies produced against poliovirus in cases who received doses of OPV or IPV. Serum samples (n = 1186) were collected yearly between 2013 and 2016 as part of the National Epidemiological Surveillance of Vaccine–Preventable Disease. The neutralizing antibody titers for Sabin strain types 1, 2, and 3 in 224 children, aged between 0 and 90 months, were assessed. Seropositive rates after vaccination with OPV or IPV were more than 90%. Neutralizing antibody titers for Sabin type 1 after vaccination with IPV were lower than those with OPV, while those for Sabin types 2 and 3 after vaccination with IPV were significantly higher than those with OPV. Analyses of antibody titer dynamics revealed that the decay of antibody titers for Sabin types 1, 2, and 3 in cases vaccinated with IPV was steeper than those with OPV. Thus, our study showed that although IPV induced a sufficient level of neutralizing antibody, the immunity induced by IPV was not maintained as long as that by OPV. Our study suggested that a long-term survey should be conducted for polio vaccination using IPV and that it might be necessary to consider booster vaccination for IPVs.
Introduction
The world Health Assembly (WHA) adopted a resolution for the worldwide eradication of polio in 1988.Citation1 The Global Polio Eradication Initiative (GPEI) has reduced the global incidence of polio by more than 99%. The oral polio vaccine (OPV) comprising live attenuated poliovirus, such as Sabin strains, was used in the immunization program in most countries. The OPV induces effective immunity against poliovirus.Citation2
However, the OPV is a live vaccine and carries the risk of causing vaccine-associated paralytic poliomyelitis (VAPP) and polio epidemics of vaccine-derived poliovirus (VDPVs).Citation3,Citation4 The Polio Eradication and Endgame Strategic Plan 2013–2018 is a strategy aimed at attaining a polio-free world by 2018.Citation2 The plan has four objectives, one of which is to strengthen immunization systems and withdraw OPV. WHO recommended stopping immunization with trivalent OPV and introducing immunization with bivalent OPV, removing the type 2, and using at least one dose of IPV. Due to the risks associated with OPVs, globally synchronized switching from OPV to inactivated polio vaccine (IPV) has been set into motion.
The conventional IPV (cIPV) is derived from virulent strains of polioviruses,Citation5 and as an alternative, the Sabin strain-derived IPV (sIPV) has been developed as a safer IPV than cIPV and licensed.Citation6-Citation8 Since the development of safer IPVs was recommended by the WHA and the Sabin strains are expected to reduce the overall biosafety risk, the sIPV has been approved for production in certain developing countries.Citation9
In Japan, a large polio outbreak occurred in 1960. This outbreak ceased by immunization campaigns with trivalent OPV, which was introduced into the national immunization program in 1964. The last reported polio case was of a 7-year-old child, which was due to the wild poliovirus, in 1980. High vaccination coverage was maintained at >90%, and two doses of trivalent OPV established a polio-free status. The OPV was discontinued in August 2012; subsequently, the trivalent OPV was replaced with standalone cIPV in September 2012. In November 2012, sIPV-containing diphtheria-tetanus-acellular pertussis combination vaccines were first introduced into the national immunization program, and polio vaccination schedule has been revised as four doses of IPV instead of the two doses of OPV.Citation10
In Japan, children aged between 3 and 90 months were immunized with two doses of OPV at intervals longer than 6 weeks. After the introduction of the IPV in the national immunization program, children aged between 3 and 90 months are immunized with three doses of IPV at intervals of 20–56 days, as the primary vaccination, followed by the fourth dose at least 6 months later.Citation10 At present, no booster vaccination of IPV is included in the national immunization program in Japan. The booster vaccination with cIPV is being performed in several countries, but the booster vaccination for IPV is still under consideration in Japan. Japan was first to incorporate sIPV into routine immunization in 2012,Citation10 before it was marketed worldwide. Therefore, reports on the immunity induced by sIPV are limited. Moreover, the period for which the neutralizing antibody titers need to be maintained without booster vaccination remains unknown. Thus, significant characteristics of sIPV, such as the time period for which the antibody titer must be maintained in order to confer sufficient protection, remain uncharacterized. We aimed to address these gaps in the understanding of the immunity resulting from vaccination using IPV.
