ABSTRACT
Hepatitis B e antigen (HBeAg) has been considered to cause immunotolerance to hepatitis B virus (HBV) in newborn infants after fetal HBeAg exposure. This study compared anti-HBs responses to hepatitis B vaccination in infants who were born to HBeAg-positive and -negative mothers respectively, to investigate whether fetal HBeAg exposure may induce immunotolerance to HBV. Totally 265 infants who received recommended neonatal immunoprophylaxis against hepatitis B and had no HBV infection were included. Anti-HBs levels were compared between 124 infants with cord blood positive HBeAg and 141 infants with cord blood negative HBeAg at 7–12 months of age. The infants in two groups had similar age at the follow-up (10.0 ± 2.3 vs 10.1 ± 2.3 months, P = 0.590). Overall, 259 (97.7%) of 265 infants achieved anti-HBs levels (mIU/ml) ≥10 and 6 (2.3%) others had anti-HBs <10. Of 124 HBeAg-positive infants at birth, 46.0%, 39.5%, 12.1%, and 2.4% had anti-HBs levels (mIU/ml) ≥1000, 100–999.9, 10–99.9, and <10, respectively. Of 141 HBeAg-negative infants at birth, 35.5%, 48.9%, 13.5%, and 2.1% showed ≥1000, 100–999.9, 10–99.9, and <10, respectively. The proportions of each anti-HBs level between the two groups were comparable (all P > 0.05). Additionally, the distribution of anti-HBs response levels were also comparable in infants with high and low HBeAg levels (P = 0.818). In conclusions, the fetal HBeAg exposure does not inhibit the antibody response to neonatal hepatitis B vaccination. The data suggest that HBeAg appears not inducing immunotolerance to HBV.
Introduction
Hepatitis B virus (HBV) infection remains a serious global public health problem. Mother-to-infant transmission is the most common form of HBV infection in endemic regions and often leads to chronicity. Before the availability of hepatitis B immunoglobulin (HBIG) and hepatitis B vaccine, chronic HBV infection occurred in 70–90% and 10–30% of infants born to hepatitis B e antigen (HBeAg) positive and HBeAg negative mothers respectively.Citation1-Citation3 HBeAg is a low-molecular-weight (15.5 kD) soluble antigen, which is not a component of HBV, but a derivative of hepatitis B core antigen (HBcAg) that is secreted into the circulation during viral replication.Citation4 HBeAg can traverse the placenta, making the fetus exposed to this antigen. It has been considered that the fetal HBeAg exposure can cause partial tolerance of newborn infants’ immune system to HBV, leading to the impaired immune functions for clearing the virus after neonatal exposure to HBV during the birth process and finally resulting in chronic infection.Citation5-Citation7 While this hypothesis is supported by some evidence,Citation6,Citation8,Citation9 the role of HBeAg in inducing neonatal immunologic tolerance to HBV remains to be controversial.Citation10,Citation11
Since the availability of HBIG and hepatitis B vaccine, administration of combined passive-active immunoprophylaxis in infants within 12 hour after birth, followed by two additional doses of vaccine at the age of 1 and 6 months respectively, mother-to-infant transmission of HBV has been reduced from 70–90% to 5–10% and from 10–30% to nearly zero in infants born to HBeAg-positive and -negative carrier mothers respectively,Citation12-Citation14 demonstrating the high efficiency of current immunoprophylaxis. As HBeAg can transplacentally transfer from mothers to their fetuses, the neonates born to HBeAg-positive or -negative mothers have different HBeAg status. This leaves us an opportunity to compare the antibody responses to hepatitis B vaccination in these two infant groups, in whom their cord blood samples were positive and negative for HBeAg respectively, and to investigate whether fetal HBeAg exposure can induce the immunologic tolerance to HBV.
