ABSTRACT
Invasive meningococcal disease is rare and potentially devastating but often vaccine-preventable. Evaluation of meningococcal vaccine effectiveness is impractical owing to relatively low disease incidence; protection is therefore estimated using serum bactericidal antibody (SBA) assays. Original experiments on natural immunity established a titer of ≥4 as the correlate of protection for SBA assays using human complement (hSBA), but human complement is relatively difficult to obtain and standardize. Use of baby rabbit complement (rSBA assays), per standard guidelines for serogroups A and C, generally results in comparatively higher titers. Postlicensure effectiveness data for serogroup C conjugate vaccines support acceptance of rSBA titers ≥8 as the correlate of protection for this serogroup, but no thresholds have been formally established for serogroups A, W, and Y. Studies evaluating MenACWY-TT (Nimenrix®; Pfizer Inc, Sandwich, UK) immunogenicity have used both hSBA and rSBA assays, and ultimately suggest that rSBA may be more appropriate for these measurements.
Introduction
Meningococcal disease is caused by the Gram-negative bacterium Neisseria meningitidis, an obligate human pathogen.Citation1 The bacterium may colonize the nasopharynx in an asymptomatic state known as carriage.Citation1 Occasionally meningococci will invade the bloodstream to cause invasive meningococcal disease (IMD); IMD can manifest as septicemia and/or meningitis, which occur when the bacteria primarily proliferate in the blood or cerebrospinal fluid, respectively.Citation1 Incidence of IMD is generally low but varies by region; for instance, in 2016, incidence in the European Union was 0.64 per 100,000 populationCitation2 whereas in the United States it was 0.12 per 100,000.Citation3 Despite low incidence, IMD can progress in a matter of hours and can be fatal in approximately 7% to 23% of cases;Citation4–Citation7 a substantial percentage of survivors suffer permanent sequelae such as limb loss, neurologic deficits, or hearing impairment.Citation8 IMD disproportionately affects certain age groups: infants and young children, adolescents and young adults, and older adults (≥65 years of age).Citation4,Citation5
Although 12 meningococcal serogroups have been identified, the majority of disease is caused by serogroups A, B, C, W, and Y.Citation9 Vaccines are available to prevent disease caused by each of these serogroups. There are 3 currently licensed conjugate vaccines targeting meningococcal serogroups A, C, W, and Y (MenACWY vaccines); each vaccine includes capsular polysaccharides from each of the 4 serogroups individually conjugated to a carrier protein. These quadrivalent conjugate vaccines include MenACWY-D (Menactra®; Sanofi Pasteur, Swiftwater, PA, USA), which uses diphtheria toxoid (D) as the carrier protein;Citation10 MenACWY-CRM197 (Menveo®; GlaxoSmithKline, Rixensart, Belgium), which uses a non-toxic mutant of diphtheria protein, CRM197;11 and MenACWY-TT (Nimenrix®; Pfizer Inc, Sandwich, UK), which uses tetanus toxoid (TT).Citation12 Several monovalent meningococcal conjugate vaccines targeting a single serogroup are also available, including MenC-TT (Neis-Vac-C™; Pfizer Ltd, Kent, UK)Citation13 and MenC-CRM197 (Menjugate®; GlaxoSmithKline Vaccines Srl, Siena, Italy),Citation14 which both contain serogroup C polysaccharides and use TT and CRM197, respectively, as carrier proteins. MenA-TT (MenAfriVac; Serum Institute of India, Pune, India) is a monovalent serogroup A meningococcal vaccine that uses TT as a carrier protein.Citation15 In addition, Hib-MenC-TT (Menitorix®; GlaxoSmithKline) is a combination conjugate vaccine containing both MenC, conjugated to TT, and Haemophilus influenzae type b.Citation16 Although serogroup B meningococcal vaccines based on capsular polysaccharides are poorly immunogenic,Citation17,Citation18 MenB vaccines targeting conserved subcapsular antigens have become available in recent years; these include MenB-FHbp (Trumenba®, bivalent rLP2086; Pfizer Inc, Philadelphia, PA)Citation19 and 4CMenB (Bexsero®, MenB-4C; GlaxoSmithKline Vaccines Srl).Citation20
As discussed in more detail below, the serum bactericidal antibody (SBA) assay has become a surrogate method for evaluating meningococcal vaccine efficacy. This article reviews the use of SBA assays in development of different meningococcal vaccines, with particular focus on differences between assays using human (hSBA) or baby rabbit (rSBA) complement. Studies evaluating immune responses to MenACWY-TT are presented in detail to highlight such differences and provide an update to an earlier review of MenACWY-TT studies.Citation21 Postlicensure effectiveness findings are also presented in the context of immunogenicity studies.
The SBA assay
Because meningococcal disease is currently relatively rare, studies evaluating efficacy of meningococcal vaccines would require impractically large sample sizes.Citation22 For this reason, it became necessary to develop a surrogate measure with which efficacy could be more easily determined.
