ABSTRACT
Seasonal influenza is a very common disease. Yearly vaccination of at-risk population groups is a well-recognized cost-effective/cost-saving preventive measure. It is, however, unclear which available alternative has the most favorable economic profile. Some available options are: trivalent (TIV) and quadrivalent (QIV) inactivated vaccines, adjuvanted TIV (aTIV). Because of immunosenescence, aTIV has been specifically developed for elderly. The present study aimed at assessing the available evidence of aTIV use in elderly from the economic perspective. A systematic literature review targeting aTIV economic evaluations in adults aged ≥65 years was performed using Medline via Ovid, Embase, DARE and NHS/EED. Of a total of 3,654 papers screened, 18 studies (13 full papers, 5 conference abstracts) were included. It emerged that compared with both non-vaccination or non-adjuvanted vaccines, aTIV was cost-effective or cost-saving. The vaccinations strategies incorporating aTIV based on age and/or risk profile are associated with the most favorable economic outcomes.
Introduction
Seasonal influenza is a common disease affecting every year up to 10% of adults and up to 30% of children.Citation1 Among other communicable diseases, influenza ranks at the top in terms of its burden. Indeed, it has been estimated that in Europe the average annual burden of influenza amounts to 81.8/100,000 disability-adjusted life years (DALYs) (cf. tuberculosis, which ranked second, estimated 53.5/100,000 DALYs, i.e., 1.53 times less than influenza).Citation2 The enormous population burden of influenza is, however, highly dependent on age. While the infection attack rate is generally the highest in the pediatric population,Citation3 about 90% of influenza-related deaths occur in the elderly.Citation4
Annual influenza vaccination is a cornerstone Public Health measure that is able to significantly reduce the burden of disease.Citation1,Citation5 In the last 70–80 years, several types of influenza vaccine types have been developed, passing from live attenuated to inactivated, whole-virion to subunit, monovalent to quadrivalent, unadjuvanted to adjuvanted, egg-based to recombinant and cell-based, standard-dose to high-dose.Citation1,Citation6,Citation7 The current worldwide armamentarium of available options includes: trivalent (TIV) and quadrivalent (QIV) inactivated vaccines, adjuvanted TIV (aTIV), high-dose TIV (hdTIV), intradermal TIV (idTIV), cell culture-derived QIV (ccQIV), recombinant QIV (rQIV), and some others.Citation7 However, the number of available alternatives is highly different among single countries, being the highest choice in North America, while in some countries only one vaccine type is available. This is likely a result of the different commercial attractiveness of single markets.
It is well-known that compared with younger age groups, standard influenza vaccines like TIV have suboptimal immunogenicity and efficacy in the elderlyCitation8,Citation9 owing to the phenomenon of immunosenescence and a relatively high prevalence of chronic conditions and multi-morbidity among seniors. In order to address these issues, three types of vaccines have been specifically developed for the elderly, namely aTIV, idTIV (15mcg) and hdTIV. It is worth noting that all of these are trivalent. While idTIV enhances immunogenicity thanks to the particular immunological environment of the derma,Citation10 hdTIV elicits a more robust immune response through the quadruple antigen content.Citation11 By contrast, aTIV takes advantage of the MF59® adjuvant that induces strong and long-lasting T- and B-cell responses against both vaccine-like and drifted/heterotypic strains.Citation12
It is now widely acknowledged that annual influenza vaccination is a good value for money; this has been demonstrated in several systematic reviews of economic studies.Citation13–Citation15 It is particularly cost-effective/cost-saving in the elderly;Citation13 indeed, seniors are the main internationally recognized target group for annual immunization campaigns.Citation1,Citation16
Systematic reviews of economic evaluations are becoming increasingly common. It has been arguedCitation17 that, differently from systematic reviews/meta-analyses of clinical efficacy data, systematic reviews of economic studies should not produce a single result, rather they should inform decision-makers (in a robust and transparent manner) on the potential impact of different resource allocations and how these may vary from setting to setting. For instance, a systematic review of cost-effectiveness studies on replacing TIV with QIVCitation18 has established that the incremental cost-utility ratio (ICUR) of QIV vs TIV ranged from likely not cost-effective [≥$140,000 per quality-adjusted life year (QALY) gained] to cost-saving.
Considering the above, the present study aimed at systematically assessing the available pharmacoeconomic evidence behind the use of aTIV in the elderly population; indeed, among the three aforementioned elderly-specific vaccines, aTIV was licensed first in 1997,Citation12 but no comprehensive reviews have been conducted so far. The published systematic evidenceCitation13–Citation15 suggests that influenza vaccination is cost-effective/cost-saving in the elderly but it is unclear which of the different available alternativesCitation7 has the most favorable economic profile. Available systematic reviews on economic assessments of influenza vaccination programs have included a few studiesCitation13,Citation15 that specifically addressed aTIV or other elderly-specific vaccines. We, however, hypothesized a larger pool of available evidence on the cost-effectiveness of aTIV, given that it has been on the market over the last 20 years. Moreover, our study was prompted by the recent decision of Public Health EnglandCitation19 on the preferential use of aTIV in the over-65 age group. Similarly, the very recent normative document issued by the Italian Ministry of HealthCitation20 advocated for the preferential use of aTIV in the oldest age group. In summary, the objective of this study was to assess the economic profile of using aTIV in the elderly population as compared to both “doing nothing” and available alternatives and to figure out how this depends on the study setting. The present study should therefore aid decision-makers of different jurisdictions (aTIV is available in more than 30 countriesCitation21) in establishing an optimal influenza immunization strategy in the elderly.
Methods
Compliance with international reporting standards
The study was carried out by consulting the Joanna Briggs Institute (JBI) guidelines on conducting systematic reviews of economic evaluation evidence.Citation22 Our findings were reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.Citation23 The internal protocol (in Italian) is available upon request.
Eligibility criteria
The primary studies of interest were model-based economic evaluations including both full [e.g., cost-effectiveness (CEA), cost-utility (CUA), budget impact (BIA), cost-benefit (CBA) analyses] and partial [e.g., cost-consequences analysis (CCA)] economic assessments. However, study-based economic evaluations were also potentially eligible candidates for inclusion.
According to the current indication of aTIV,Citation21 the study population included adults aged ≥65 years independently of age subgroup (e.g., 65–74 or ≥75 years), risk profile (e.g., at low or high risk of developing influenza-related complications), institutionalization (e.g., community-dwelling or nursing home residents). aTIV comparators could include both non-vaccination or either competitor.
