ABSTRACT
In 2016, an outbreak of mumps occurred in a primary school in China with a student population having high vaccination coverage. An unmatched case-control study was performed to identify risk factors contributing to this outbreak, and a retrospective cohort study was conducted to evaluate the effectiveness of mumps-containing vaccine (MuCV). A total of 97 cases were identified during the outbreak, and the overall attack rate was 8.2%. Among students with confirmed vaccination status, 90% had received at least one dose of MuCV. Cases were more likely than non-cases to report taking the school bus during the epidemic period (adjusted OR = 2.3, 95% CI: 1.4–3.7). Vaccine effectiveness (VE) was higher for two-dose MuCV (76%, 95% CI:49–89%) than for one-dose MuCV (59%, 95% CI: 36–74%. The protection afforded by both one-dose and two-dose MuCV waned over time, from 82% among students vaccinated within 5 years to 41% among those vaccinated more than 10 years previously for one-dose VE, and from 90% to 25% over the same time period for two-dose VE. We found that outbreaks of mumps can occur in schools despite high coverage of one-dose MuCV vaccination. Although the VE of both two-dose and one-dose MuCV wanes over time, the overall VE for two-dose MuCV was superior than that of one-dose MuCV. Therefore, a two-dose MuCV schedule through routine services is likely needed in order to control mumps epidemics in China.
Introduction
Mumps is an infectious disease caused by the mumps virus and manifesting clinically as swollen salivary glands, fever, and headache.Citation1 Mumps is a vaccine-preventable disease, and mumps-containing vaccine (MuCV) is used in almost all 194 WHO member countries.Citation2 As the effectiveness of the mumps vaccine is about 80%, two doses of the vaccine are generally needed to achieve herd immunity.Citation1 Moreover, a third dose of the measles–mumps–rubella (MMR) vaccine has been recommended to improve control over mumps outbreaks.Citation3,Citation4 By the end of 2016, 121 out of 194 WHO member countries used the MMR vaccine for routine immunization, and 102 countries implemented the two-dose MMR vaccination.Citation2,Citation5 Consequently, the incidence of mumps has fallen dramatically, and mumps has been to some extent overlooked in recent years. However, large outbreaks occurring in highly immunized populations and in some industrialized countries have sparked renewed interest in mumps.Citation6-Citation9
In China, both genotypes F (99.0%) and G (1.0%) were identified between 2013 and 2015, genotype F being the predominant genotype over the previous 20-year period.Citation10 However, the mumps vaccine is manufactured using the S79 strain, which is derived through further attenuation from the US Jeryl Lynn strain, which itself belongs to genotype A.11,Citation12 This may suggest a mismatch of the vaccine virus strain with the circulating outbreak strains. However, mumps has been nearly eliminated despite the possible mismatch between the genotype of circulating viruses and that of the vaccine virus in many countries,Citation13-Citation15 indicating that antigenic mismatch between epidemic strains and vaccine strains has not greatly hindered vaccine effectiveness.
Three types of MuCV have been used to control mumps since 1990: measles and mumps combined vaccine (MM), monovalent mumps vaccine (MuV), and MMR vaccine. In practice, mumps vaccination in China was voluntary in 1990 and was not included in routine immunization, resulting in low vaccination coverage at that time.Citation10 After the policy for one-dose MMR vaccination targeting children aged 18–24 months was introduced in the Expanded Program on Immunization (EPI) in 2008, reports of mumps cases declined slightly, and the natural epidemic pattern of mumps has changed since that time.Citation10,Citation16 However, mumps has continued to spread in vaccinated individuals over the last decade, and outbreaks occurring predominantly in schoolsCitation17,Citation18 have prompted concerns about the effectiveness of the one-dose MMR vaccine immunization strategy currently used in China. The occurrence of continuous outbreaks highlights the need for introducing a two-dose immunization schedule in China.
From October 2016 to January 2017, an outbreak of mumps occurred in a school in Anhui Province. We investigated the outbreak to estimate mumps vaccine effectiveness (VE) and to detect potential risk factors for infection during the outbreak.