Results
Demographic characteristic and anti-HBs response
Based on the HBeAg status in the umbilical cord blood samples at birth, the infants were divided into two groups, 124 infants with positive HBeAg and 141 infants with negative HBeAg at birth. The demographics and baseline characteristics of the two group infants are shown in . Overall, the variables were comparable between the two groups. The infants with positive HBeAg at birth had the median HBeAg level 1.39 log10 S/CO (range 0.04–3.05) in the cord blood, much lower than the levels (median level was 2.80 log10 S/CO, range 0.14–3.53, P < 0.05) in their mothers.
Table 1. Demographics and baseline characteristics of infants.
Overall, 259 (97.7%) of 265 infants achieved anti-HBs ≥10 mIU/ml. Of them, 34 (12.8%), 118 (44.5%), and 107 (40.4%) had anti-HBs levels 10.0–99.9, 100–999.9, and ≥1000 mIU/ml, respectively, and were accordingly defined as low, medium, and high responders, respectively. Additionally, six (2.3%) other infants had anti-HBs levels <10 mIU/ml (ranged 6.04–7.92), and were defined as non-responders.
Comparison of anti-HBs responses between HBeAg-positive and -negative infants at birth
To investigate whether fetal exposure to HBeAg has influence on the antibody response to neonatal vaccination against hepatitis B, we compared anti-HBs levels in infants with different status of HBeAg. Overall, the median anti-HBs levels in infants with positive and negative HBeAg in umbilical cord blood at birth were 351.7 mIU/ml and 293.12 mIU/ml respectively (P = 0.049). The detailed anti-HBs titers of non-, low-, medium-, and high-responders were comparable between the two groups (). The distributions of responders in infants with different HBeAg status at birth are shown in . The proportions of high, medium, low, and non responders to hepatitis B vaccine in neonates with positive HBeAg in cord blood were comparable to that in neonates with negative HBeAg in their cord blood. In addition, based on the median HBeAg titer (1.39 log10 S/CO) in cord blood, we compared the anti-HBs response levels between infants with HBeAg titer ≥1.39 and those with HBeAg titer <1.39 (). The distributions of anti-HBs response levels were also comparable in infants with different HBeAg levels.
Table 2. Anti-HBs titers of infants with different HBeAg status.
Figure 1. Proportion of different responders in infants with different hepatitis B e antigen (HBeAg) status.
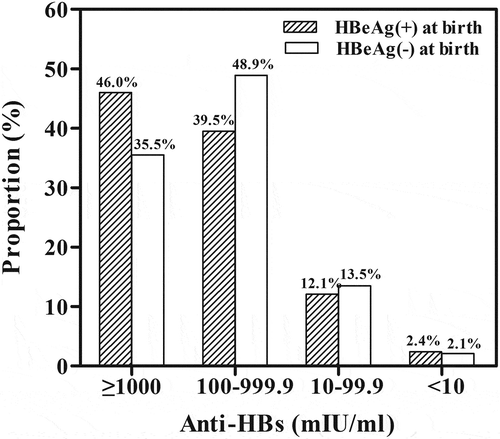
Table 3. The frequency of different responders with different HBeAg levels.
Discussion
Combined use of passive and active vaccination against hepatitis B is the most effective way to prevent mother-to-child transmission of HBV. Immunologic tolerance has been considered to be one of the factors affected the production of anti-HBs after vaccination against hepatitis B.Citation15 Studies have suggested that maternal HBeAg can transfer through placenta and induce T-cell tolerance in utero.Citation5,Citation16 Exposure of immature immune system and early life to transplacental HBeAg could induce immunotolerance in infants. Although some researchers suggested that HBV exposure triggers a state of “trained immunity” that “challenges the role of immune tolerance in vrial persistence after neonatal infection” in utero, other investigators considered that the concept of immune tolerance of HBeAg is alive and well.Citation17
In the previous study, we found that as high as 89% of the newborn infants born to HBeAg positive mothers were also HBeAg positive in their cord blood samples,Citation18 which is similar to the reported transplacental HBeAg transfer proportion (94.3%, 182/193).Citation19 In the present study, after vaccination against hepatitis B based the standard protocol, the anti-HBs positive rate was as high as 97.7%, which is similar to the positive rate of 98.2% at 7 month and 97.1% at 12 month in China reported by Wei KP et al.Citation13 The birth weigh as well as the median anti-HBs levels in infants in the present study are also comparable to those in Wei KP et al’s report.Citation13 Additionally, the 265 infants included in the present study were derived from stratified cluster sampling method, but not deliberately selected from the infants with high or low anti-HBs responses. Thus, we considered that the results obtained in these infants can generally represent those in general infant populations. In the present study, we compared the anti-HBs response to hepatitis B vaccination between newborn infants with positive HBeAg in umbilical blood and those with negative HBeAg in umbilical blood. No differences in anti-HBs response rates and antibody levels were found between infants with different HBeAg status, indicating transplacental HBeAg in fetus did not inhibit the immune responses to hepatitis B vaccine. Therefore, our present study provides evidence against the concept of HBeAg as an immunotolerance in the transmission of HBV from mothers to infants.
