?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: While receptive anal sex is an established risk factor for anal human papillomavirus (HPV) infection and squamous cell carcinoma of the anus (SCCA), people with anal HPV infection and SCCA commonly report no lifetime receptive anal sex suggesting other factors may also increase risk for anal HPV infection and persistence. Given potential associations between obesity and conditions that may cause perianal or anal canal lesions, we hypothesized that body mass index (BMI) was associated with HPV infection.
Methods: Genotyping for 36 HPV types was conducted on anal canal specimens from men, ages 18–70, from Brazil, Mexico, and the USA. Eligibility included no history of genital warts or HIV. Evaluable specimens were collected from 328 men having sex with men (MSM) and 1348 men having sex with women (MSW) who reported no lifetime receptive anal sex. Prevalence of anal HPV infection and six-month persistence by BMI were estimated in addition to adjusted prevalence ratios for the association between BMI and HPV infection.
Results: Among MSW, obese men had a higher prevalence of HPV-16 in the anal canal (3.1%), compared to normal weight men (1.3%) although 95% CI overlapped. Among MSM, prevalence of HPV decreased with increasing BMI. A similar pattern was observed for persistence. After adjustment for confounders, obese MSW had 2.4 times higher odds of HPV-16 compared to normal weight men.
Conclusions: BMI may be positively associated with anal HPV (especially HPV-16) among MSW and negatively associated with anal HPV among MSM which supports continued universal HPV vaccination programs.
Introduction
Squamous cell carcinoma of the anus (SCCA) occurs in men and women who report no receptive anal sex.Citation1 These cases occur within the well-documented increasing incidence of anal cancer in high-resource countries since the mid-20th century.Citation2 Much of the increasing incidence of SCCA has been attributed to the HIV epidemic,Citation3 changing human behavior, e.g., an increased number of sexual partners, and increased cigarette smoking.Citation4–Citation7
SCCA is primarily caused by sexually transmitted human papillomavirus (HPV) which is commonly found in the anal canal of women and men regardless of sexual orientation.Citation8,Citation9 High-risk HPV genotypes are responsible for the large majority of anal cancers. HPV-16, specifically, is responsible for 86% of SCCA among HIV-negative persons. Prevalence of these types is much higher among men who have sex with men compared to men who have sex with women.Citation10,Citation11
Persistent HPV infection in the anal canal is a formative biological event increasing risk for SCCA. It precedes the development of anal intraepithelial neoplasia 2/3 (AIN 2/3) which is a precursor of SCCA. HPV vaccination, either with Gardasil® (Merck & Co., Whitehouse Station, NJ USA) which protects against HPV-16, HPV-18, HPV-6, and HPV-11 (4vHPV), or Gardasil 9® (Merck & Co., Whitehouse Station, NJ USA) which protects against the four Gardasil types plus an additional five high-risk HPV types (HPV-31, HPV-33, HPV-45, HPV-52, HPV-58) (9vHPV), is known to prevent AIN 2/3. Both Gardasil and Gardasil 9 are licensed for use in the United States although Gardasil 9 has supplanted Gardasil.Citation12–Citation14 In addition, Cervarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium) (2vHPV), which protects against HPV-16 and HPV-18, is used in some countries.Citation15
HPV persistence can occur when the virus enters an epidermal basal cell, a process that requires macro or micro tears in the epidermis,Citation16 e.g., through receptive anal sex; however, other sexual behaviors and health conditions may also increase risk for anal fissures and micro tears including Crohn’s Disease and low-fiber diets.Citation17–Citation22 In turn, these conditions are also associated with, or an effect of, overweight and obesity; therefore, we hypothesized overweight and obese persons were associated with prevalence and persistence of anal HPV infection due to increased risk of micro and macro anal epithelial tears.
Results
Among both MSM and MSW, BMI was associated with age (p < 0.001) with increased BMI among older age groups (). Among MSM there were no other characteristics associated with BMI except duration of relationship with primary partner and number of male anal sex partners in the last 3 months. As BMI increased among MSM, they reported fewer recent sexual partners. For example, among normal weight MSM, 40.4% of men reported ≥2 male anal sex partners in the last 3 months while only 18.6% of obese men reported ≥2 partners. In contrast, among MSW there was no association between number of female sex partners and BMI. For example, 24.3% of normal weight MSW reported ≥2 female sex partners in the last 6 months while 19.2% of obese MSW reported ≥2 female sex partners (p = 0.16).
Table 1. Selected baseline characteristics of men who have sex with men (MSM) and men who have sex with women (MSW) by BMI in the HIM study, No. (%).
Among normal weight men, the prevalence of HPV-16 was much higher among MSM than MSW (11.5%; 95% CI, 7.2%-17.0% and 1.3%; 95% CI, 0.5%-2.6%, respectively); however, for obese men, prevalence was more comparable for MSM and MSW (4.7%; 95% CI, 0.6%-15.8% and 3.1%; 95% CI, 1.3%-5.9%, respectively).