Methods
Study design
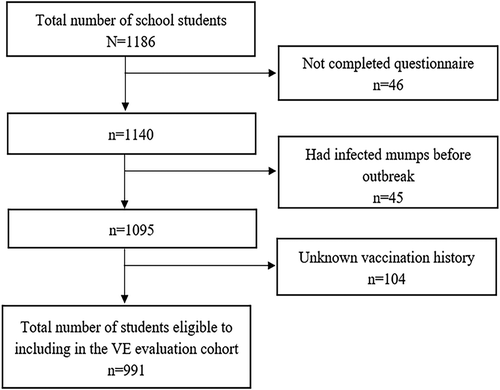
We conducted an unmatched case-control study to identify risk factors contributing to the 2016–2017 outbreak in Lu’an, Anhui Province. A retrospective cohort study was then conducted to evaluate the effectiveness of MuCV during this outbreak. A total of 149 students with mumps history of mumps before the outbreak and unknown vaccination history were excluded from the cohort. We designed a structured questionnaire that was sent to parents of all of the students in the school where the outbreak occurred. Data collected in the questionnaire included demographic characteristics, vaccination status, clinical characteristics, possible risk factors, and previous incidence of mumps.
Case definition and ascertainment
For this school-based outbreak, we defined a clinical case of mumps as any student in the school having acute onset of unilateral or bilateral parotid gland swelling lasting ≥ 2 days and without other apparent cause between October 1, 2016 and January 31, 2017. We reviewed data on mumps cases from: (1) cases reported to the National Notifiable Communicable Disease Report System (NNDRS), (2) hospital medical records, and (3) affirmative answer in the questionnaire completed by parents or care-givers for the item: “Has your child gotten mumps after October 2016?”. After collecting case status for each student in the cohort, we verified whether each case had been reported in the NNDRS. For cases that were not reported in the NNDRS, we interviewed parents by telephone to confirm case status and collected additional information (e.g., date of illness onset and principal residence, etc.). Since we did not conduct laboratory tests, all of the cases were defined based on clinical diagnosis or self-report, and the outbreak strains were not characterized or sequenced.
Vaccination status
We collected information on vaccination status primarily from vaccination certificates provided by students. Students with mumps onset occurring within two weeks of vaccination were considered to be unvaccinated. For students who did not have vaccination certificates, we searched in the Anhui Immunization Information Management System (AIIMS) by child’s name, parents’ names, and birth dates to obtain vaccination status. All of the data on vaccination status and timing of vaccinations were reviewed and confirmed by EPI staff from Lu’an Municipal Centers for Disease Control and Prevention (CDC).
Data analysis
Data from the structured questionnaires were doubly entered using Epidata software version 3.1 (Epidata Association, Odense, Denmark), and statistical analyses were performed using SPSS version 10.01 (Statistical Product and Service Solutions, Chicago, IL, US). We performed univariate analyses to determine the unadjusted ORs and 95% confidence intervals (CI) for case status in relation to vaccination status and other risk factors. Covariates significantly associated with disease (P < 0.05) in univariate analysis were included in a multivariate logistic regression model. We used a stepwise regression to create the most parsimonious multivariate model by eliminating covariates that were not significant (P > 0.05). To evaluate VE, we used the formula VE = [(ARU − ARV)/ARU] × 100%, where ARV and ARU are the attack rate of vaccinated students and unvaccinated students respectively. A P-value < 0.05 was considered to be statistically significant.
Results
Outbreak description
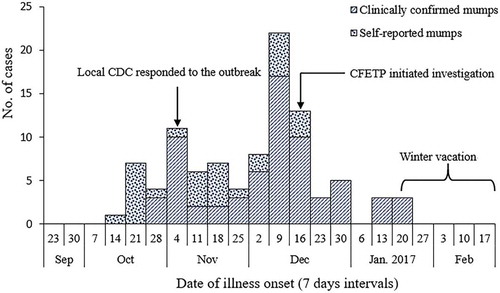
A total of 97 mumps cases (67 clinically confirmed and 30 self-reported cases) were reported from October 2016 to January 2017. The overall attack rate (AR) was 8.2% (97/1186). All of the cases were students aged between 6–15 years (median age 8 years), and 62.9% (61/97) were male. As shown in , this outbreak lasted roughly 4 months and the onset of illness peaked from December 9–22, 2016. Signs and symptoms experienced by cases included parotid gland swelling (100%), fever (22.7%), headache (13.4%), lethargy (7.2%), and vomiting (4.1%). In addition, 9.3% of cases experienced complications, including meningitis, orchitis, and hearing damage.