In 1969, Goldschneider and colleagues published a seminal paper describing the use of SBA assays and the correlation of results with susceptibility to meningococcal disease.Citation23 To perform the SBA assay, sera from subjects were serially diluted and incubated with a suspension containing a given meningococcal strain; in most experiments, exogenous human complement lacking bactericidal activity to the tested strains was then added (Citation24). Bactericidal activity was determined based on the efficiency of bacterial killing compared with controls, with SBA titers defined as the highest dilution of sera at which ≥50% killing occurred. The authors tested sera from newly enlisted military recruits for SBA activity against circulating serogroup C meningococcal strains that later infected 54 recruits; 5.6% of cases and 82.2% of controls (10 randomly selected men in the same training platoon for each given case) had baseline sera with SBA titers ≥4. These findings suggested that SBA titers ≥4 might be indicative of protection from serogroup C IMD. Much higher percentages of controls compared with serogroup C cases also had SBA titers ≥4 against serogroup A and B strains,Citation23 indicating that SBA activity was mediated by subcapsular antigens in addition to capsular polysaccharides.Citation25 Goldschneider and colleagues also demonstrated that the presence of SBA titers ≥4 against representative strains from serogroups A, B, and C was inversely proportional to IMD incidence across age groups (through age 26 years), providing additional indirect evidence for the correlation of SBA titers ≥4 and protection from IMD.Citation23
Figure 1. Schematic illustrating serum bactericidal antibody assay using either human or baby rabbit complement. For serogroups A and C, the World Health Organization guidelines stipulate that complement sourced from baby rabbits should be used.Citation24 For the subject shown, the SBA titer would be 8. SBA = serum bactericidal antibody. Figure has been adapted with permission from Gandhi A, Balmer P, York LJ. Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba®). Postgrad Med. 2016;128(6):548–556
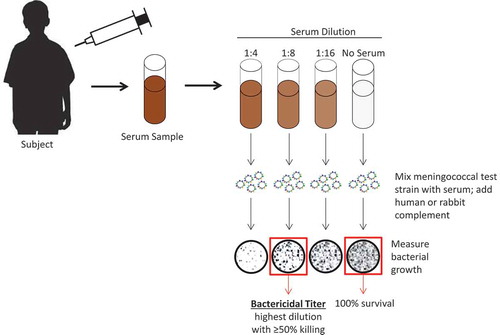
Because evaluating meningococcal vaccine efficacy in clinical trials is impractical owing to the low incidence rate of IMD, the SBA assay became especially useful as a surrogate measure of vaccine efficacy. Several years after the publication of the experiments by Goldschneider and colleagues, the World Health Organization (WHO) stipulated the use of SBA assays using baby rabbit complement to demonstrate efficacy for potential licensure of meningococcal polysaccharide vaccines targeting serogroups A and C.24 Guidelines specified that vaccines should induce a ≥4-fold rise in SBA titers in ≥90% of tested subjects.
Sources of complement used for the SBA assay
The original experiments establishing the correlation between SBA and protection from IMD used human complement (ie, sera containing complement proteins) for performing the SBA assay.Citation23 However, finding suitable human donors can be challenging. Ideally, serum from agammaglobulinemic individuals should be used because it lacks antibodies capable of contributing to bactericidal activity in the assay, but this is a relatively rare condition and most of these individuals receive treatment containing replacement immunoglobulins.Citation26,Citation27 Human complement must therefore be sourced from the relatively few individuals who lack intrinsic bactericidal activity against meningococcus but still exhibit normal complement hemolytic activity (CH50).Citation26,Citation28 Furthermore, each human donor must be screened for each meningococcal strain to be tested before each donation to ensure that natural immunity has not been acquired.Citation28 The difficult task of standardization of human complement across laboratories presents an additional challenge to the use of hSBA assays.Citation29
For serogroup A and C vaccines, complement sourced from baby rabbits was used as an alternative to human complement because it was more easily available in large batches and subject to standardization; results with human and baby rabbit complement also seemed to correlate with one another in initial studies.Citation24,Citation29 rSBA assays thus became the standard surrogate for evaluating meningococcal vaccine efficacy and subsequent licensure. A 1997 study by the US Centers for Disease Control and Prevention (CDC) demonstrated the reproducibility of a standardized rSBA assay method, in which the critical parameters of target strains, incubation times, and complement sources were specified, for serogroups A and C in multiple laboratories across different countries.Citation30 Of note, interlaboratory variability still exceeded intralaboratory variability, indicating that additional parameters contributed to assay results.Citation30
Over time, it became apparent that results from rSBA and hSBA assay analyses did not necessarily correlate with one another. For serogroup C, one study using sera from toddlers and young children vaccinated with MenC-CRM197 identified rSBA titers ≥128 as reliable predictors of hSBA titers ≥4 (using complement from adult donors; ≥80% sensitivity), but most subjects with hSBA titers ≥4 had rSBA titers ≤128.Citation27 Another study for serogroup C demonstrated that rSBA cutoffs of <8 and ≥128 reliably predicted proportions of subjects with hSBA titers <4 and ≥4, respectively, but rSBA titers between 8 and 64 were poorly predictive.Citation31 A more recent study similarly found higher titers measured by rSBA compared with hSBA assays; results additionally demonstrated that although rSBA and hSBA titers were reasonably correlated for serogroup C, they were not correlated for serogroups A and Y.Citation32 It is not clear whether the general discrepancy between rSBA and hSBA results reflect overestimation of rSBA assays, underestimation of hSBA assays, or both;Citation32,Citation33 however, the correlation of rSBA titers ≥8 with vaccine effectiveness for serogroup C in postlicensure studiesCitation34 (discussed in the “Correlation of Observed Protection With Serology” section below) suggests that the ≥4 cutoff for hSBA may be overly conservative.Citation25 The differences between rSBA and hSBA titers are thought to be due, at least partially, to meningococcal factor H binding protein, which binds specifically to human factor H to ultimately enable evasion of complement-mediated killing.Citation35,Citation36 Relatedly, it has been shown that human antibody subclasses may differentially interact with human and rabbit complement.Citation37
It is important to note that specific rSBA and hSBA assays usually differ from one another beyond the source of complement. For example, meningococcal test strains may differ across laboratories.Citation21,Citation33 These factors are also important to consider when comparing different assays and are discussed in greater detail in the “Assays and Strains Used in Different Laboratories” section below.