This report is a surveillance study on the immunity induced by IPV and OPV, after the introduction of sIPV into the routine immunization program in Japan. This study assessed and compared the neutralizing antibodies produced against the poliovirus in children who were vaccinated with doses of OPV, cIPV, or sIPV, in order to verify the vaccine immunogenicity of sIPV on polio.
Results
Study population
From 2013 to 2016, Serum samples were collected from 1186 individuals aged between 0 and 69 years. In total, 224 children age between 0 and 90 months from 1186 individuals participated in the poliomyelitis immunization program in Japan were selected. Profiles of children less than 90 months old are shown in . The children vaccinated with OPV were 34.8% (78/224), and those with IPV were 53.6% (120/224). The median age of children vaccinated with OPV, cIPV, and sIPV was 67.5, 35.5, and 17.5 months, respectively.
Table 1. Oral polio vaccine (OPV), conventional inactivated polio vaccine (cIPV), and Sabin strain-derived IPV (sIPV)-vaccinated cases of less than 90 months of age.
Seropositive rates and geometric mean antibody titers
The age distribution of seropositive rates and geometric mean antibody titers (GMTs) among the 1186 cases are shown in . The seropositive rates for Sabin type 1 and type 2 were over 80% in most ages; however, those for Sabin type 1 among individuals in the age groups of 30’s and 40’s were less than 80% (). The declining trend in seropositive rates and GMTs with age for Sabin type 1 and 2 was similar, but that for Sabin type 3 was different. Specifically, the seropositive rates of Sabin type 3 in individuals aged between 5 years and 50’s were less than 80% (), and GMTs in those aged more than 4 years were less than 32 ().
Figure 1. Seropositive rates (a) and geometric mean antibody titers (GMTs) (b) of cases vaccinated with Sabin type 1, 2, and 3. Seropositive rates and GMTs were plotted by age and age groups. Seropositivity was represented for titers of 1:8 or more. Age and age groups were plotted for age groups between 0 to 9 years and for every 10 years thereafter. Type 1: Sabin type 1; Type 2: Sabin type 2; Type 3: Sabin type 3.
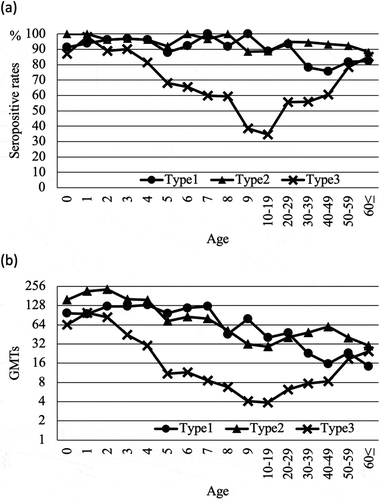
Seropositive rates and GMTs in children aged between 0 and 90 months
The cases vaccinated with OPV were compared to the cases vaccinated with IPV (). All seropositive rates were more than 90%, except for Sabin type 3 in children vaccinated with OPV (88.3%). There was a significant difference in the seropositive rates for Sabin type 1 between children vaccinated with OPV and those vaccinated using IPV. The GMTs for Sabin type 1 in children vaccinated with IPV (83.5) were lower than those with OPV (179.4), while GMTs for Sabin types 2 and 3 after vaccination with IPV (173.8 and 76.1) were higher than those of OPV (118.2 and 16.3). Significant differences were found in the GMTs for Sabin types 1, 2, and 3 between children with vaccinated OPV and IPV.
Table 2. Seropositive rates and geometric mean antibody titers (GMTs) for oral polio vaccine (OPV) and inactivated polio vaccine (IPV).
Children vaccinated with two doses of OPV were compared to those vaccinated with four doses of IPV to confirm whether there are differences in seropositive rates and GMTs after the completion of vaccination. After vaccination with two doses of OPV, the GMTs were 69, and in the case of vaccination with four doses of IPV, the GMTs were 54. All seropositive rates after vaccination with two doses of OPV or four doses of IPV were more than 90% (). There were no significant differences between two doses of OPV and four doses of IPV in terms of the seropositive rates for Sabin types 1, 2, and 3. The GMTs for Sabin type 1 after vaccination with four doses of IPV (131.3) were lower than those after two doses of OPV (174.8); however, there were no significant differences. The GMTs for Sabin types 2 and 3 in children vaccinated with four doses of IPV (287.4 and 143.6) were significantly higher than those after vaccination with two doses of OPV (110.1 and 17.5; ).