Among the 97 cases, 57.7% (56/97) of cases went to school via school bus. From October 1–November 10, 2016 (first wave of the epidemic), 90.5% (19/21) of cases took the school bus to school before onset of illness. The index case was a 7-year-old boy who had received a one-dose MMR vaccine. The source of his infection was unknown. He received no medical care for the infection and continued to go to school by school bus after the onset of illness. Fourteen patients (14.4% of cases) were not promptly isolated, and 8 of these students (57.1%) still took the school bus to school after illness onset. Cases were identified in 23 school classes (96% of all classes at the school) during the outbreak. The highest AR within a class was 21% and the lowest was 2%.
Vaccination status
Out of 1186 students at the school, 1140 had completed questionnaires (response rate of 96.1%). Vaccination status was verified for 1026 students (90.0%) through vaccination certificates (n = 930) or AIIMS (n = 96). Before the outbreak, 919 students (89.6%) had received at least one dose of MuCV. The distribution of the number of previous MuCV vaccine doses (0, 1, 2, 3) was 107 (10.4%), 747 (72.8%), 155 (15.1%), and 17 (1.7%), respectively.
Of the 97 cases, 60 (61.9%) had received one dose of MuCV, 8 (8.2%) had received two doses, and 21 (21.6%) had not been immunized. Vaccination status was unknown for the other 8 cases (8.2%). Of the 68 cases who had received at least one dose of MuCV, 64 (94.1%) had received their most recent dose more than 5 years before the outbreak.
Risk factors
The final multivariable regression model included two significant covariates: taking the school bus to school and MuCV status. We found that cases had 2.3 times the odds (95% CI: 1.4–3.7) of going to school by school bus compared to non-cases. Since 90% of students had received at least one dose of MuCV, we calculated the odds of being a case associated with different time intervals since last MuCV immunization compared with unvaccinated students. We found that students who received MuCV had reduced odds of mumps (). In addition, a significant dose–response relationship was observed between increasing time interval since the last dose of MuCV vaccination and mumps infection (χCitation2 = 7.712, p = 0.005). We also found that vaccination coverage was 91.8% (346/377) among students who took the school bus and 88.3% (573/649) among students who did not take the school bus, a difference that was not statistically significant (χCitation2 = 3.105, p = 0.078).
Table 1. Analyses for risk factors for mumps infection during an outbreak in Lu’an, China, 2016–2017.
MuCV effectiveness
We enrolled 991 cohort members from the school after excluding 149 respondents with pervious mumps history or unknown immunization history (). The cohort included 89 cases and 902 non-cases. None of the individuals with a history of mumps developed the disease during this outbreak. The overall AR in unvaccinated students was 20.4% (21/103), compared to 7.7% (68/888) in vaccinated students, and the overall VE for MuCV was 62% (95% CI: 41–76%). The VE was 59% (95% CI: 36–74%) for one-dose MuCV, 76% (95% CI: 49–89%) for two-dose MuCV, and 100% for three-dose MuCV (). For one-dose MuCV, the VE differed by the time since vaccination. The estimated VE was 82% (95% CI: 40–94%) for students vaccinated within 5 years, 60% (95% CI: 35–75%) for students vaccinated between 5 and 10 years previously, and 41% (95% CI: −11–69%) for students vaccinated more than 10 years previously. Similar results were observed for students who had received two-dose MuCV. In this group, effectiveness was 90% (95% CI: 26–99%) among students who had received the second dose within 5 years before the outbreak, compared to 73% (95% CI: 31–89%) among those who had received the second dose between 5 and 10 years before the outbreak.
Table 2. Unadjusted vaccine effectiveness by MuCV dosage and vaccination interval, Lu’an, China, 2016–2017.