Of note, a modified version of the hSBA assay was developed in recent years; this high-throughput, automatable method involves using a colored indicator of cell metabolic activity in a liquid medium that can be correlated with hSBA titers.Citation38 Assay results have been demonstrated to correlate with those from conventional hSBA assays,Citation38 and some recent analyses have used this modified assay.Citation39,Citation40
Serogroup B vaccines
A 1983 publication demonstrated that subjects with high rSBA titers against serogroup B meningococcal strains had much lower or even nonexistent bactericidal activity to the same strains in hSBA assays.Citation41 This discrepancy is likely related to anti-MenB polysaccharide antibodies being primarily of the immunoglobulin M subclass, which features relatively low avidity that can further be affected by complement source among other variables.Citation41
An international meeting in 2006 emphasized that human complement was the only acceptable source for SBA assays evaluating MenB vaccines.Citation42 Serogroup B vaccine licensure has generally relied on the percentages of subjects with hSBA titers of ≥4 or ≥4-fold rises in hSBA titers from pre- to postvaccination;Citation43 the more recent licensure of subcapsular-based MenB vaccines has therefore been based on hSBA data.Citation19,Citation20,Citation44,Citation45
Effectiveness data from outer membrane vesicle vaccines have been shown to correlate with hSBA serology.Citation46,Citation47 For a more recently licensed subcapsular-based vaccine, 4CMenB, effectiveness was estimated at 82.9% (95% CI: 24.1, 95.2) among UK infants;Citation48,Citation49 this high percentage validated the use of hSBA assays for predicting vaccine-induced protection.
Complement sources used in studies of serogroup A, C, W, and Y vaccines
In the United Kingdom, MenC conjugate vaccines were licensed on the basis of robust immune responses demonstrated in the rSBA assay; efficacy studies were not required because of demonstrated correlations between efficacy and serologic correlates of protection for meningococcal polysaccharide vaccines in toddlers.Citation31,Citation50 A MenC conjugate vaccine program was subsequently broadly implemented in 1999 on the basis of these immunogenicity data; titers ≥8 were proposed to indicate protectionCitation31,Citation50 and were demonstrated to inversely correlate with disease.Citation51 Coupled with postlicensure data (discussed in greater detail later), these findings led rSBA titers ≥8 to become the generally accepted correlate of protection for MenC conjugate vaccines.Citation43
The clinical development program of MenA-TT followed the UK MenC program and used the rSBA assay.Citation52 Licensure was obtained in India in late 2009 and for countries in the African meningitis belt in 2010 after prequalification by WHO.Citation52 Similarly, recently published results from a phase 1 study of an investigational MenACWXY vaccine intended to target increased rates of serogroup X disease in Africa used rSBA assays for immunogenicity evaluations.Citation53
MenACWY conjugate vaccines have often been licensed on the basis of either hSBA or rSBA assay data, or both. MenACWY-D, the first MenACWY conjugate vaccine licensed by the US Food and Drug Administration, was licensed in 2005 primarily on the basis of rSBA assay data.Citation54 On the other hand, in 2010, MenACWY-CRM197 was approved in the United States at least partially on the basis of hSBA assay data.Citation55 In Europe, MenACWY-CRM197 was licensed in 2009 on the basis of hSBA assay data, although some rSBA assay data were also included in the assessment report.Citation56 Conversely, European licensure of MenACWY-TT in 2012 relied primarily on rSBA assay results, with some hSBA assay data included in the assessment report.Citation57
Complement sources used in MenACWY-TT studies
Studies evaluating immune responses to MenACWY-TT have used rSBA or hSBA assays or both (Tables S1–S5).Citation58–Citation79 In a number of studies spanning multiple age groups, hSBA titers for serogroup A rapidly or steeply declined after vaccination,Citation60,Citation65,Citation67–Citation69,Citation76,Citation78 whereas those studies that evaluated both hSBA and rSBA did not generally observe similar decreases in rSBA titers ( and ).Citation65,Citation68,Citation69,Citation76,Citation78 For unknown reasons, several of these studies also observed somewhat more rapid declines in hSBA for other serogroups compared with rSBA titers.Citation68,Citation76,Citation78 These findings, coupled with previously described data identifying rSBA titers between 8 and 64 as poorly predictive of hSBA titers ≥4 for serogroup C31 (despite subsequent correlation of MenC rSBA titers ≥8 with effectivenessCitation34), suggest that hSBA assay results may underestimate immune responses for MenACWY-TT.Citation25
Figure 2. Percentages of infants/toddlers with rSBA titers ≥8 or hSBA titers ≥4 for serogroups A, C, W, and Y at various time points after vaccination with either 3 primary doses of MenACWY-TT (at 2, 4, and 6 months of age) followed by a booster dose at 15–18 months of age (3 + 1 schedule) or 1 primary dose of MenACWY-TT at 6 months of age followed by a booster dose at 15–18 months of age (1 + 1 schedule). Data are plotted as percentages along with 95% CIs. hSBA = serum bactericidal antibody assay using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y conjugate vaccine using tetanus toxoid as a carrier protein; rSBA = serum bactericidal antibody assay using rabbit complement. Data are from Dbaibo G, Tinoco Favila JC, Traskine M, Jastorff A, Van der Wielen M. Immunogenicity and safety of MenACWY-TT, a meningococcal conjugate vaccine, co-administered with routine childhood vaccine in healthy infants: a phase III, randomized study. Vaccine. 2018;36(28):4102–4111
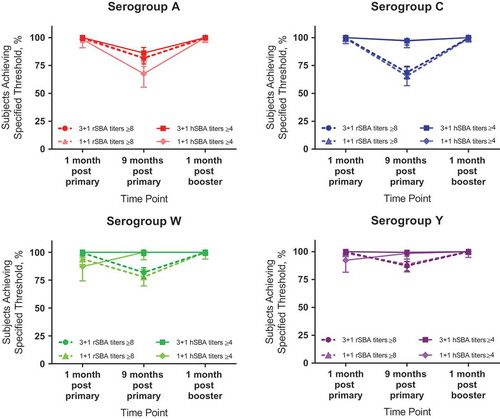
Figure 3. Percentages of toddlers with rSBA titers ≥8 or hSBA titers ≥4 for serogroups A, C, W, and Y at various time points before or after vaccination with 1 dose of MenACWY-TT at 12 to 23 months of age. Data are plotted as percentages along with 95% CIs. hSBA = serum bactericidal antibody assay using human complement; MenACWY-TT = meningococcal serogroups A, C, W, and Y conjugate vaccine using tetanus toxoid as a carrier protein; pre = prevaccination; rSBA = serum bactericidal antibody assay using rabbit complement. Data are from Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–1903
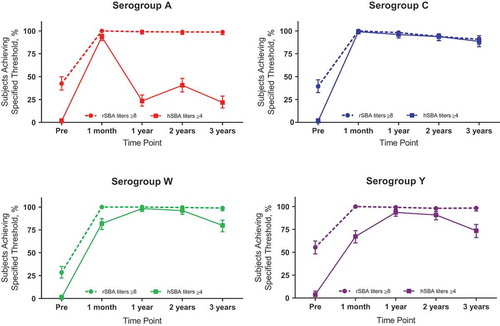
Additionally, several MenACWY-TT studies indicated lower initial immune responses in hSBA assays to serogroups W and Y compared with rSBA assays;Citation62,Citation68,Citation76,Citation78 this was especially evident in toddlers given a single initial dose ( and ).Citation62,Citation76,Citation78 However, when evaluated at later time points, hSBA titers increased over time despite a lack of additional dosing.Citation68,Citation76,Citation78 In contrast, rSBA titers were generally high after initial vaccination and gradually declined when later time points were evaluated.Citation58,Citation61–Citation66,Citation68–Citation79 Such observations from earlier studies have been noted in a previous review by Findlow and Borrow,Citation21 and similar findings from the newer studies provide further evidence of these persistent trends.