Table 3. Seropositive rates and geometric mean antibody titers (GMTs) for two doses of oral polio vaccine (OPV) and four doses of inactivated polio vaccines (IPVs).
We compared the vaccination with four doses of cIPV with vaccination using four doses of sIPV to confirm the difference between the IPVs. Sixteen children were vaccinated with four doses of cIPV and thirty-two children were vaccinated with four doses of sIPV. The seropositive rates in children vaccinated with four doses of cIPV or sIPV were 100% (). The GMTs for Sabin type 1, 2, and 3 after vaccination with four doses of sIPV (173.3, 479.8, and 245.1, respectively) were higher than those after four doses of cIPV (94.5, 145.8, and 83.0, respectively); the GMTs for Sabin types 2 and 3 were significantly higher than those after four doses of cIPV.
We compared the vaccination with two doses of OPV with that using four doses of sIPV to confirm the difference between Sabin-derived IPV and OPV. Sixty-nine children were vaccinated with two doses of OPV, and thirty-two children were vaccinated with four doses of sIPV. All seropositive rates in children vaccinated with two doses of OPV or four doses of sIPV were 100%, except those for Sabin type 3 after vaccination with two doses of OPV. There were significant differences in the seropositive rates for Sabin type 3 between children vaccinated with two doses of OPV and those vaccinated with four doses of sIPV. The GMTs for Sabin types 2 and 3 after vaccination with four doses of sIPV (479.8 and 245.1) were significantly higher than those after two doses of OPV (110.1 and 17.5; )
Decay of neutralizing antibody titers induced by vaccination with two doses of OPV or four doses of IPV
Both vaccination with two doses of OPV and vaccination with four doses of IPV had high seropositive rates for Sabin types 1, 2, and 3.
The regression lines of neutralizing antibody titers for Sabin types 1, 2, and 3 after vaccination with two doses of OPV and those after four doses of IPV are depicted in . The slope of the regression line for children vaccinated with four doses of IPV was steeper than that in children vaccinated with two doses of OPV. There were significant differences in the regression slopes (p < 0.05) between children vaccinated with four doses of IPV and those vaccinated with two doses of OPV. The decay of neutralizing antibodies for Sabin types 1, 2, and 3 after vaccination with four doses of IPV was faster than that after vaccination with two doses of OPV.
Figure 2. Regression line of neutralizing antibody titers induced by vaccination with two doses of oral polio vaccine (OPV), four doses of inactivated polio vaccine (IPV), or four doses of Sabin strain-derived inactivated polio vaccine (sIPV). The relationship between neutralizing antibody titers and elapsed time after the vaccination schedule completion was depicted by least-squares curve-fitting method. p values less than 0.05 were considered significant. Each regression line was statistically significant. There were significant differences (p < 0.05) between regression slopes of two doses of OPV and those of four doses of IPV for Sabin types 1, 2, and 3. In addition, regression slopes of two doses of OPV and those of four doses of sIPV for Sabin type 3 were significantly different (p < 0.05). Circle: two doses of OPV; open circle: four doses of IPV; open square: four doses of sIPV; dotted line: regression line of two doses of OPV; alternate long and short dashed line: regression line of four doses of IPV; dashed line: regression line of four doses of sIPV. Regression line of two doses of OPV for Sabin type 1; y = −0.0284x + 8.8741 RCitation2 = 0.0917, Sabin type 2; y = −0.0298x + 8.1965 RCitation2 = 0.0917, Sabin type 3; y = −0.0435x + 6.092 RCitation2 = 0.1052. Regression line of four doses of IPV for Sabin type 1; y = −0.1263x + 8.7408 RCitation2 = 0.3972, Sabin type 2; y = −0.0917x + 9.5512 RCitation2 = 0.331, Sabin type 3; y = −0.1196x + 8.9806 RCitation2 = 0.3445. Regression line of four doses of sIPV for Sabin type 1; y = −0.0789x + 8.1942 RCitation2 = 0.1848, Sabin type 2; y = −0.0568x + 9.0626 RCitation2 = 0.2078, Sabin type 3; y = −0.1234x + 9.3093 RCitation2 = 0.4052.