Discussion
We reported on a field investigation of a mumps outbreak occurring in a school with 1186 students in China. In this school setting, 90% of students with known vaccination status had received at least one dose of MuCV in childhood, suggesting a high herd immunity level before the outbreak. However, our data showed that the overall VE for MuCV was only 62% in this school, although the VE of two-dose MuCV (76%) was superior to that of one-dose MuCV (59%). Furthermore, we observed that the protection offered by both one-dose and two-dose MuCV waned over time. Finally, our analyses illustrated that taking the school bus to school during the epidemic period and waning immunity conferred by MuCV were likely contributors to this outbreak.
Although the efficacy for the Jeryl Lynn strain of mumps vaccine was estimated at more than 95% in early randomized clinical trials,Citation19,Citation20 varying effectiveness has been observed in epidemic or outbreak conditions.Citation8,Citation18 The US CDC found that the average vaccine effectiveness for one dose of the Jeryl Lynn strain of mumps vaccine is 78% (range: 49–91%), compared with 88% (range: 66–95%) for two-dose vaccine.Citation21 A meta-analysis to assess VE of MuCV in the Chinese population found an overall VE of 69% (95% CI: 51–80%) among school students.Citation12 In our study, we found that a two-dose mumps vaccination conferred higher protection than one-dose vaccination, similar to findings from previous studies.Citation16,Citation22 Our results are also in agreement with a sero-epidemiological survey on mump antibodies.Citation23 In China, despite implementation of large-scale immunization with one-dose MMR in 2008, the incidence of mumps has not declined substantially since then.Citation10,Citation24 In the school where our study outbreak occurred, although 17% of parents had paid extra for the second or third dose of MuCV for their children, the coverage was not enough to prevent the outbreak. However, the incidence of mumps and occurrence of outbreaks have declined in some more developed provinces in China where two-dose MMR vaccine has been introduced into EPI.Citation16,Citation24 These results suggest that a two-dose MMR vaccination schedule through routine services is likely to be needed in order to contain the prevalence of mumps and reduce ongoing outbreaks in China. Changes in epidemic patterns of mumps after introduction of a two-dose vaccine schedule also need to be carefully considered in China.
We found that the protection afforded by both one-dose and two-dose MuCV waned over time, with VE decreasing from 82% to 41% for one-dose MuCV and from 90% to 25% for two-dose MuCV. This finding confirms that students who had their last MuCV dose more than 10 years before the outbreak had an increased risk of mumps.Citation3 Fortunately, the VE for MuCV was excellent within a 5-year timeframe, thus offering a favorable opportunity to implement the two-dose immunization strategy. There are at least two options for China to implement this strategy: using MMR vaccine as a substitute for the MM vaccine at 8 months of age, and using a second dose of MMR vaccine within 5 years of administration of the first dose. Considering that the majority of mumps outbreaks in China occur in primary schoolsCitation17,Citation18 and in light of the waning immunity for two-dose MuCV over time,Citation4,Citation25 we recommend that the second MuCV dose be given at 6 years of age.
We found that 90% of students in the school where the outbreak occurred had received at least one dose of MuCV. However, large mumps outbreaks may still occur and spread despite high vaccination coverage. Potential reasons for the occurrence of outbreaks in highly vaccinated populations include waning immunity conferred by vaccinationCitation3,Citation25,Citation26 and intense proximity in semi-closed populations.Citation27,Citation28 The herd immunity threshold for mumps has been estimated to be within the range of 70–90%.Citation29 Consequently, if we assume VE of 80% for mumps vaccines, two doses of MuCV are likely needed in order to achieve herd immunity.Citation1 However, our estimated VE for MuCV was under this theoretical threshold, especially within 5–10 years, where it ranged from 60–73%. This may have been sufficient to account for the outbreak under investigation in our study. Additionally, with the loss of vaccine-induced immunity to mumps over time, the intense exposure on school buses overwhelmed the protection afforded by the vaccine.
Our study had several limitations. First and foremost, all of the cases were identified by clinical symptoms without laboratory confirmation, thus we may have underestimated the VE for MuCV. Second, some of the VE estimates were unstable owing to the small number of cases in some subgroups, as reflected by the wide confidence intervals. Despite these limitations, our study provides valuable evidence to guide the mumps immunization strategy for China.