These observations collectively suggest that rSBA assays are a more appropriate method than hSBA assays for measuring immune responses and antibody persistence to MenACWY-TT, particularly for serogroup A. In general, hSBA assay results presented in MenACWY-TT studies do not show a clear trend across serogroups.Citation60,Citation62,Citation65,Citation67–Citation69,Citation74,Citation76,Citation78
Correlation of observed protection with serology
Postlicensure studies of vaccine effectiveness have served as critical sources for validation and refinement of the use of SBA assays. For MenC vaccines implemented in the United Kingdom beginning in 1999, a study performed before vaccine introduction indicated that the proportions of individuals with rSBA titers ≥8 were inversely correlated with disease incidence across age groups.Citation51 Subsequently, effectiveness data in multiple age groups (ranging from 90.1–100%) suggested that the percentage of subjects with rSBA titers of ≥128 dramatically underestimated effectiveness; titers of ≥4 or ≥8 were more reflective of observed effectiveness.Citation34 A later study found that overall vaccine effectiveness estimates were comparatively slightly lower for routine vaccination (83%) but similar for catch-up programs; effectiveness was generally higher within 1 year of vaccination compared with later time points.Citation80
In 2011, WHO noted that although use of hSBA titers ≥4 or rSBA titers ≥8 have been used for vaccine licensure for serogroups A, W, and Y, these thresholds have not been formally correlated with protection for these serogroups.Citation81 In 2010, the CDC described a vaccine effectiveness study for MenACWY-D that found a decrease in effectiveness from 91% at 1 year postvaccination to 58% at 2 to 5 years postvaccination.Citation82 These findings corresponded with serologic results available at the time from 5 different studies, which relied on both hSBA and rSBA assays and used a variety of cutoffs (4 or 8 for hSBA and 128 for rSBA assays). These analyses led the CDC to recommend a booster MenACWY vaccine dose at age 16 years in order to maximize protection during the peak risk period of 16–21 years. A more recent study similarly found that effectiveness of MenACWY-D for serogroups C and Y declined over time since vaccination (from 79% within 1 year of vaccination to 61% between 3–8 years of vaccination);Citation83 these data parallel serologic results indicating decreasing proportions of subjects with hSBA titers ≥8 over time.Citation84 Additionally, for MenA-TT, serologic data collected 1 month postvaccination from 2 studies, 1 in toddlers 12 to 23 months of age and 1 in subjects 2 to 29 years of age, in various regions in Africa in 2006 indicated that 96% of toddlers and 78% of older subjects had ≥4-fold increases in rSBA titers.Citation85 Impact data indicated that MenA incidence decreased by 94% in vaccinated areas compared with unvaccinated areas in 2012,Citation86 and a study analyzing data from 2011 to 2015 found a >99% reduction in confirmed MenA cases in African countries following MenA-TT vaccination campaigns.Citation87 These findings support the use of rSBA data for MenA-TT vaccine licensure.