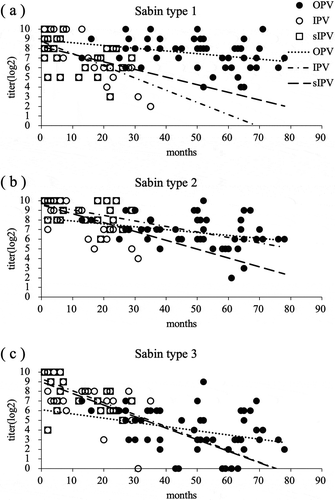
The regression lines of the neutralizing antibody titers for Sabin types 1, 2, and 3 after vaccination with two doses of OPV and after vaccination with four doses of sIPV are shown in . The slope of the regression line for children vaccinated with four doses of sIPV was steeper than that in children vaccinated with two doses of OPV. The regression slope for Sabin type 3 was significantly different (p < 0.05) between children vaccinated with four doses of sIPV and those vaccinated with two doses of OPV. The decay of neutralizing antibodies for Sabin types 1, 2, and 3 after vaccination with four doses of sIPV was faster than that after vaccination with two doses of OPV, similar to that after vaccination with cIPV; however, decay of neutralizing antibodies for Sabin types 1, 2, and 3 after vaccination with sIPV was slower than that after vaccination with cIPV.
Discussion
In this report, we analyzed the seropositive rates and GMTs induced by OPV or IPV, after the introduction of routine sIPV immunization in Japan.
The seropositive rates for Sabin type 1 and 2 were over 80% in almost all age groups, except the seropositive rates for Sabin type 1 in the 30’s and 40’s age groups, which was consistent with the low seropositive rates for Sabin type 1 in the population born in between 1975 and 1977 in Japan.Citation11 The seropositive rates for Sabin type 3 were less than 80% in groups aged between 5 and 50 years and less than 40% in groups aged between 9 and 10 years. The increased seropositive rates in groups aged over 50 years old might be a booster response due to the outbreak of poliomyelitis in 1960 and 1961 in Japan.Citation10
The GMTs for Sabin type 1 and 2 were over 8 in all age groups. On the other hand, those for Sabin type 3 in groups aged between 8 and 30 years were less than 8. Since the neutralizing antibody titer of 1:8 or more for poliomyelitis is sufficient for protection from infection,Citation12 our results revealed that individuals of all ages possessed antibody titers sufficient for protection against poliovirus type 1 and 2 infection, but insufficient for protection against poliovirus type 3.
Furthermore, both the lower seropositive rate and GMT for Sabin type 3 were considered to result from viral interference. Sabin type 3 is believed to be the most susceptible to viral interference among the various types of Sabin vaccines.Citation13,Citation14 Furthermore, it was suggested that IPV has higher immunity-inducing ability in the case of Sabin 2 and 3 than OPV. IPV might elicit antibody responses against all vaccine antigens with no significant interference among the types included in the live vaccine. The GMTs for Sabin type 1, 2, and 3 after vaccination with four doses of cIPV were lower than those with sIPV, which might be due to the longer periods post vaccination and the different challenge viruses that were used for the neutralizing tests.
The antibody titers for Sabin type 1, 2, and 3 after vaccination with four doses IPV decreased faster than those after two doses OPV and the antibody titers for Sabin type 3 after four doses of sIPV decreased faster than that after two doses of OPV. This suggests that the decay tendency of the neutralizing antibody titers differs between IPV and OPV. Since IPV, particularly sIPV, is the newly introduced vaccine, further assessment of antibody persistence is necessary. In many countries such as the United States and Germany,Citation15-Citation17 booster vaccination for cIPV is routinely carried out for children aged over 4 years. However, no booster vaccination of IPV is performed in Japan. In addition, it was shown that children given the booster vaccination at the age of 10 years maintained higher antibody titer at the age of 18 years than those given the same at the age of 6 years.Citation17 It is also suggested that booster vaccination should be conducted at 4 years of age or order.Citation16 On the other hand, most currently available reports on IPV are based on cIPV and there are no reports on the evaluation of the antibody titer during long-term follow-up after sIPV vaccination. A long-term survey is needed for the polio vaccination program using sIPV in Japan. Depending on the results of surveillance, it might be necessary to consider the booster vaccination for IPV and adding it into the list of routine vaccination.