In conclusion, this study showed that mumps control remains a challenge in China in spite of high coverage of one-dose MuCV. To contain the prevalence of mumps in China, a two-dose MMR schedule through routine services is likely needed.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
Wei QIN and Yao WANG participated in the data analysis and drafted the manuscript. Hong SU critically reviewed and supervised development of the paper. Shao-yu XIE, Kai-chun LI, and Wei QIN designed the retrospective cohort study and the questionnaire. Tao YANG, Xiao-kang XU, Xiang-mei MENG, Chang-jun ZHAO, and Shao-yi LI performed the field epidemiological investigation. All of the authors contributed to the review and revision of the final manuscript.
Ethical considerations
Ethical clearance was not required as this outbreak investigation was urgent and considered to be a public health response.
Acknowledgments
We would like to acknowledge the contributions of all of the participants in the outbreak investigation, especially public health workers from the Jin’an CDC and Zhongdian Township Health Hospital. Many thanks for the support from the Field Epidemiology Training Program of the China CDC.
References
- Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008;371(9616):932–44. doi:10.1016/S0140-6736(08)60419-5.
- Ma C, Hao LX, Su QR, Wen N, Fan CX, Yang H, Wang HQ, Li L. Vaccination schedules, reported vaccination coverage rates, and incidences of measles, mumps and rubella of the 194 member states of the world health organization. Chin J Vaccines Immunization. 2015;21(3):241–7+254. (In Chinese).
- Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, Marin M, Riley J, Feikin DR, Patel M, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. N Engl J Med. 2017;377(10):947–56. doi:10.1056/NEJMoa1703309.
- Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. Sci Transl Med. 2018;10(433):S25. doi:10.1126/scitranslmed.aao4496.
- Beleni AI, Borgmann S. Mumps in the vaccination age: global epidemiology and the situation in Germany. Int J Environ Res Public Health. 2018;15(8):1618. doi:10.3390/ijerph15061188.
- Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. Br Med J. 2005;330(7500):1132–35. doi:10.1136/bmj.330.7500.1132.
- Dayan GH, Quinlisk MP, Parker AA, Barskey AE, Harris ML, Schwartz JM, Hunt K, Finley CG, Leschinsky DP, O’Keefe AL, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358(15):1580–89. doi:10.1056/NEJMoa0706589.
- Domínguez A, Torner N, Castilla J, Batalla J, Godoy P, Guevara M, Carnicer D, Caylà J, Rius C, Jansà JM. Mumps vaccine effectiveness in highly immunized populations. Vaccine. 2010;28(20):3567–70. doi:10.1016/j.vaccine.2010.02.107.
- Anis E, Grotto I, Moerman L, Warshavsky B, Slater PE, Lev B. Mumps outbreak in Israel’s highly vaccinated society: are two doses enough. Epidemiol Infect. 2012;140(3):439–46. doi:10.1017/S095026881100063X.
- Cui A, Zhu Z, Hu Y, Deng X, Sun Z, Zhang Y, Mao N, Xu S, Fang X, Gao H, et al. Mumps epidemiology and mumps virus genotypes circulating in mainland China during 2013–2015. PLoS One. 2017;12(1):e0169561. doi:10.1371/journal.pone.0169561.
- Cui A, Zhu Z, Mao N, Si Y, Ma Y, Hu Y, Deng X, Wang L, Zeng L, Zhang Y, et al. Assessment of one-dose mumps-containing vaccine effectiveness on wild-type genotype F mumps viruses circulating in mainland China. Vaccine. 2018;36(38):5725–31. doi:10.1016/j.vaccine.2018.08.028.
- Wang H, Hu Y, Zhang G, Zheng J, Li L, An Z. Meta-analysis of vaccine effectiveness of mumps-containing vaccine under different immunization strategies in China. Vaccine. 2014;32(37):4806–12. doi:10.1016/j.vaccine.2014.05.061.