Papers on economic evaluations of (i) the pediatric formulation of aTIV, (ii) previously available adjuvanted vaccines (e.g., virosomal), (iii) off-label (i.e., age <65 years) use of aTIV, (iv) highly selected population groups non-representative to the general population and (v) pandemic/pre-pandemic adjuvanted vaccines were excluded. Moreover, when a study was first presented at a conference or published in a “grey literature” modality but then published in a peer-reviewed journal, we considered only the latter record.
Search strategy
The automatic search strategy was adapted from previous systematic reviews on aTIVCitation24,Citation25 and those on economic evaluations of influenza vaccinesCitation13,Citation18 and is reported in Supplementary file 1. Medline via Ovid, Embase, Database of Abstracts of Reviews of Effects (DARE) and the NHS Economic Evaluation Database (EED) were the primary information sources. Considering a relatively small amount of output produced by the DARE/NHS EED databases and for the purpose of increasing sensitivity, only terms relative to aTIV (“MF59”, “adjuvanted influenza vaccine”) were searched. No time, language or other restrictions were applied.
We then proceeded with searching for conference abstracts and grey literature (at www.abstract-archive.org and www.opengrey.eu) using the same simplified search strategy. The subsequent manual search included the following modalities: (i) standard reference checking; (ii) screening of the most pertinent journals (Vaccine, Human Vaccines and Immunotheraputics, Expert Review of Vaccines, Journal of Vaccines and Vaccination, Influenza and Other Respiratory Viruses, The European Journal of Health Economics, Expert Review of Pharmacoeconomics and Outcomes Research, Health Affairs, Health Economics, Journal of Health Economics, PharmacoEconomics, Quality of Life Research, Value in Health); and (iii) searches for articles in Google Scholar that cited the included papers. Finally, in a post-hoc modality, we cross-checked the reference lists of all known systematic reviews on economic evaluations of influenza vaccination in the elderly.Citation13,Citation18
Potentially eligible records from all information sources were pooled into a spreadsheet and screened for duplicates. As a standard procedure, titles and abstracts were then evaluated to remove clearly irrelevant records; subsequently full texts of potentially eligible studies were assessed for inclusion and exclusion criteria. The search was performed on 12–16/07/2018 independently by IL and cross-checked by IL and AS; any disagreement was solved by consensus.
Data extraction and abstraction
In order to extract potentially useful information, the JBI data extraction form for economic evaluationsCitation22 was first consulted. It was then adapted to stay in line with the study objectives. In particular, the following key data were extracted and abstracted: authors, publication year, publication type (journal article or conference abstract), country/territory, currency and reference year, study population and setting, type of economic evaluation and key features of model structure/key assumptions, strategies evaluated and/or comparators, perspective, time horizon and discount rate, methods for principal data extraction/imputation, primary outcome measures, funding source, main results (both the base case and uncertainty analyses) and authors’ conclusions.
We would like to point out the following notes on our extraction/abstraction strategy. First, while the inclusion of conference abstracts that were not published in full may sometimes mitigate the publication bias,Citation26 the information available in the abstracts is provided in limited detail; moreover, the peer-review process of contributions submitted to conferences may be less rigorous.Citation27 Indeed, a recent systematic review by de Boer et al.Citation18 excluded conference abstracts. We, however, decided to retain the abstracts with the aim of (i) providing the reader the broadest horizon possible and (ii) checking whether the unpublished abstracts differ somehow from the published full texts (i.e., non-positive results are often less likely to be published in full). In any case, in order to circumscribe the intrinsic shortcomings of the conference abstracts we reported them separately from ordinary journal articles.
Second, a note should be made for what concerns the study intervention/strategy. We decided to dichotomize the included studies into “comparison of aTIV with non-vaccination and/or competitors” and “comparison of Public Health strategies”. Indeed, a Public Health vaccination strategy could compare different scenarios, in which different vaccine types are administered to different population groups (e.g., QIV to subjects aged 18–64 years and aTIV to subjects aged ≥65 years) or in different proportions to account for real world market splits (a usual case of BIA). Indeed, aTIV is a vaccine specifically designed for the over 65, while unadjuvanted TIVs/QIVs may be administered to everyone aged of 6 months and above. However, from our personal experience, dynamic models usually include all age-cohorts to see the indirect effect of influenza vaccination (e.g., reduction of influenza cases in the elderly by vaccinating children). By contrast, in a head-to-head comparison of aTIV with “doing nothing” or other competitors, it is intrinsically assumed that a given intervention (i.e., either vaccine or non-vaccination) is given to 100% of a given population group. Therefore, we decided that the variable “study intervention/strategy” should be split into the aforementioned categories as the between-category comparison would be difficult to interpret or even misleading.
Third, all costs were extracted and reported in their original currency. However, in order to make results more comparable, the costs were first trended to 2017 and then exchanged to 2017 US dollars (2017$) by using data of the Organization for Economic Co-operation and Development (OECD),Citation28,Citation29 as described earlier.Citation18 If the exact costing year was not explicitly reported, it was imputed as a difference between the year of publication and 3 years.Citation18
Data were extracted/abstracted by IL and cross-checked by IL and AS; any disagreement was solved by discussion.
Quality appraisal
The quality assessment was performed by using the JBI appraisal checklist for economic evaluations.Citation22 This tool evaluates the quality of economic evaluations through 11 items with four possible response options, namely “Yes”, “No”, “Unclear” and “Not applicable”. The JBI appraisal tool covers different domains, including the available comparators, (e.g., Is there comprehensive description of alternatives?), effectiveness (e.g., Has clinical effectiveness been established?), costs and outcomes (e.g., Are costs and outcomes valued credibly?), etc. The tool also provides an explicit guideline on voting procedure.
The quality appraisal of the included studies was independently conducted by IL and MT; the inter-rater agreement was measured by Cohen’s κ. The identified disagreements were solved by consensus. The quality of conference abstracts was not assessed.
It is worth noting that no study was removed on the basis of a low score obtained by applying the JBI checklist, but pertinent remarks were made in the text.
As suggested by a peer-reviewer, we performed a post-hoc quality reporting assessment of the included full papers by using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.Citation30
Overall synthesis of results
All studies were summarized qualitatively. In order to summarize the key findings of included studies, whenever possible, we followed the 3-by-3 dominance ranking matrix proposed by JBI.Citation22 Briefly, this scheme envisages three categories (always aTIV vs comparator), namely “Strong dominance” [i.e., (i) aTIV is more effective and less costly; or (ii) aTIV is effective and less costly; or (iii) aTIV has equal cost and is more effective], “Weak dominance” [i.e., (iv) aTIV is equally costly and effective; or (v) aTIV is more effective and more costly; or (vi) aTIV is less effective and less costly] and “Non-dominance” [(vii) aTIV more costly and less effective; or (viii) aTIV is equally as costly and less effective; or (ix) aTIV is more costly and as effective]. Considering that the 3-by-3 dominance ranking matrix takes into account only incremental cost-effectiveness measures, the results of BIAs were not summarized using this tool.