Studies on the prenatal diagnosis by amniocentesis or percutaneous umbilical blood sampling showed the presence of HBeAg in amniotic fluid and fetal blood samples at gestation weeks 16–32,Citation20,Citation21 demonstrating that HBeAg can pass through placenta to fetus in the second trimester. Although it is infeasible to determine when transplacental HBeAg transfer is initiated, the presence of HBeAg in the cord blood at birth indicated that the fetuses had been exposed to this antigen for at least several weeks in utero, rather than exposure just around partus, However, the infants with positive HBeAg at birth did not show any inhibition of anti-HBs response to hepatitis B vaccine, compared with the infants with negative HBeAg at birth. This suggests that in utero exposure to HBeAg may not induce immunotolerance to HBsAg.
Our study had some limitations. First, we did not include infants who were born to HBsAg negative pregnant women. As a result, we could not compare the anti-HBs response to hepatitis B vaccine between infants born to HBV-infected and non-infected mothers. However, the findings that as high as 97.7% (259/265) infants had anti-HBs ≥10 mIU/ml at 7–12 months of age indicate that the anti-HBs response to neonatal hepatitis B vaccination in this cohort infants born to HBV carrier mothers was overall comparable to that in infants born to non-HBV infected mothers.Citation22 Second, we did not further quantitate the anti-HBs levles in samples that were beyond the upper detection limit (1000 mIU/ml), leaving the exact concentrations of anti-HBs unknown. However, it is generally accepted that vaccinees with anti-HBs higher than 1000 mIU/ml are considered to be high responders and will have long protection duration.Citation23,Citation24
In conclusion, the present study revealed that the fetal exposure to maternal HBeAg did not induce immunotolerance to neonatal hepatitis B vaccination. The results suggest that the transplacentally acquired maternal HBeAg in utero may be not associated with the pathogenesis of chronic HBV infection after neonatal exposure to HBV.
Materials and methods
Study subjects
The subjects in the present study included two groups of infants by stratified cluster sampling method. One group was composed of 124 infants born to mothers with positive for both hepatitis B surface antigen (HBsAg) and HBeAg, and they were also HBeAg positive in umbilical cord blood samples collected at birth. These infants were offsprings of consecutive pregnant women who served as a control group in a prospective study to evaluate the safety and efficacy of telbivudine used during the trimester to prevent perinatal HBV infection in Jiangsu province; their mothers were not treated with any antiviral treatment before and during pregnancy.Citation18 The other group comprised 141 infants born to mothers with positive HBsAg and negative HBeAg, and they were also HBeAg negative in their umbilical cord blood collected at birth.Citation25 Infants who met any of followings were excluded: 1) mothers with co-infection of hepatitis A, C, E or HIV; 2) low birth weight; 3) premature birth; 4) incompletion or not adherence of recommended 0, 1 and 6 months schedule of hepatitis B vaccination; 5) positive for antibody against hepatitis B core antigen (anti-HBc) or for HBsAg at 7–12 month of age.