For MenACWY-TT in particular, recent effectiveness data against serogroup W following the 2014 introduction of the vaccine into the Chilean national immunization program for toddlers indicated that effectiveness was 100% and 92.3% in 2015 and 2016, respectively.Citation88 MenACWY-TT is also being used in a nationwide program in England that began in 2015 and mainly targets adolescents.Citation89,Citation90 In the first year of the program, there was a 69% reduction in MenW cases among 2015 school leavers, the first cohort to be vaccinated at general medical practices, despite only 36.6% vaccine coverage.Citation89 The epidemiologic year 2017/2018 was the first year since 2011/2012 that MenW cases declined overall; decreases observed in age groups other than those targeted for vaccination suggest that herd protection has played a role in this reduction.Citation91
Assays and strains used in different laboratories
Approaches to SBA assays, including strain selection, have varied among different laboratories. The standardized method for MenA and MenC rSBA assays published in 1997 specified use of specific strains for these assays.Citation30 Of note, a single representative strain for each serogroup can be used in the case of a vaccine targeting a serogroup-specific capsular polysaccharide;Citation92 the strain recommended for serogroup C evaluations is the same one originally used in the Goldschneider experiments.Citation23,Citation30 For vaccines targeting subcapsular antigens which are not serogroup-specific (ie, currently available MenB vaccines), the choice of strains required for SBA assays to access broad protection is more complicated.Citation92
In 2011, GlaxoSmithKline laboratories reported that use of a MenA strain (strain 3125) different from that specified in the standardized methodCitation30 (strain F8238) might be more appropriate for evaluation of MenA immune responses.Citation93 This suggestion was based on strain 3125 belonging to an immunotype more commonly associated with invasive MenA strains, whereas the strain F8238 immunotype was more commonly associated with carrier strains. Studies across multiple age groups indicated higher levels of natural immunity to strain F8238 as compared with strain 3125 when evaluated in rSBA. By contrast, postvaccination immune responses were similar for the 2 strains, indicating that strain 3125 more accurately captured vaccine-induced protection and that rSBA results using strain F8238 might be artificially high. A recent MenACWY-TT study indicates continued use of strain 3125.Citation69 As might be expected, differences in strain selection and other aspects of protocol dictate the inherent complexity in comparing results across different laboratories.Citation21,Citation33 Relatedly, rSBA assays for MenACWY-TT immunogenicity assessments began being performed by Public Health England rather than GlaxoSmithKline laboratories beginning around 2011,Citation94,Citation95 with one study directly noting the resulting difficulties in correlating immunogenicity results from the 2 laboratories.Citation74
Discussion
Serum bactericidal antibody assays are the accepted surrogate measure of efficacy for meningococcal vaccines.Citation43 Parameters used in SBA assays can vary, with the choice of rabbit or human complement often having a profound effect on study results.Citation27,Citation31,Citation32,Citation41 Current guidelines specify use of rSBA assays for serogroups A and C vaccines and hSBA assays for serogroup B subcapsular vaccines.Citation24,Citation42
Postlicensure effectiveness studies for vaccines targeting serogroups C, A, and W support the use of rSBA for licensure of these vaccines. The correlation between effectiveness and rSBA titers ≥1:8 was formally established for MenC vaccines both before and after their introduction in the United Kingdom.Citation34,Citation51 Subsequently, the widespread use of MenA-TT in Africa enabled postlicensure evaluations of impact which paralleled estimates of protection based on rSBA data.Citation85–Citation87 More recently, for serogroup W, recent data demonstrating high impact and effectiveness following MenACWY-TT vaccination programs in England and ChileCitation88,Citation89,Citation91 support the primary use of rSBA data for licensure of this vaccine.Citation57 Based on these observations for serogroups C, A, and W, it is likely that rSBA data is similarly accurate for meningococcal conjugate vaccines targeting serogroup Y.
In contrast to rSBA data, hSBA data from recent MenACWY-TT studies corroborate previously published data questioning the validity of these results for evaluating immunogenicity of this vaccine.Citation21 Specific concerns relate to lower responses to primary vaccination as measured in hSBA compared with rSBA assays,Citation62,Citation65,Citation68,Citation76,Citation78 particularly for toddlers after 1 dose.Citation62,Citation76,Citation78 Even more strikingly, some studies feature a more rapid decline of hSBA compared with rSBA titers, which is particularly notable for serogroup A, as well as an increase over time in initially lower responses for serogroups W and Y despite lack of additional dosing.Citation60,Citation65,Citation67–Citation69,Citation76,Citation78 There is no readily available explanation for these perplexing hSBA assay results, which are also in conflict with observed effectiveness data.Citation88,Citation89,Citation91 These findings collectively suggest that rSBA assays are the appropriate method for measuring immune responses in MenACWY-TT studies, whereas hSBA assays may be less relevant for these evaluations. Data from MenACWY-CRM197Citation96 and MenA-TTCitation97 studies also demonstrate serogroup A titers that rapidly wane in hSBA compared with rSBA. Rapidly waning serogroup A hSBA titers have also been observed for MenACWY-D.Citation84 These data suggest that observations regarding MenACWY-TT may be extrapolated to meningococcal conjugate vaccines using diverse carrier proteins, in that rSBA assays may be preferable to hSBA assays for immunogenicity evaluations.Citation96
Meningococcal vaccine immunogenicity evaluations remain limited by several considerations. As mentioned, standardization of the hSBA assay continues to be challenging,Citation28,Citation29 and despite more formal standardization of the rSBA assay with regard to certain parameters,Citation30 laboratories likely differ with regard to others. Additionally, although rSBA titers ≥8 are generally considered to correlate with protection for MenACWY vaccines, these have only been formally correlated with effectiveness for serogroup C.81
Disclosure of potential conflicts of interest
J Findlow and P Balmer are employees of Pfizer Inc and may hold stock/stock options. R Borrow performs contract research on behalf of Public Health England for GSK, Pfizer, and Sanofi Pasteur.
Supplemental Material
Download Zip (460.1 KB)Acknowledgments
Medical writing support was provided by Judith Kandel, PhD, of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group Company, and was funded by Pfizer Inc.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13(1):144–66. table of contents
- European Centre for Disease Prevention and Control. Surveillance atlas of infectious diseases. [ accessed 2017 Aug 15]. https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report. 2016. [ accessed 2017 Nov 29]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf.
- Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report. 2015. [ accessed 2018 June 19]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf.
- European Centre for Disease Prevention and Control. Annual epidemiological report for 2015: invasive meningococcal disease. [ accessed 2018 Oct 11]. https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-meningococcal-disease.pdf.
- Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. doi:10.1016/S0140-6736(06)67932-4.
- Parikh SR, Campbell H, Gray SJ, Beebeejaun K, Ribeiro S, Borrow R, Ramsay ME, Ladhani SN. Epidemiology, clinical presentation, risk factors, intensive care admission and outcomes of invasive meningococcal disease in England, 2010-2015. Vaccine. 2018;36(26):3876–81. doi:10.1016/j.vaccine.2018.02.038.
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(suppl 2):B3–B9. doi:10.1016/j.vaccine.2011.12.062.
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):11–17. doi:10.1186/1478-7954-11-17.
- Menactra® (meningococcal [groups A, C, Y and W-135] polysaccharide diphtheria toxoid conjugate vaccine), Full prescribing information. Swiftwater (PA): Sanofi Pasteur Inc.; 2016.