Results
Study selection process
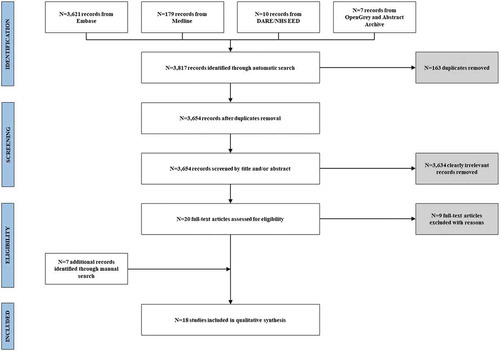
The initial automatic search produced a total of 3,817 results. After duplicate removal (N = 163), a total of 3,654 titles and abstracts were screened and clearly irrelevant records (N = 3,634) were removed. The full text assessment allowed including 11 papers. The list of excluded studies is provided in Supplementary file 2. Furthermore, two potentially eligible studies and five conference abstracts were identified through manual search. A total of 18 studies, corresponding to 13 full papersCitation31-Citation43 and 5 conference abstractsCitation44-Citation48 were included in the qualitative assessment. The whole process of study selection is illustrated in .
Characteristics of the included studies: full papers
The main characteristics of the included studies are reported in . Most studies (N = 10) were written in English,Citation31,Citation32,Citation34–Citation37,Citation39–Citation41,Citation43 while the remaining two in SpanishCitation38,Citation42 and one in Italian,Citation33 respectively. All studies were conducted in high-income countries, namely Italy (N = 5),Citation32,Citation33,Citation36,Citation41,Citation43 the US (N = 3),Citation34,Citation37,Citation40 Spain (N = 2),Citation38,Citation42 Canada (N = 2)Citation35,Citation39 and France (N = 1).Citation31 Most studies (N = 8)Citation31–Citation33,Citation35,Citation37,Citation41–Citation43 were funded/supported by pharmaceutical companies and/or included industry employees as authors; three studiesCitation34,Citation39,Citation40 were funded/supported by a public body, while the funding source of other two studiesCitation36,Citation38 was not clearly stated. The study population was set to individuals aged 65 years and older in 10 studies,Citation32–Citation34,Citation36,Citation38–Citation43 while in the earliest studyCitation31 it consisted of the elderly at high risk. The over 65 population, in fact, can be considered at high risk of developing complications due to influenza because it has at least one serious chronic disease (diabetes, heart attack, angina pectoris, other heart disease, stroke, chronic bronchitis, chronic obstructive pulmonary disease, liver cirrhosis, malignancy, Alzheimer’s, senile dementia, parkinsonism, renal failure). The remaining two studiesCitation35,Citation37 included all age cohorts (people aged <65 years could receive TIV and/or QIV).
Table 1. Main characteristics of the included studies.
From the point of view of modelling characteristics, most studies could be classified as CEAs/CUAs (N = 8)Citation31–Citation35,Citation37,Citation40,Citation43 and BIAs (N = 4).Citation36,Citation38,Citation41,Citation42 One studyCitation39 was declared by the author as CEA; however, it seemed to be a CCA with some elements of CBA. All ICURs in CUAs were expressed as cost/QALY gained. Among CEAs the following outcomes were used: cost/life year gained,Citation31 cost/death avoided,Citation31,Citation33 cost per episode of influenza-like illness (ILI) avoidedCitation33 and incremental net benefit (INB).Citation32 A note on BIAs should be also made. Only the most recent BIA by Barbieri et al.Citation41 considered a “realistic” scenario where each of the alternatives available to the elderly was “weighted” by its market share, while the remaining three BIAsCitation36,Citation38,Citation42 assumed a universal use aTIV vs TIV and/or non-vaccination. Most models were static, conducted from the third payer’s perspective in a time horizon of one year (one influenza season). At least one type of uncertainty analysis was reported in all studies but one.Citation39 Three studies were classified as “comparison of Public Health strategies”, while the remaining studies compared aTIV with other competitors and/or non-vaccination ().
Characteristics of the included studies: conference abstracts
All the five abstractsCitation44-Citation48 were CUAs. Four studiesCitation44-Citation47 included an industry employee as an author, while the funding source was unclear in the fifth.Citation48 All the models were likely conducted from the third payer’s perspective of the National Healthcare System (NHS) of Germany,Citation44 Spain,Citation45 Italy,Citation46 the UKCitation47 and Canada.Citation48 Two modelsCitation47,Citation48 were dynamic, included all-age cohorts and compared different Public Health strategies, while all abstracts by Petri et al.Citation44–Citation46 were static, included only the elderly population and compared single vaccines (TIV, aTIV, idTIV and QIV) and non-vaccination.
Comparison between aTIV and non-vaccination
ativ was explicitly compared to “doing nothing” in five full papers,Citation32,Citation33,Citation36,Citation39,Citation43 mostly in the context of ItalyCitation32,Citation33,Citation36,Citation43 (). Compared with non-vaccination, aTIV seems to give good value for money at least from the third payer perspective being cost-savingCitation32,Citation33 or cost-effective.Citation43 The main ICUR drivers were natural influenza attack rate, aTIV effectiveness, aTIV acquisition price, vaccine administration cost, cost of hospitalization due to respiratory complications and QALY loss due to an episode of influenza. Likewise, its universal use in the elderly was associated with net budget savingsCitation36 ().
Table 2. Key findings of the included studies (full papers): adjuvanted trivalent inactivated vaccine (aTIV) vs non-vaccination.