All infants were injected with HBIG (100 IU) and the first dose of the hepatitis B vaccine (yeast recombinant HBsAg, 10 μg) within 24 h of birth, and received the two other vaccine doses at the ages of 1 and 6 months respectively. The infants also received other routine vaccinations as recommended by China Expanded Program on Immunization.
This study was approved by the institutional review boards (IRB) of Nanjing Drum Tower Hospital. As serum samples used in the present study were collected in two previous investigations,Citation18,Citation25 in which the mothers consented to participation and gave the written informed consent for themselves and their infants, the exemption of written informed consent in this study was approved by the IRB of Nanjing Drum Tower Hospital.
Laboratory tests and outcome assessment
Hepatitis B serologic markers, including HBsAg, antibody against HBsAg (anti-HBs), HBeAg, antibody against HBeAg (anti-HBe), and anti-HBc, were measured with microparticle enzyme immunoassay (Architect, Abbott, North Chicago). The infants with anti-HBs levels <10, 10–99.9, 100–999.9, and ≥1000 mIU/ml were respectively defined as non-, low-, medium-, and high-responders as described elsewhere.Citation23,Citation24
Statistical analysis
Normally distributed continuous variables were shown as mean ± SD and compared using Student’s test. Non-normally distributed quantitative data were expressed as median and range and compared using Mann-Whitney U test. Categorical variables were compared by Chi-square or Fisher’s exact tests. Level of HBeAg was expressed as a logarithm of measured value (log10 S/CO). The anti-HBs were shown as geometric mean concentration (GMC) followed by minimum and maximum values and compared by Mann-Whitney U tests. All statistical analyses were conducted using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P < 0.05 indicated statistically significant difference.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Xu ZY, Liu CB, Francis DP, Purcell RH, Gun ZL, Duan SC, Chen RJ, Margolis HS, Huang CH, Maynard JE. Prevention of perinatal acquisition of hepatitis B virus carriage using vaccine: preliminary report of a randomized, double-blind placebo-controlled and comparative trial. Pediatrics. 1985;76:713–18.
- Hou J, Liu Z, Gu F. Epidemiology and Prevention of Hepatitis B Virus Infection. Int J Med Sci. 2005;2(1):50–57. doi:10.7150/ijms.2.50.
- Li Z, Hou X, Cao G. Is mother-to-infant transmission the most important factor for persistent HBV infection? Emerg Microbes Infect. 2015;4(5):e30. doi:10.1038/emi.2015.30.
- Zhou YH. Treatment of chronic Hepatitis B infection. JAMA. 2018;320(11):1201. doi:10.1001/jama.2018.10007.
- Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990;87(17):6599–603. doi:10.1073/pnas.87.17.6599.
- Milich DR. Do T cells “see” the hepatitis B core and e antigens differently? Gastroenterology. 1999;116(3):765–68. doi:10.1016/S0016-5085(99)70203-9.
- Trehanpati N, Hissar S, Shrivastav S, Sarin SK. Immunological mechanisms of hepatitis B virus persistence in newborns. Indian J Med Res. 2013;138:700–10.
- Milich DR, Chen MK, Hughes JL, Jones JE. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J Immunol. 1998;160:2013–21.
- Prendergast AJ, Klenerman P, Goulder PJ. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol. 2012;12(9):636–48. doi:10.1038/nri3277.
- Reifenberg K, Deutschle T, Wild J, Hanano R, Gastrock-Balitsch I, Schirmbeck R, Schlicht HJ. The hepatitis B virus e antigen cannot pass the murine placenta efficiently and does not induce CTL immune tolerance in H-2b mice in utero. Virology. 1998;243(1):45–53. doi:10.1006/viro.1998.9033.
- Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol. 2015;12(3):258–63. doi:10.1038/cmi.2014.79.
- Cheung KW, Seto MTY, Kan ASY, Wong D, Kou KO, So PL, Lau WL, Jalal K, Chee YY, Wong RMS, et al. Immunoprophylaxis failure of infants born to Hepatitis B carrier mothers following routine vaccination. Clin Gastroenterol Hepatol. 2018;16(1):144–45. doi:10.1016/j.cgh.2017.07.013.