- Menveo® (meningococcal [groups A, C, Y and W-135] oligosaccharide diphtheria CRM197 conjugate vaccine). Full prescribing information, GSK Vaccines S.r.l. Sovicille, Italy; 2017.
- Nimenrix (meningococcal group A, C, W-135 and Y conjugate vaccine). Summary of product characteristics. Belgium: Pfizer Manufacturing Belgium N.V.; 2017.
- NeisVac-C (meningococcal C conjugate vaccine), Full prescribing information. New York (NY): Pfizer Inc; 2015.
- Menjugate 10 micrograms suspension for injection (meningococcal group C conjugate vaccine). Summary of product characteristics, novartis vaccines and diagnostics S.r.l. Sovicille, Italy; 2016.
- World Health Organization. Meningococcal A conjugate vaccine: updated guidance, February 2015. Wkly Epidemiol Rec. 2015;90(8):57–68.
- Menitorix (Haemophilus type B and meningococcal group C conjugate vaccine), Summary of product characteristics. Middlesex (UK): GlaxoSmithKline; 2016.
- Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, Berman SL, Lowenthal JP. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–21.
- Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22(9–10):1087–96. doi:10.1016/j.vaccine.2003.10.005.
- Trumenba® (meningococcal group B vaccine), Full prescribing information. Philadelphia (PA): Pfizer Inc; 2018.
- Bexsero® (MenB-4C). Full prescribing information. Mississauga (ON): GlaxoSmithKline Inc; 2017.
- Findlow H, Borrow R. Immunogenicity and safety of a meningococcal serogroup A, C, Y and W glycoconjugate vaccine, ACWY-TT. Adv Ther. 2013;30(5):431–58. doi:10.1007/s12325-013-0032-5.
- O’Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74(1):15–30. doi:10.1007/s40265-013-0155-7.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26.
- World Health Organization. WHO Expert Committee on Biological Standardization: Twenty-seventh Report. [ accessed 2018 Aug 3]. http://apps.who.int/iris/bitstream/handle/10665/37954/WHO_TRS_594.pdf?sequence=1&isAllowed=y.
- Borrow R, Miller E. Surrogates of protection. In: Frosch M, Maiden M, editors. Handbook of meningococcal disease. Weinheim, Germany: Wiley-VCH; 2006. p. 323–51.
- Borrow R, Carlone GM. Serogroup B and C serum bactericidal assays. Methods Mol Med. 2001;66:289–304. doi:10.1385/1-59259-148-5:289.
- Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol. 2001;8(3):616–23. doi:10.1128/cdli.8.3.616-623.2001.
- Keiser PB, Gill CJ. Defining efficacy in meningococcal vaccine trials. Future Sci. 2012;2:589–601.
- World Health Organization. Standardization and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup A/C vaccines. [ accessed 2017 Feb 2]. http://apps.who.int/iris/bitstream/10665/66298/1/WHO_V%26B_99.19.pdf.
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, Arhin FF, Devi SJ, Frasch CE, Huang JC, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4(2):156–67.
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69(3):1568–73. doi:10.1128/IAI.69.3.1568-1573.2001.
- Gill CJ, Ram S, Welsch JA, Detora L, Anemona A. Correlation between serum bactericidal activity against Neisseria meningitidis serogroups A, C, W-135 and Y measured using human versus rabbit serum as the complement source. Vaccine. 2011;30(1):29–34. doi:10.1016/j.vaccine.2011.10.068.
- McIntosh ED, Broker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays - the role of complement in infection and immunity. Vaccine. 2015;33(36):4414–21. doi:10.1016/j.vaccine.2015.07.019.
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–86. doi:10.1128/CDLI.10.5.780-786.2003.
- Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, Tang CM. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–75.
- Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77(2):764–69. doi:10.1128/IAI.01191-08.
- Jones S. Mechanisms responsible for differential bactericidal activities of human and rabbit complement against Neisseria meningitidis [PhD thesis]. School of Medicine, Cardiff University. [ accessed 2018 October 24] https://orca.cf.ac.uk/98819/1/JonesSPhD2017.pdf.
- Mak PA, Santos GF, Masterman KA, Janes J, Wacknov B, Vienken K, Giuliani M, Herman AE, Cooke M, Mbow ML, et al. Development of an automated, high-throughput bactericidal assay that measures cellular respiration as a survival readout for Neisseria meningitidis. Clin Vaccine Immunol. 2011;18(8):1252–60. doi:10.1128/CVI.05028-11.
- Block SL, Szenborn L, Daly W, Jackowska T, D’Agostino D, Han L, Dull PM, Smolenov I. A comparative evaluation of two investigational meningococcal ABCWY vaccine formulations: results of a phase 2 randomized, controlled trial. Vaccine. 2015;33(21):2500–10. doi:10.1016/j.vaccine.2015.03.001.
- Welsch JA, Senders S, Essink B, Klein T, Smolenov I, Pedotti P, Barbi S, Verma B, Toneatto D. Breadth of coverage against a panel of 110 invasive disease isolates, immunogenicity and safety for 2 and 3 doses of an investigational MenABCWY vaccine in US adolescents–Results from a randomized, controlled, observer-blind phase II study. Vaccine. 2018;36(35):5309–17. doi:10.1016/j.vaccine.2018.07.016.
- Zollinger WD, Mandrell RE. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–64.
- Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24(24):5093–107.
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–27. doi:10.1016/j.vaccine.2005.01.051.
- European Medicines Agency. Annex I: summary of product characteristics (Trumenba). [ accessed 2018 Nov 7]. https://www.ema.europa.eu/documents/product-information/trumenba-epar-product-information_en.pdf.
- European Medicines Agency. Annex I: summary of product characteristics (Bexsero). [ accessed 2018 Nov 7]. https://www.ema.europa.eu/documents/product-information/bexsero-epar-product-information_en.pdf.
- Milagres LG, Ramos SR, Sacchi CT, Melles CE, Vieira VS, Sato H, Brito GS, Moraes JC, Frasch CE. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun. 1994;62:4419–24.