Results of the comparison with non-vaccination were also reported in three conference abstracts: aTIV has been found to be a cost-effective strategy in Germany [ICUR: €925/QALY (2017$1,094/QALY)]Citation44 and Italy [ICUR: €12,305 (2017$14,061)/QALY]Citation46 and dominant in Spain [incremental costs: -€0.48 (2017$0.55)/person, incremental QALYs: 0.0016/person).Citation45
Comparison between aTIV and other vaccine types
TIV was the most common active comparator used in all included full papers (). Among the included CEAs/CUAs aTIV was deemed cost-savingCitation32-Citation34 or cost-effective.Citation31,Citation43 The earliest paper by Piercy et al.Citation31 is the only to report an incremental cost-effectiveness ratio (ICER) separately for each virus strain: it was the lowest for A(H3N2) and the highest for A(H1N1). In the sensitivity analysis the following variables drove significantly the results: influenza attack rate, aTIV and TIV effectiveness, and acquisition price of both vaccines. From the point of view of budget impact, the use of aTIV was associated with net savings in ItalyCitation33,Citation36 and Spain.Citation38,Citation42 Three conference abstracts by Petri et al.Citation44–Citation46 reported consistent results. In Germany,Citation44 it was stated that aTIV was dominant over TIV at a relative price increase of up to 31% [€9.20 (2017$10.88) and €12.06 (2017$14.26), respectively]. It was analogously cost-effective [at the willingness-to-pay (WTP) threshold of €30,000/QALY] in SpainCitation45 and ItalyCitation46 with an incremental price of 352% [€3.10 (2017$3.54) vs €14.00 (2017$15.96)] and 156% [€6.15 (2017$7.03) vs €15.75 (2017$16.30)], respectively.
Table 3. Key findings of the included studies (full papers): adjuvanted trivalent inactivated vaccine (aTIV) vs non-adjuvanted trivalent inactivated vaccine (TIV).
A head-to-head comparison aTIV vs idTIV was reported in a full paper by Capri et al..Citation43 From the Italian NHS perspective, aTIV dominated idTIV in the base case scenario. In the probabilistic sensitivity analysis, the acceptability curves of four vaccines (TIV, aTIV, idTIV and QIV) showed that at the threshold of €30,000/QALY idTIV had the probability of 25.5% to be the best choice, while the same probability for aTIV was 58.8%.
A full paper by Capri et al.Citation43 reported results on a direct comparison aTIV vs QIV. Considering that there was no effectiveness estimate for QIV in the elderly, the latter was assumed to be more effective than TIV by 35% in case of B lineage mismatch (relative to B lineage strain included in trivalent formulations); the frequency of B lineage mismatch was set to 61.8%. In the base case scenario, QIV was clearly dominated by aTIV from the Italian NHS perspective [incremental costs: -€5.2 (2017$5.86)/person, incremental QALYs: 2x10−7/person]. In the probabilistic sensitivity analysis, QIV remained constantly dominated by aTIV independently from the WTP threshold set.
Finally, Raviotta et al.Citation40 compared different vaccines available to the US elderly; however, aTIV was used as a comparator only in a secondary analysis (and therefore several relevant data were not described) since at the time the study was conducted, aTIV was not available in the US. In the primary analysis, hdTIV was found to be cost-effective in comparison with non-vaccination, TIV and QIV. The authors then conducted a secondary two-way sensitivity analysis by varying the relative effectiveness of aTIV vs TIV in a range of 0–50% and its price in the range of $10–50. At the WTP threshold of $100,000/QALY, aTIV was not favored if its relative effectiveness increase was <15% but was favored if its relative effectiveness was ≥32%. Moreover, if it is assumed that the relative effectiveness vs TIV was the same as hdTIV vs TIV (24.2%), it would be favored if its acquisition price was less than $31.20 (2017$32.29).
Comparison between different public health strategies
Fisman et al.Citation35 compared the universal use of TIV in Canada with two alternative strategies, namely (i) use of aTIV in the elderly and TIV in children aged <6 years and (ii) use of aTIV in both children and the elderly. Moreover, each scenario could also include the immunization with TIV of subjects aged 6–64 years. Compared with the universal offering of TIV to the entire population aged >0.5 years, the strategy in which aTIV was used in the elderly while TIV in persons aged 0.5–64 years produced an ICUR of C$2,111 (2017$1,853)/QALY gained. When aTIV offer was extended to children, the ICUR dropped to <C$500 (2017$439)/QALY gained. The latter strategy was also highly cost-effective [C$1,612 (2017$1,415)/QALY gained] when compared with that of TIV to people aged 0.5–64 years and aTIV to the elderly. A similar picture was seen in the comparison of scenarios when people aged 6–64 years were excluded from the vaccination offer [TIV to children and the elderly vs TIV to children and aTIV to the elderly: C$1,424 (2017$1,250)/QALY; TIV to children and the elderly vs aTIV to both children and the elderly: <C$300 (2017$263)/QALY; aTIV to both children and the elderly vs TIV to children and aTIV to the elderly: C$1,190 (2017$1,045)/QALY].
The US study by Mullikin et al.Citation37 compared the differential use of QIV and aTIV (administered to subjects aged <65 and ≥65 years, respectively) to the universal all-age use of QIV and TIV. This comparison was made using nine scenarios according to the intensity of seasonal influenza activity (low, average and high) and matching (low, average and high correspondence between circulating and vaccine strains) levels. From the societal perspective, the differential use of QIV/aTIV was dominant over QIV independently from influenza intensity and match level. QIV/aTIV versus TIV was the dominant strategy in all but three scenarios where the former was deemed cost-effective [ICURs of $9,980 (2017$10,649)/QALY, $28,761 (2017$30,690)/QALY and $1,955 (2017$2,086)/QALY in the scenarios low intensity + average match, low intensity + high match and average intensity + high match, respectively]. A similar picture was seen when only direct costs were considered. The differential use of QIV/aTIV was constantly dominant over QIV. Compared with the universal use of TIV, the strategy QIV/aTIV was dominant in three scenarios (average intensity + low match, high intensity + low match, high intensity + average match), while it was cost-effective in other six scenarios with the ICUR range of $1,619–34,404 (2017$1,728–36,712)/QALY gained.
A recent BIA by Barbieri et al.Citation41 compared the real-world strategy with the observed market share of all available vaccines for the elderly in Italy (TIV, aTIV, idTIV and QIV) with a hypothetical strategy in which each vaccine is administered according to the age (65–74 and ≥75 years) and risk profile (low and high risks). Switching to the alternative strategy was associated with a significant reduction in terms of occurrence of influenza-related events and slightly higher total vaccination campaign costs (+1.5%) that, however, were offset by a reduction of costs associated with lower number of influenza events and therefore generating net budget savings. In the scenario analyses where QIV took the whole quotum of TIV in low-risk people aged 65–74 years (with or without QIV price reduction of 20%), only a slight increase in total costs was seen [€0.07–0.24 (2017$0.08–0.27)/person].