- Wei KP, Zhu FC, Liu JX, Yan L, Lu Y, Zhai XJ, Chang ZJ, Zeng Y, Li J, Zhuang H. The efficacy of two different dosages of hepatitis B immunoglobulin combined with hepatitis B vaccine in preventing mother-to-child transmission of hepatitis B virus: A prospective cohort study. Vaccine. 2018;36(2):256–63. doi:10.1016/j.vaccine.2017.11.037.
- Liu J, Xu B, Chen T, Chen J, Feng J, Xu C, Liu L, Hu Y, Zhou YH. Presence of hepatitis B virus markers in umbilical cord blood: exposure to or infection with the virus? Dig Liver Dis. 2018;pii:S1590-8658(18)31222-2. doi:10.1016/j.dld.2018.11.003.
- Yang S, Tian G, Cui Y, Ding C, Deng M, Yu C, Xu K, Ren J, Yao J, Li Y, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251. doi:10.1038/srep27251.
- Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38(5):1075–86. doi:10.1053/jhep.2003.50453.
- Milich DR. The concept of immune tolerance in chronic Hepatitis B virus infection is alive and well. Gastroenterology. 2016;151(5):801–804. doi:10.1053/j.gastro.2016.09.037.
- Hu Y, Xu C, Xu B, Hu L, Liu Q, Chen J, Liu J, Liu L, Yang J, Chen T, et al. Safety and efficacy of telbivudine in late pregnancy to prevent mother-to-child transmission of hepatitis B virus: A multicenter prospective cohort study. J Viral Hepat. 2018;25(4):429–37. doi:10.1111/jvh.12834.
- Zhang L, Gui XE, Wang B, Fan JY, Cao Q, Mullane K, Liang XL. Serological positive markers of hepatitis B virus in femoral venous blood or umbilical cord blood should not be evidence of in-utero infection among neonates. BMC Infect Dis. 2016;16(1):408. doi:10.1186/s12879-016-1754-1.
- Zhu YY, Mao YZ, Wu WL, Cai QX, Lin XH. Does hepatitis B virus prenatal transmission result in postnatal immunoprophylaxis failure? Clin Vaccine Immunol. 2010;17(12):1836–41. doi:10.1128/CVI.00168-10.
- Feng J, Li J, Liu JL, Zhu HY, Zhu XY, Zhou YH, Hu Y. Influence of amniocentesis on risk of mother-to-child transmission of hepatitis B virus. Chin J Perinat Med. 2015;18(11):823–27. (In Chinese). doi:10.3760/cma.j.issn.1007-9408.2015.11.006.
- Hu Y, Wu Q, Xu B, Zhou Z, Wang Z, Zhou YH. Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine. 2008;26(48):6064–67. doi:10.1016/j.vaccine.2008.09.014.
- Zou H, Chen Y, Duan Z, Zhang H. Protective effect of hepatitis B vaccine combined with two-dose hepatitis B immunoglobulin on infants born to HBsAg-positive mothers. PLoS One. 2011;6(10):e26748. doi:10.1371/journal.pone.0026748.
- Wang ZZ, Gao YH, Wang P, Wei L, Xie CP, Yang ZX, Lan J, Fang ZL, Zeng Y, Yan L, et al. Comparison of immunogenicity between hepatitis B vaccines with different dosages and schedules among healthy young adults in China: a 2-year follow-up study. Hum Vaccin Immunother. 2018;14(6):1475–82. doi:10.1080/21645515.2018.1438090.
- Liu J, Bi Y, Xu C, Liu L, Xu B, Chen T, Chen J, Pan M, Hu Y, Zhou YH. Kinetic changes of viremia and viral antigens of Hepatitis B virus during and after pregnancy. Medicine (Baltimore). 2015;94(45):e2001. doi:10.1097/MD.0000000000002001.