There are some limitations of this study. The median age of subjects vaccinated with OPV, cIPV, and sIPV was quite different, because of the change in the immunization program. However, as the age of immunization is the same, effect of age differences on this study will be less. The tested neutralizing antibody titers were against Sabin strains and not for the wild type strains. However, it has been reported that the neutralizing antibody obtained by sIPV exerts the same effect on the wild type strains in Phase II and Phase III clinical studies.Citation6
In order to maximize the effect of introducing IPV, it is important to ensure the stable supply of IPV. For manufacturing IPV in middle- and low-income countries, sIPV derived from Sabin poliovirus strains, which are attenuated strains, is safer than the cIPV, derived from wild type poliovirus strains. However, further studies on sIPV are required for the use of the safer vaccine.
In conclusion, this is a surveillance study on the immunity induced by IPV and OPV, after the introduction of Japanese routine immunization program using the first licensed sIPV. Immunogenicity of sIPV in polio needs to be monitored in order to evaluate its effectiveness. Our study highlights the necessity to consider booster vaccination for IPV.
Materials and methods
Study populations
This study was conducted as a part of the National Epidemiological Surveillance of Vaccine-Preventable Disease (NESVPD) in Japan. The NESVPD included the surveillance of poliomyelitis and was carried out by the National Institute of Infectious Diseases (NIID) and the Ministry of Health, Labour and Welfare, in collaboration with the prefectural governments and prefectural public health laboratories. Cases were recruited between 2013 and 2016 in the Chiba Prefecture in Japan and the profiles included vaccination history, which were obtained from healthy individuals with their informed consent. Seropositive rates and GMTs were analyzed for children aged from 0 to 90 months who were included in the immunization program.
This study was conducted according to the ethical guidelines for medical and health research involving human subjects in Japan on the basis of the immunization law in Japan. The study procedures were conducted in accordance with the Helsinki Declaration of 1975.
Cell culture
RD-A, a Human embryo rhabdomyosarcoma cell line, gifted from the National Institute of Infectious Diseases, was used for the neutralizing test. Eagle’s Minimum Essential Medium (M4655-500ML, Merck) supplemented with 10% fetal calf serum was used as the growth medium.
Titration
The neutralizing antibody titers against polioviruses were determined using the neutralizing test. Challenge viruses were Sabin strains of types 1, 2, and 3. The dilution ranging from 1:4 to 1:1024 were used. Serially diluted serum was incubated with challenge viruses at 50% cell culture infectious dose (CCID50) of 100, at 36ºC for 3 hours; then, RD-A cells were added to each well. The titer of neutralizing antibody that exhibited 50% inhibition of the cytopathic effect (CPE) was measured after 7 days. Neutralizing antibody titers of 1:8 or more were defined as seropositive.Citation12
Statistics
Seropositive rates, geometric mean antibody titers (GMTs), and their two-sided 95% confidence intervals (CIs) were calculated. p values were calculated using the student’s t test. P values less than 0.05 were considered statistically significant. The correlation between the neutralizing antibody titers and time elapsed after vaccination was plotted for each Sabin type. Each regression line was depicted by the least-squares curve-fitting method from the plot diagram. We estimated the decay of neutralizing antibody titers induced by vaccination with two doses of OPV, four doses of IPV, or four doses of sIPV by regression analysis using the least-square curve-fitting methods. The relationship between neutralizing antibody titers and elapsed time after the vaccination schedule completion was plotted for each Sabin type. The differences between the regression slopes were analyzed using the student’s t test. P values less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all the staff at the division of virology, Chiba prefectural Institute of public health.
References
- World Health Organization. Global eradication of poliomyelitis by the year 2000. Forty first World Health Assembly, resolution 41.28. WHO, Geneva, Switzerland, 1988.
- Polio Grobal Eradication Initiative. Polio eradication & endgame strategic plan 2013–2018. WHO, Geneva, Switzerland, 2013.