Finally, two conference abstracts compared different Public Health strategies in the UKCitation47 and Canada,Citation48 by using dynamic modelling approach. Nguyen et al.Citation47 compared the current (not well-defined) UK strategy with a hypothetical one where aTIV is preferentially used in people aged ≥65 years. At a WTP threshold of £20,000 (2017$26,667)/QALY and aTIV price of £16 (2017$21.3), the strategy would be highly cost-effective with an ICUR of £3,540 (2017$4,720)/QALY. In the probabilistic sensitivity analysis, the ICUR was <£19,048 (2017$25,397)/QALY in 97% of simulations. It was also estimated that the use of aTIV would be cost-neutral to the UK NHS when aTIV price is within a range of £10–13 (2017$13.3–17.3). A recent conference abstract by Fisman et al.Citation48 has reported that in Canada, over a 10-year period, a differential vaccination strategy (aTIV for both infants and the elderly, QIV for children and TIV for adults) would produce 325,000 QALYs gained and C$1.33 (2017$1.04) billion in savings, as compared with non-vaccination.
Quality assessment of the included full papers
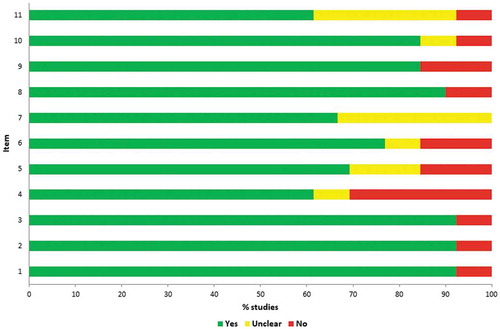
Although the absolute agreement in judging single quality attributes was relatively high (79.2%), Cohen’s κ was deemed only moderate (0.46). Following the reconciliation process, it was observed that the overall quality of the included studies could be judged moderate to good; each item was met by at least 60% of studies (). However, only two studiesCitation31,Citation43 were deemed to meet all quality criteria. A notable exception was the study by GarlapatiCitation39 that did not meet 8 out of 11 quality attributes (Supplementary file 3). That study was identified through manual search and was published in a journal not currently indexed in either Medline or Embase. There were no obvious patterns relating the study quality to the year and language of publication, funding source and overall study findings.
Figure 2. Quality appraisal of the included studies (Joanna Briggs Institute checklist).
Notes: Item 1, Is there a well-defined question?; Item 2, Is there comprehensive description of alternatives?; Item 3, Are all important and relevant costs and outcomes for each alternative identified?; Item 4, Has clinical effectiveness been established? Item 5, Are costs and outcomes measured accurately? Item 6, Are costs and outcomes valued credibly? Item 7, Are costs and outcomes adjusted for differential timing? Item 8, Is there an incremental analysis of costs and consequences? Item 9, Were sensitivity analyses conducted to investigate uncertainty in estimates of cost or consequences? Item 10, Do study results include all issues of concern to users? Item 11, Are the results generalizable to the setting of interest in the review?
The CHEERS checklist produced almost similar results (Supplementary file 4). The most frequent regarded reporting of currency, price date and conversion (item 9); conflict of interest (item 24); study setting and location (item 5) and analytical methods used (item 17). Indeed, these items were not reported/clearly reported by 69%, 54%, 38% and 38% of included studies.
Overall synthesis of results
The JBI 3-by-3 dominance ranking matrix showed that all pairwise comparisons could be categorized as strong or weak dominance of aTIV (Supplementary file 5). In particular, compared with non-vaccination, aTIV was more effective and less costly in 4 comparisons and more effective and more costly in 2 comparisons. When compared with TIVs, aTIV established a strong dominance in 6 comparisons and a weak dominance in 2 comparisons. aTIV was directly compared to idTIV and QIV only in the study by Capri et al.;Citation43 aTIV was strongly dominant over both comparisons (Supplementary file 5).
By analyzing the studies that compared different Public Health vaccination strategies, it seems that the strategies incorporating aTIV on the basis of age and/or risk profile are associated with the most favorable economic outcomes.
Discussion
The present paper is the first to comprehensively assess the economic profile of the use of aTIV in the elderly population. Our main conclusion is that aTIV is “good value for money” independently from the comparator evaluated. The concept of “value for money” is central to health policy (especially among the universal healthcare systems) and the main reason for that is related to accountability. In particular, payers should be reassured that their money is being spent wisely, while patients should be reassured that their claims on the NHS are being treated fairly and consistently.Citation49
In line with this concept, a comparison with non-vaccination revealed that aTIV was highly cost-effective or cost-saving in all included studies; this finding is consistent with the previously published systematic evidence on the overall economic value of influenza vaccination in the elderly.Citation13 Similarly, aTIV has been found to be cost-effective/dominant in comparison with TIV and dominant over QIV and idTIV. From the point of view of “real-world” Public Health strategies, the available evidence suggests that there is a need to establish an optimal combination of existing vaccines that is based on age and/or risk profile of the target population groups. The latter finding is in line with recently formulated guidelines issued by health authorities in the UK,Citation19 ItalyCitation20 and Australia.Citation50 In particular, in the UK general practitioners and community pharmacists were advised to preferentially use aTIV in all people aged ≥65 years and QIV in people at risk aged 18–64 years.Citation19 The most recent Italian recommendations issued by the Ministry of HealthCitation20 suggested that both aTIV and QIV may be used in people aged ≥65 years. However, given that the A(H3N2) subtype has the highest burden in the oldest age group (≥75 years) and the evidence of better effectiveness of aTIV in this particular age group, aTIV should be preferred over both QIV and TIV among over-75 elderly people. In other younger target populations, QIV should be preferred over TIV (unless the former is unavailable).Citation20 Similarly, in AustraliaCitation50 both aTIV and hdTIV are preferentially recommended over QIV in ≥65-year-olds, while QIVs are to be used in all target groups <64 years. In summary, our review advocates the recent policy changes from the health economics point of view. Furthermore, a somewhat similar theoretical concept of the appropriateness of the use of different influenza vaccines has been proposed by Bonanni et al..Citation51 It is, however, interesting to note that in the US, although hdTIV, aTIV and QIV are available, the Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2018–19 Influenza Season for the elderly population does not express any preference for the type of vaccine to be used. For persons aged ≥65 years, any TIV age-appropriate formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or QIV are acceptable options.Citation52 Additional data concerning these vaccines from studies examining immunogenicity and non-laboratory-confirmed influenza outcomes are needed. The main problem in the US is the lack of robust studies so ACIP will continue to review data concerning the efficacy and effectiveness of these vaccines as more information emerges and will state new recommendations.