- Holst J, Feiring B, Fuglesang JE, Hoiby EA, Nokleby H, Aaberge IS, Rosenqvist E. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine. 2003;21:734–37.
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–82. doi:10.1016/S0140-6736(16)31921-3.
- Frosi G, Biolchi A, Sapio ML, Rigat F, Gilchrist S, Lucidarme J, Findlow J, Borrow R, Pizza M, Giuliani MM, et al. Bactericidal antibody against a representative epidemiological meningococcal serogroup B panel confirms that MATS underestimates 4CMenB vaccine strain coverage. Vaccine. 2013;31(43):4968–74. doi:10.1016/j.vaccine.2013.08.006.
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(suppl 1):S58–S67. doi:10.1016/S0264-410X(01)00299-7.
- Trotter C, Borrow R, Andrews N, Miller E. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine. 2003;21:1094–98.
- Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac(TM). Hum Vaccin Immunother. 2012;8(6):715–24. doi:10.4161/hv.19619.
- Chen WH, Neuzil KM, Boyce CR, Pasetti MF, Reymann MK, Martellet L, Hosken N, LaForce FM, Dhere RM, Pisal SS, et al. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect Dis. 2018;18(10):1088–96. doi:10.1016/S1473-3099(18)30400-6.
- Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54:1–21.
- Centers for Disease Control and Prevention. Licensure of a meningococcal conjugate vaccine (Menveo) and guidance for use - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):273.
- European Medicines Agency. Assessment report for Menveo. [ accessed 2018 Oct 23]. https://www.ema.europa.eu/documents/assessment-report/menveo-epar-public-assessment-report_en.pdf.
- European Medicines Agency. Assessment report: Nimenrix. [ accessed 2018 Oct 23]. https://www.ema.europa.eu/documents/assessment-report/nimenrix-epar-public-assessment-report_en.pdf.
- Aplasca-De Los Reyes MR, Dimaano E, Macalalad N, Dbaibo G, Bianco V, Baine Y, Miller J. The investigational meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (ACWY-TT) and the seasonal influenza virus vaccine are immunogenic and well-tolerated when co-administered in adults. Hum Vaccin Immunother. 2012;8(7):881–87. doi:10.4161/hv.20212.
- Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J. 2011;30(3):e41–e48. doi:10.1097/INF.0b013e3182054ab9.
- Baxter R, Baine Y, Kolhe D, Baccarini CI, Miller JM, Van der Wielen M. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J. 2015;34(11):1236–43. doi:10.1097/INF.0000000000000866.
- Bermal N, Huang LM, Dubey A, Jain H, Bavdekar A, Lin TY, Bianco V, Baine Y, Miller JM. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7:239–47.
- Cutland CL, Nolan T, Halperin SA, Kurugol Z, Ahmed K, Perrett KP, Richmond P, Marshall HS, Ceyhan M, Kolhe D, et al. Immunogenicity and safety of one or two doses of the quadrivalent meningococcal vaccine MenACWY-TT given alone or with the 13-valent pneumococcal conjugate vaccine in toddlers: a phase III, open-label, randomised study. Vaccine. 2018;36(14):1908–16. doi:10.1016/j.vaccine.2018.02.013.
- Dbaibo G, El-Ayoubi N, Ghanem S, Hajar F, Bianco V, Miller JM, Mesaros N. Immunogenicity and safety of a quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) administered to adults aged 56 years and older: results of an open-label, randomized, controlled trial. Drugs Aging. 2013;30(5):309–19. doi:10.1007/s40266-013-0065-0.
- Dbaibo G, Macalalad N, Aplasca-De Los Reyes MR, Dimaano E, Bianco V, Baine Y, Miller J. The immunogenicity and safety of an investigational meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (ACWY-TT) compared with a licensed meningococcal tetravalent polysaccharide vaccine: a randomized, controlled non-inferiority study. Hum Vaccin Immunother. 2012;8(7):873–80. doi:10.4161/hv.20211.
- Dbaibo G, Tinoco Favila JC, Traskine M, Jastorff A, Van der Wielen M. Immunogenicity and safety of MenACWY-TT, a meningococcal conjugate vaccine, co-administered with routine childhood vaccine in healthy infants: a phase III, randomized study. Vaccine. 2018;36(28):4102–11. doi:10.1016/j.vaccine.2018.05.046.
- Dbaibo G, Van der Wielen M, Reda M, Medlej F, Tabet C, Boutriau D, Sumbul A, Anis S, Miller JM. The tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic with a clinically acceptable safety profile in subjects previously vaccinated with a tetravalent polysaccharide vaccine. Int J Infect Dis. 2012;16(8):e608–e615. doi:10.1016/j.ijid.2012.04.006.
- Halperin SA, Baine Y, Domachowske JB, Aggarwal N, Simon M, Langley JM, McNeil SA, Friedland LR, Bianco V, Baccarini CI, et al. Comparison of the safety and immunogenicity of a novel quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine and a marketed quadrivalent meningococcal ACWY-diphtheria toxoid conjugate vaccine in healthy individuals 10–25 years of age. J Pediatric Infect Dis Soc. 2014;3(1):33–42. doi:10.1093/jpids/pit058.
- Klein NP, Baine Y, Bianco V, Lestrate PR, Naz A, Blatter M, Friedland LR, Miller JM. One or two doses of quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine is immunogenic in 9- to 12-month-old children. Pediatr Infect Dis J. 2013;32(7):760–67. doi:10.1097/INF.0b013e31828693c5.
- Knuf M, Helm K, Kolhe D, Van Der Wielen M, Baine Y. Antibody persistence and booster response 68 months after vaccination at 2–10 years of age with one dose of MenACWY-TT conjugate vaccine. Vaccine. 2018;36(23):3286–95. doi:10.1016/j.vaccine.2018.04.064.