- WHO. Polio vaccines: WHO position paper – March, 2016. Wkly Epidemiol Rec. 2016;91(12):145–68. PMID:27039410.
- Platt LR, Estívariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis. 2014;210 Suppl 1:S380–9. doi:10.1093/infdis/jiu184. PubMed PMID: 25316859.
- Murdin AD, Barreto L, Plotkin S. Inactivated poliovirus vaccine: past and present experience. Vaccine. 1996;14(8):735–46. PubMed PMID: 8817819.
- Okada K, Miyazaki C, Kino Y, Ozaki T, Hirose M, Ueda K. Phase II and III clinical studies of diphtheria-tetanus-acellular pertussis vaccine containing inactivated polio vaccine derived from sabin strains (DTaP-sIPV). J Infect Dis. 2013;208(2):275–83. Epub 2013/ 04/08. doi:10.1093/infdis/jit155. PubMed PMID: 23568174.
- Liao G, Li R, Li C, Sun M, Li Y, Chu J, Jiang S, Li Q. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: a phase II, randomized, positive-controlled trial. J Infect Dis. 2012;205(2):237–43. Epub 2011/ 12/08. doi:10.1093/infdis/jir723. PubMed PMID: 22158682.
- Resik S, Tejeda A, Fonseca M, Alemañi N, Diaz M, Martinez Y, Garcia G, Okayasu H, Burton A, Bakker WA, et al. Reactogenicity and immunogenicity of inactivated poliovirus vaccine produced from Sabin strains: a phase I Trial in healthy adults in Cuba. Vaccine. 2014;32(42):5399–404. Epub 2014/ 08/12. doi:10.1016/j.vaccine.2014.07.109. PubMed PMID: 25131734.
- Okayasu H, Sein C, Hamidi A, Bakker WA, Sutter RW. Development of inactivated poliovirus vaccine from Sabin strains: A progress report. Biologicals. 2016;44(6):581–87. Epub 2016/ 10/05. doi:10.1016/j.biologicals.2016.08.005. PubMed PMID: 27720268.
- Shimizu H. Development and introduction of inactivated poliovirus vaccines derived from Sabin strains in Japan. Vaccine. 2016;34(16):1975–85. Epub 2014/ 11/21. doi:10.1016/j.vaccine.2014.11.015. PubMed PMID: 25448090.
- National Institute of Infectious Diseases. Poliomyelitis, Japan, 1962–1995. IASR. 1997;18(1):28–29.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. Epub 2010/05/12. doi:10.1128/CVI.00131-10. PubMed PMID: 20463105; PubMed Central PMCID: PMCPMC2897268.34.
- Okayasu H, Sutter RW, Czerkinsky C, Ogra PL. Mucosal immunity and poliovirus vaccines: impact on wild poliovirus infection and transmission. Vaccine. 2011;29(46):8205–14. Epub 2011/ 09/05. doi:10.1016/j.vaccine.2011.08.059. PubMed PMID: 21896299.
- Maldonado YA, Peña-Cruz V, de la Luz Sanchez M, Logan L, Blandón S, Cantwell MF, Matsui SM, Millan-Velasco F, Valdespino JL, Sepulveda J. Host and viral factors affecting the decreased immunogenicity of Sabin type 3 vaccine after administration of trivalent oral polio vaccine to rural Mayan children. J Infect Dis. 1997;175(3):545–53. doi:10.1093/infdis/175.3.545. PubMed PMID: 9041324.
- Knuf M, Vetter V, Celzo F, Ramakrishnan G, Van Der Meeren O, Jacquet JM. Repeated administration of a reduced-antigen-content diphtheria-tetanus-acellular pertussis and poliomyelitis vaccine (dTpa-IPV; Boostrix™ IPV). Hum Vaccin. 2010;6(7):554–61. doi:10.4161/hv.6.7.11760. PubMed PMID: 20448468.
- Centers for Disease Control and Prevention (U.S.). Poliomyelitis. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington (DC) Public Health Foundation, 2015. p. 297–310.
- Böttiger M. Polio immunity to killed vaccine: an 18-year follow-up. Vaccine. 1990;8(5):443–45. doi:10.1016/0264-410X(90)90244-G. PubMed PMID: 2251870.