The present systematic review has both strengths and limitations. The main strength is that we were able to analyze a significantly higher number of original studies on the economic profile of aTIV than any previously published systematic review focusing on influenza vaccines of any type.Citation13-Citation15 This was likely achieved thanks to a sufficiently high sensitivity and specificity of the adopted search strategy. Another strength concerns the fact that the included studies had similar conclusions and therefore the overall review summary and interpretation were straightforward. The main drawback was that the inter-rater agreement of the quality appraisal was not high (even if it was judged moderate). The most probable explanation is that both raters studied the checklist independently and did not agree before how to vote. However, to make comparisons, we were not able to identify any assessment on the reliability and other psychometric properties of the JBI checklist. However, our post-hoc application of the CHEERS checklist produced roughly comparable to the JBI tool results; indeed, the latter is partially based on the former.
We then would like to discuss the principal limitations of the included papers. First, most comparisons were made with TIV and non-vaccination, while aTIV was compared to idTIV, hdTIV and QIV only in two studies. This is not surprising since about half of studies were conducted in the period when only TIV and aTIV were available. However, given that newer vaccine formulations have recently entered the market of some jurisdictions (e.g. ccQIV and rQIV), novel models should be constructed. Indeed, the recent observational study by the Food and Drug Administration showed that ccQIV and hdTIV were more effective in preventing hospitalizations for influenza-related events than standard egg-based QIV by 11% and 8% in the US elderly.Citation53 Second, the reporting quality should be improved. One may observe that several studies were published about 10–15 years ago when economic evaluations of the vaccines had just commenced. Third, most studies were conducted from the third payer perspective only. This is likely due to the fact that most of the studies included were carried out in countries with National and/or Regional tender policies, like Italy and Spain. However, the recent recommendation issued by the World Health Organization (WHO) on the economic evaluation of influenza vaccinationCitation54 has stated that the societal perspective should be ideally adopted, while (where possible) other perspectives should be also reported separately. Indeed, the third payer perspective underestimates indirect costs even in the case of elderly since the latter may contribute to the economy by looking after grandchildren during influenza epidemics.Citation43 Forth, most included studies somehow (directly or indirectly) involved the industry. The same pattern has been established by the recently published systematic review of cost-effectiveness studies on QIV.Citation18 This so-called “funding” or “sponsorship” biasCitation55 may skew study outcomes in favor of the sponsoring company’s product. However, the exact mechanisms and level of influence of the funding bias are still largely unknown and inconclusive.Citation56 In any case, we believe that despite a limited budget available to governmental and Regional Public Health authorities (especially on the condition of healthcare spending review seen in recent years) independent economic evaluations of the available influenza vaccine formulations should be performed and made readily available to all relevant stakeholders given that annual influenza vaccination in the elderly is a well-recognized, cost-effective and targetable goal.Citation43 Indeed, independently funded economic studies would further reassure both taxpayers and patients. The fifth limitation identified by the authors is conditional and regards the fact that most of the included studies were static and not dynamic. While the static models are relatively easy to construct and interpret, they do not account for herd protection effects;Citation54 this, indeed, underestimates the total vaccine-induced savings. However, most models specifically targeted the elderly, where herd protection is less important, while all age-class CUAs models were dynamic. Moreover, static models usually produce more conservative estimates.Citation18 Therefore, this limitation has a limited impact on our findings.
Apart from the above-mentioned suggestions for future research, the authors would like to acknowledge that most economic evaluations of QIV (identified through the systematic review by de Boer et al.Citation18) compared the latter to a generic category of “TIVs”. It is therefore not clear whether the “TIV” category was composed of only standard unadjuvanted TIV or included all trivalent formulations together (i.e., TIV plus enhanced elderly specific aTIV, idTIV and hdTIV). Indeed, the second hypothesis is unacceptable given that enhanced trivalent elderly-specific vaccine formulations have been found more immunogenic and effective than both TIVs and QIVs.Citation25,Citation57-Citation60 Compared with trivalent vaccine formulations QIV is expected to provide a higher effectiveness by covering both B lineages. Type B lineage mismatch is a frequent event and the degree of cross-lineage protection provided by TIV is still controversial.Citation61,Citation62 On the other hand, a recent systematic review and meta-regression analysis by Beyer et al.Citation63 has established that the cross-lineage protection highly depends on age of vaccinees; QIV is therefore expected to provide significant benefits in younger populations, while its advantage over TIV is rather limited in the elderly.Citation63
To conclude, aTIV use in people aged 65 years and over seems to be a good value for money and it may be cost-saving in some jurisdictions. Future economic evaluations should be up-to-date and include either available alternatives. Health authorities should give general practitioners the opportunity to choose the most appropriate vaccines tailored to specific patients. In this regard, an availability of clinical guidelines on which specific vaccine type is to be administered to a specific population group (such as those recently issued in the UK,Citation19 ItalyCitation20 and AustraliaCitation50) may be a core component. Indeed, formal guideline development expertise among physicians may represent a bottom-up approach by training end-users of such guidelines in order to develop their own explicit evidence-based guidelines which meet nationally recognized criteria.Citation64
Disclosure of potential conflicts of interest
IL has been serving as consultant for Seqirus srl since January 2019 for an unrelated project. AS, AN, MT declare no conflict of interest.
Supplemental Material
Download Zip (62 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- World Health Organization (WHO). Vaccines against influenza WHO position paper – november 2012. Wkly Epidemiol Rec. 2012;87:461–76.
- Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, Maringhini G, van Lier A, Haagsma JA, Havelaar AH, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the burden of communicable diseases in Europe study, European union and European economic area countries, 2009 to 2013. Euro Surveill. 2018 Apr;23(16). doi:10.2807/1560-7917.ES.2018.23.16.17-00454. PubMed PMID: 29692315; PubMed Central PMCID: PMC5915974.
- Panatto D, Signori A, Lai PL, Gasparini R, Amicizia D. Heterogeneous estimates of influenza virus types A and B in the elderly: results of a meta-regression analysis. Influenza Other Respir Viruses. 2018 Mar 2;12:533–43. doi:10.1111/irv.12550. Epub ahead of print.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 Jan 8;289(2):179–86.
- de Lusignan S, Correa A, Ellis J, Pebody R. Influenza vaccination: in the UK and across Europe. Br J Gen Pract. 2016 Sep;66(650):452–53. doi:10.3399/bjgp16X686677.
- Hannoun C The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013 Sep;12(9):1085–94. doi:10.1586/14760584.2013.824709. Epub 2013 Sep 12.
- Treanor JJ. Clinical practice. Influenza vaccination. N Engl J Med. 2016;375:1261–68. doi:10.1056/NEJMcp1512870. PMID:27682035.
- Demicheli V, Jefferson T, Di Pietrantonj C, Ferroni E, Thorning S, Thomas RE, Rivetti A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2018 Feb 1;2:CD004876. doi:10.1002/14651858.CD004876.pub4. Review. PubMed PMID: 29388197.
- Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: support for policies to vaccinate elderly people against influenza. Vaccine. 2013;31(50):6030–33. doi:10.1016/j.vaccine.2013.09.063.
- Bragazzi NL, Orsi A, Ansaldi F, Gasparini R, Icardi G. Fluzone® intra-dermal (Intanza®/Istivac® Intra-dermal): an updated overview. Hum Vaccin Immunother. 2016 Oct 2;12(10):2616–27. doi:10.1080/21645515.2016.1187343. Epub 2016 May 31.
- Robertson CA, DiazGranados CA, Decker MD, Chit A, Mercer M, Greenberg DP. Fluzone® high-dose influenza vaccine. Expert Rev Vaccines. 2016 Dec;15(12):1495–505. doi:10.1080/14760584.2016.1254044. Epub 2016 Nov 14.
- O’Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011 Apr;10(4):447–62. doi:10.1586/erv.11.23.
- Shields GE, Elvidge J, Davies LM. A systematic review of economic evaluations of seasonal influenza vaccination for the elderly population in the European Union. BMJ Open. 2017 Jun 10;7(6):e014847. doi:10.1136/bmjopen-2016-014847.
- Ting EEK, Sander B, Ungar WJ. Systematic review of the cost-effectiveness of influenza immunization programs. Vaccine. 2017 Apr 4;35(15):1828–43. doi:10.1016/j.vaccine.2017.02.044. Epub 2017 Mar 9. Review. PubMed PMID: 28284681.
- D’Angiolella LS, Lafranconi A, Cortesi PA, Rota S, Cesana G, Mantovani LG Costs and effectiveness of influenza vaccination: a systematic review. Ann Ist Super Sanita. 2018 Jan-Mar;54(1):49–57. doi:10.4415/ANN_18_01_10. PubMed PMID: 29616674.
- European Centre for Disease Prevention and Control (ECDC). Seasonal influenza vaccination and antiviral use in Europe – overview of vaccination recommendations and coverage rates in the EU Member States for the 2013–14 and 2014–15 influenza seasons. Stockholm: ECDC; 2016. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Seasonal-influenza-vaccination-antiviral-use-europe.pdf.
- Donaldson C, Mugford M, Vale L, eds. Evidence-based Health Economic: from effectiveness to efficiency in systematic review. London, UK: BMJ Books; 2002.
- de Boer PT, van Maanen BM, Damm O, Ultsch B, Dolk FCK, Crépey P, Pitman R, Wilschut JC, Postma MJ A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev Pharmacoecon Outcomes Res. 2017 Jun;17(3):249–65. doi:10.1080/14737167.2017.1343145. Epub 2017 Jun 28.
- Public Health England (PHE). Guidance. Summary of data to support the choice of influenza vaccination for adults in primary care. https://www.gov.uk/government/publications/flu-vaccination-supporting-data-for-adult-vaccines/summary-of-data-to-support-the-choice-of-influenza-vaccination-for-adults-in-primary-care.
- Italian Ministry of Health. Prevention and control of influenza: recommendations for the season 2018/19. http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2018&codLeg=64381&parte=1%20&serie=null.
- Fluad®. Prescribing information. http://www.fluad.com/Common/docs/FLUAD_Package_Insert.pdf
- Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. 2015 Sep;13(3):170–78. doi:10.1097/XEB.0000000000000063.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
- An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Literature review on pediatric Fluad® influenza vaccine use in children 6-72 months of age. http://www.phac-aspc.gc.ca/naci-ccni/assets/pdf/pediatric-pediatrique-fluad-eng.pdf
- Domnich A, Arata L, Amicizia D, Puig-Barberà J, Gasparini R, Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine. 2017 Jan 23;35(4):513–20. doi:10.1016/j.vaccine.2016.12.011. Epub 2016 Dec 23. Review. PubMed PMID: 28024956.
- Schmucker CM, Blümle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, von Elm E, Briel M, Meerpohl JJ. OPEN consortium. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS One. 2017 Apr 25;12(4):e0176210. doi:10.1371/journal.pone.0176210. eCollection 2017.
- Burton HE, Mitchell SA, Watt M. A systematic literature review of economic evaluations of antibiotic treatments for Clostridium difficile infection. Pharmacoeconomics. 2017 Nov;35(11):1123–40. doi:10.1007/s40273-017-0540-2.
- Organization for Economic Co-operation and Development (OECD). Consumer price indices. https://stats.oecd.org/Index.aspx?DataSetCode=PRICES_CPI&_ga=2.40857698.1932218757.1546681581-1435710676.1546681579#
- Organization for Economic Co-operation and Development (OECD). Exchange rates. https://data.oecd.org/conversion/exchange-rates.htm#indicator-chart
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. doi:10.1016/j.jval.2013.02.010.
- Piercy J, Ryan J, Megas F. Economic evaluation of MF59 adjuvanted vaccine against influenza in the high-risk elderly population in France. J Med Econ. 2004;7:1–18. doi:10.3111/200407001018.
- Baio G, Pammolli F, Baldo V, Trivello R. Object-oriented influence diagram for cost-effectiveness analysis of influenza vaccination in the Italian elderly population. Expert Rev Pharmacoecon Outcomes Res. 2006;6(3):293–301. doi:10.1586/14737167.6.3.293.
- Iannazzo S, Sacchi V. Valutazione farmacoeconomica dei programmi di vaccinazione influenzale nella popolazione anziana italiana. Farmacoeconomia E Percorsi Terapeutici. 2009;10(2):59–72. doi:10.7175/fe.v10i2.164.
- Lee BY, Ercius AK, Smith KJ. A predictive model of the economic effects of an influenza vaccine adjuvant for the older adult (age 65 and over) population. Vaccine. 2009;27(16):2251–57. doi:10.1016/j.vaccine.2009.02.024.
- Fisman DN, Tuite AR. Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada. PLoS One. 2011;6(11):e27420. doi:10.1371/journal.pone.0027420.
- Iannazzo S. Pharmacoeconomic evaluation of the MF59-adjuvanted influenza vaccine in the elderly population in Italy. J Prev Med Hyg. 2011;52:1–8.
- Mullikin M, Tan L, Jansen JP, Van Ranst M, Farkas N, Petri E. A Novel dynamic model for health economic analysis of influenza vaccination in the elderly. Infect Dis Ther. 2015;4(4):459–87. doi:10.1007/s40121-015-0076-8. Erratum in: Infect Dis Ther. 2015;4(4):489–90.