- Davidkin I, Kontio M, Paunio M, Peltola H. MMR vaccination and disease elimination: the finnish experience. Expert Rev Vaccines. 2010;9(9):1045–53. doi:10.1586/erv.10.99.
- Galazka AM, Robertson SE, Kraigher A. Mumps and mumps vaccine: a global review. Bull World Health Organ. 1999;77(1):3–14.
- Slater PE, Anis E, Leventhal A. The control of mumps in Israel. Eur J Epidemiol. 1999;15(8):765–67.
- Ma R, Lu L, Zhou T, Pan J, Chen M, Pang X. Mumps disease in Beijing in the era of two-dose vaccination policy, 2005–2016. Vaccine. 2018;36(19):2589–95. doi:10.1016/j.vaccine.2018.03.074.
- Man W, Jin-Kou Z, Tao W, Li-Xin H, Chao M, Qi-Ru S, Hui-Ming L. Mumps-containing vaccine effectiveness during outbreaks in two schools in Guangdong, China, 2012. Western Pac Surveill Response J. 2012;3(4):29–32. doi:10.5365/WPSAR.2012.3.4.012.
- Ma C, Liu Y, Tang J, Jia H, Qin W, Su Y, Wang H, Hao L. Assessment of mumps-containing vaccine effectiveness during an outbreak: importance to introduce the 2-dose schedule for China. Hum Vaccin Immunother. 2018;14(6):1392–97. doi:10.1080/21645515.2018.1428508.
- Sugg WC, Finger JA, Levine RH, Pagano JS. Field evaluation of live virus mumps vaccine. J Pediatr. 1968;72(4):461–66.
- Hilleman MR, Weibel RE, Buynak EB, Stokes J, Whitman JE. Live attenuated mumps-virus vaccine. IV. Protective efficacy as measured in a field evaluation. N Engl J Med. 1967;276(5):252–58. doi:10.1056/NEJM196702022760502.
- Centres for Disease Control and Prevention (CDC). VPD surveillance manual. 5th ed. 2012. Chapter 9: Mumps. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt09-mumps.pdf.
- Deeks SL, Lim GH, Simpson MA, Gagné L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. CMAJ. 2011;183(9):1014–20. doi:10.1503/cmaj.101371.
- Pang H, Zhou Y, Zhao W, Jiang Q. Seroprevalence and determinants associated with mumps antibodies after 20 years of MMR vaccination in urban area of Shanghai, China. Int J Environ Res Public Health. 2018;15(10):2089. doi:10.3390/ijerph15061188.
- Su QR, Liu J, Ma C, Fan CX, Wen N, Luo HM, Wang HQ, Li L, Hao LX. Epidemic profile of mumps in China during 2004–2013. Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50(7):611–14. In Chinese. doi:10.3760/cma.j.issn.0253-9624.2016.07.009.
- Vygen S, Fischer A, Meurice L, Mounchetrou NI, Gregoris M, Ndiaye B, Ghenassia A, Poujol I, Stahl JP, Antona D, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveillance. 2016;21(10):30156. doi:10.2807/1560-7917.ES.2016.21.10.30156.
- Clemmons NS, Redd SB, Gastanaduy PA, Marin M, Patel M, Fiebelkorn AP. Characteristics of large mumps outbreaks in the United States during July 2010-December 2015. Clin Infect Dis. 2018. doi:10.1093/cid/ciy779.
- Barskey AE, Schulte C, Rosen JB, Handschur EF, Rausch-Phung E, Doll MK, Cummings KP, Alleyne EO, High P, Lawler J, et al. Mumps outbreak in orthodox Jewish communities in the United States. N Engl J Med. 2012;367(18):1704–13. doi:10.1056/NEJMoa1202865.
- Sane J, Gouma S, Koopmans M, de Melker H, Swaan C, van Binnendijk R, Hahné S. Epidemic of mumps among vaccinated persons, The Netherlands, 2009–2012. Emerg Infect Dis. 2014;20(4):643–48. doi:10.3201/eid2004.131681.
- Edmunds WJ, Gay NJ, Kretzschmar M, Pebody RG, Wachmann H. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: implications for modelling studies. Epidemiol Infect. 2000;125(3):635–50.