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, Fischbach T, Zinke H, Pankow-Culot H, Papaevangelou V, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix hexa is immunogenic, with an acceptable safety profile in 12–23-month-old children. Vaccine. 2011;29(25):4264–73. doi:10.1016/j.vaccine.2011.03.009.
- Knuf M, Romain O, Kindler K, Walther U, Tran PM, Pankow-Culot H, Fischbach T, Kieninger-Baum D, Bianco V, Baine Y, et al. Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2–10-year-old children: results of an open, randomised, controlled study. Eur J Pediatr. 2013;172(5):601–12. doi:10.1007/s00431-012-1924-0.
- Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, Bianco V, Van der Wielen M, Gatchalian S, Miller JM. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J. 2011;30(4):e56–e62. doi:10.1097/INF.0b013e31820e6e02.
- Merino Arribas JM, Carmona Martinez A, Horn M, Perez Porcuna XM, Otero Reigada MD, Mares Bermudez J, Centeno Malfaz F, Miranda M, Mendez M, Garcia Cabezas MA, et al. Safety and immunogenicity of the quadrivalent meningococcal serogroups A, C, W and Y tetanus toxoid conjugate vaccine coadministered with routine childhood vaccines in European infants: an open, randomized trial. Pediatr Infect Dis J. 2017;36(4):e98–e107. doi:10.1097/INF.0000000000001484.
- Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother. 2016;12(1):132–39. doi:10.1080/21645515.2015.1058457.
- Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. A randomized study to assess the immunogenicity, antibody persistence and safety of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in children aged 2–10 years. Hum Vaccin Immunother. 2012;8(12):1882–91. doi:10.4161/hv.22165.
- Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–903. doi:10.4161/hv.22166.
- Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: an open, randomized controlled trial. Vaccine. 2011;29(25):4274–84. doi:10.1016/j.vaccine.2011.03.043.
- Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence to meningococcal serogroups A, C, W and Y in toddlers two years after vaccination with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate (MenACWY-TT) vaccine as measured by bactericidal antibody assays using rabbit or human complement. Trials Vaccinol. 2014;3:121–26. doi:10.1016/j.trivac.2014.06.003.
- ClinicalTrials.gov. Lot consistency, immuno, safety of meningococcal vaccine GSK134612 given with Fluarix™ to 18–55 year-old adults. [ accessed 2018 Oct 26]. https://clinicaltrials.gov/ct2/show/results/NCT00453986?sect=X0156#outcome3.
- Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine. 2009;27(suppl 2):B20–B29. doi:10.1016/j.vaccine.2009.04.067.
- World Health Organization. Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec. 2011;86(47):521–39.
- Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines — Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60(3):72–76.
- Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):e20162193. doi:10.1542/peds.2016-2193.
- Baxter R, Reisinger K, Block SL, Izu A, Odrljin T, Dull P. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr. 2014;164(6):1409–1415 e1404. doi:10.1016/j.jpeds.2014.02.025.
- Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, Tapia M, Akinsola AK, Arduin P, Findlow H, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364(24):2293–304. doi:10.1056/NEJMoa1003812.
- Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbe M, Toralta J, Kodbesse B, Ngadoua C, Coldiron ME, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet. 2014;383(9911):40–47. doi:10.1016/S0140-6736(13)61612-8.
- Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, Ronveaux O, Preziosi MP, Stuart JM. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017;17(8):867–72. doi:10.1016/S1473-3099(17)30301-8.
- Villena R, Santolaya ME Chilean experience with serogroup W outbreak and meningococcal ACWY conjugate vaccines. Paper presented at: 14th Congress of the EMGM, European Meninogococcal and Haemophilus Disease Society; September 18–21, 2017; Prague, Czech Republic.
- Campbell H, Edelstein M, Andrews N, Borrow R, Ramsay M, Ladhani S. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015–2016. Emerg Infect Dis. 2017;23(7):1184–87. doi:10.3201/eid2307.170236.
- National Health Service. MenACWY Vaccine. [ accessed 2018 Aug 7]. https://www.nhs.uk/conditions/vaccinations/men-acwy-vaccine/.
- Liberator P, Wang X, Vuong J, Hu F, Hao L, Nunez L, McNeil L, Logan SM, Tompkins K, Jansen KU, et al. Genetic analysis and quantitation of factor H binding protein expression in US invasive meningococcal serogroup B isolates from population-based active bacterial core surveillance (2010–2012). Paper presented at: International Pathogenic Neisseria Conference (IPNC); 2014 October 12–17; Asheville, NC.
- Donald RG, Hawkins JC, Hao L, Liberator P, Jones TR, Harris SL, Perez JL, Eiden JJ, Jansen KU, Anderson AS. Meningococcal serogroup B vaccines: estimating breadth of coverage. Hum Vaccin Immunother. 2017;13(2):255–65. doi:10.1080/21645515.2017.1264750.
- Poolman JT, De Vleeschauwer I, Durant N, Devos N, Feron C, Lestrate P, Weynants V, Boutriau D. Measurement of functional anti-meningococcal serogroup A activity using strain 3125 as the target strain for serum bactericidal assay. Clin Vaccine Immunol. 2011;18(7):1108–17. doi:10.1128/CVI.00549-10.
- European Medicines Agency. Assessment report: Nimenrix. [accessed 2018 Aug 7]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/002226/WC500220577.pdf.
- European Medicines Agency. Assessment report: Nimenrix. [ accessed 2018 Aug 7]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/002226/WC500164494.pdf.
- Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12(5):1300–10. doi:10.1080/21645515.2015.1136040.
- Price GA, Hollander AM, Plikaytis BD, Mocca BT, Carlone G, Findlow H, Borrow R, Sow SO, Diallo A, Idoko OT, et al. Human complement bactericidal responses to a group A meningococcal conjugate vaccine in Africans and comparison to responses measured by 2 other group A immunoassays. Clin Infect Dis. 2015;61(Suppl 5):S554–S562. doi:10.1093/cid/civ504.