- Ruiz-Aragón J, Grande Tejadab AM, Márquez-Peláezc S, García-Cenozd M. Estimación del impacto de la vacunación antigripal con adyuvante MF59 en población mayor de 64 años para el Sistema Nacional de Salud: efectos y costes. Vacunas. 2015;16:6–11. doi:10.1016/j.vacun.2015.02.002.
- Garlapati S. Decision analytic modeling for cost-effectiveness evaluation of adjuvanted and non-adjuvanted flu vaccines in the elderly 65+. J Vaccines Vaccn. 2016;2:00028.
- Raviotta JM, Smith KJ, DePasse J, Brown ST, Shim E, Nowalk MP, Zimmerman RK. Cost-effectiveness and public health effect of influenza vaccine strategies for U.S. elderly adults. J Am Geriatr Soc. 2016;64(10):2126–31. doi:10.1111/jgs.2016.64.issue-10.
- Barbieri M, Capri S, Waure C, Boccalini S, Panatto D. Age- and risk-related appropriateness of the use of available influenza vaccines in the Italian elderly population is advantageous: results from a budget impact analysis. J Prev Med Hyg. 2017 Dec 30;58(4):E279–E287. doi:10.15167/2421-4248/jpmh2017.58.4.867. eCollection 2017 Dec. PubMed PMID: 29707658; PubMed Central PMCID: PMC5912787.
- Pérez-Rubio A, Eiros JM. Economic and Health impact of influenza vaccination with adjuvant MF59 in population over 64 years in Spain. Rev Esp Quimioter. 2018 Feb;31(1):43–52. Epub 2017 Jan 18. Spanish. PubMed PMID: 29355006.
- Capri S, Barbieri M, de Waure C, Boccalini S, Panatto D. Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population. Hum Vaccin Immunother. 2018 Jun 3;14(6):1331–41. doi:10.1080/21645515.2018.1438792. Epub 2018 Feb 26. PubMed PMID: 29425079; PubMed Central PMCID: PMC6037461.
- Petri E. Cost-effectiveness analyses of various seasonal influenza vaccines available for elderly in Germany. Eur J Public Health. 2015;25(Suppl 3):436. doi:10.1093/eurpub/ckv176.167.
- Petri E A comparison of cost-effectiveness of seasonal influenza vaccines in Spain. Presented at IVW Conference; 2015 Oct 6–9; Albufeira (Portugal).
- Petri E Health economics for seasonal influenza vaccination of older adults in Italy. Presented at Options IX conference underscores our commitment to the prevention and control of seasonal influenza, Chigago, US.
- Nguyen VH, Kelly C, Mansi JA UK health economic model demonstrates use of adjuvanted trivalent seasonal influenza vaccine in older adults to be highly cost-effective. Presented at ESWI, Riga, Latvia.
- Fisman D, Tuite A Estimating the relative cost-effectiveness of competing seasonal influenza vaccination programs in Canada: a multi-strain dynamic modeling approach. Presented at: ECCMID, Madrid, Spain.
- Smith PC Measuring value for money in healthcare: concepts and tools. https://www.who.int/pmnch/topics/economics/20091027_smith/en/
- The Australian Immunisation Handbook. 10th ed. [ accessed 2018 Feb]. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/90ADD02CDCB5D49DCA257D4D0022D886/$File/4-7-Influenza-2018-02-19.pdf.
- Bonanni P, Boccalini S, Zanobini P, Dakka N, Lorini C, Santomauro F, Bechini A. The appropriateness of the use of influenza vaccines: recommendations from the latest seasons in Italy. Hum Vaccin Immunother. 2018 Mar 4;14(3):699–705. doi:10.1080/21645515.2017.1388480. Epub 2017 Dec 1. PubMed PMID: 29059004; PubMedCentral PMCID: PMC5861775.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2018–19 influenza season. MMWR Recomm Rep. 2018;67(No.RR–3):1–20. doi:10.15585/mmwr.rr6703a1. Hanon E, Van der Most R, Del Giudice G, Rappuoli R. Short-term and mid-term solutions for influenza vaccines. Lancet Infect Dis. 2018 Aug;18(8):832-833. doi:10.1016/S1473-3099(18)30404-3. PubMed PMID: 30064669.
- Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among the U.S. elderly, 2017-18. J Infect Dis. 2018 Dec 18. doi:10.1093/infdis/jiy716.
- Newall AT, Chaiyakunapruk N, Lambach P, Hutubessy RC WHO guide on the economic evaluation of influenza vaccination. Influenza Other Respir Viruses. 2018 Mar;12(2):211–19. doi:10.1111/irv.12510. Epub 2017 Dec 27.
- Krimsky S. Do financial conflicts of interest bias research?: an inquiry into the “Funding Effect” hypothesis. Sci Technol Hum Values. 2013;38(4):566–87. doi:10.1177/0162243912456271.
- Naci H, Dias S, Ades AE. Industry sponsorship bias in research findings: a network meta-analysis of LDL cholesterol reduction in randomised trials of statins. BMJ. 2014 Oct 3;349:g5741. doi:10.1136/bmj.g5741.
- Coudeville L, Andre P, Bailleux F, Weber F, Plotkin S. A new approach to estimate vaccine efficacy based on immunogenicity data applied to influenza vaccines administered by the intradermal or intramuscular routes. Hum Vaccin. 2010;6:841–48.
- Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19(17–19):2673–80. doi:10.1016/S0264-410X(00)00499-0.
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta–analysis. Gerontology. 2003;49(3):177–84. doi:10.1159/000069172.
- Lee JKH, Lam GKL, Shin T, Kim J, Krishnan A, Greenberg DP, Chit A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018 May;17(5):435–43. doi:10.1080/14760584.2018.1471989.
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010 Feb 25;28(9):2149–56. doi:10.1016/j.vaccine.2009.11.068.
- Skowronski DM, Chambers C, De Serres G, Sabaiduc S, Winter AL, Dickinson JA, Gubbay JB, Drews SJ, Fonseca K, Charest H, et al. Vaccine effectiveness against lineage matched and mismatched influenza B viruses across 8 seasons in Canada, 2010-11 to 2017-18. Clin Infect Dis. 2018 Oct 11 . doi:10.1093/cid/ciy876.
- Beyer WEP, Palache AM, Boulfich M, Osterhaus ADME. Rationale for two influenza B lineages in seasonal vaccines: A meta-regression study on immunogenicity and controlled field trials. Vaccine. 2017 Jul 24;35(33):4167–76. doi:10.1016/j.vaccine.2017.06.038.
- World Health Organization (WHO) Regional Office for Europe. Guidelines in health care practice. http://www.euro.who.int/__data/assets/pdf_file/0011/118379/E53492.pdf