Among MSM, prevalence of anal HPV decreased with increasing BMI for any HPV type, high-risk types, low-risk types, and 9vHPV vaccine types although confidence intervals overlapped (). Among MSW (), while overweight and obese men had higher prevalence than normal weight men in 2vHPV, 4vHPV, and 9vHPV vaccine types, confidence intervals overlapped in all comparisons.
Table 2. Prevalence of anal canal HPV infection among men who have sex with men (MSM) by BMI in the HIM study.
Table 3. Prevalence of anal canal HPV infection among men who have sex with women (MSW) by BMI in the HIM study.
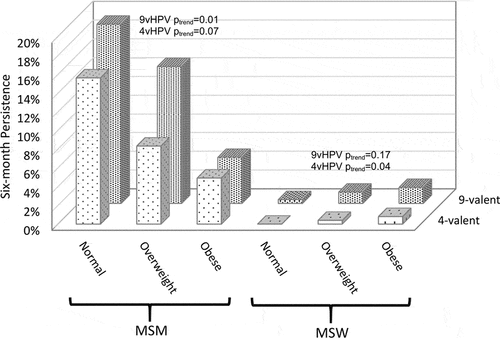
Persistence of an HPV type in the 9vHPV and 4vHPV vaccine types was substantially higher among MSM compared with MSW (). For 9vHPV and 4vHPV vaccine types, prevalence estimates for persistence declined among MSM with increasing BMI (ptrend = 0.01 and 0.07 for 9vHPV and 4vHPV types, respectively) whereas persistence increased with increasing BMI among MSW BMI (ptrend = 0.17 and 0.04 for 9vHPV and 4vHPV types, respectively).
Figure 1. Prevalence of six-month persistence for 9vHPV and 4vHPV vaccine types among men who have sex with men (MSM) and men who have sex with women (MSW) stratified by body mass index (BMI).
After controlling for age, city and number of sexual partners (), overweight and obese MSW had more than double the odds of HPV-16 infection compared to normal weight men (for example, aOR 2.40, 95% CI 0.93–6.15 for overweight MSW compared to normal weight MSW). In contrast, obese MSM had approximately one-half the odds of HPV-16 infection compared to normal weight MSM (for example, aOR 0.52, 95% CI 0.14–2.02 for obese MSM compared to normal weight MSM). Given the potential for number of sex partners to be in the causal pathway between BMI and HPV infection, and therefore ineligible for inclusion in multivariable analysis, prevalence ratios were estimated without controlling for sex partners. This change did not substantially alter prevalence estimates (supplemental table).
Table 4. Association between BMI and anal HPV prevalence in the human papillomavirus infection in men study adjusted for age, city and number of recent sexual partners.
In mediation analysis among MSW, the number of female sex partners was not associated with BMI and therefore not a potential mediator; however, number of male anal sex partners was associated with BMI among MSM (i.e., number of male anal sex partners declined as BMI increased ()) indicating that number of sexual partners may be a partial mediator of the association between BMI and HPV among MSM.
Discussion
We observed a strong positive association between increasing levels of BMI and anal HPV, especially HPV-16, among MSW and a comparably strong negative association between increasing levels of BMI and anal among MSM although in both cases 95% CIs included unity. A similar pattern was noted for persistence of HPV among both MSM and MSW. For MSW, as the number of genotypes narrowed from 13 high-risk types, to 9vHPV vaccine types, to 4vHPV vaccine types and finally HPV-16, the association with overweight and obese men became stronger.
In this cohort and a second cohort of MSW, we previously documented a consistent prevalence of HPV infection of approximately 12% on the mucosal surface of the anal canal of MSW who report no lifetime sex with men.Citation23,Citation24 Given the presence of virions, a microtear in the mucosal surface created in any manner may provide an opportunity for HPV infection.Citation25 Hence, the prevalence of anal HPV among men who acknowledge receptive anal sex is several fold higher than among men who do not acknowledge receptive anal sex.Citation9 Indeed, changes in human sexual behavior and the appearance of HIV likely underpins most, but not all, of the increased incidence in anal cancer.Citation3,Citation26–Citation28 Nevertheless, men (and women) who do not acknowledge receptive anal sex have anal HPV infection and are sometimes diagnosed with anal squamous cell carcinomas indicating other factors may also play a role.Citation6,Citation8,Citation29–Citation31
Crohn’s Disease (CD) may facilitate HPV basal cell infection due to an increased risk of lesions at the perianus or anal canal.Citation32–Citation34 While earlier efforts did not find an association between CD and anal cancer,Citation35,Citation36 three recent studies have found associations between CD and HPV infection and CD and anal cancer. A cross-sectional study assessed anal canal HPV infection in 469 gastroenterology female and male patients including 101 persons with Irritable Bowel Syndrome (IBD). Of these, 70 patients had CD, 29 had uncreative colitis and 2 persons undetermined colitis. Persons with CD had a higher prevalence of high-risk HPV types (30.0%) and HPV-16 (14.0%) compared to those without CD (p ≤ 0.005). Among CD patients with perianal involvement (n = 22), one-half had high-risk HPV types. In contrast, the prevalence of anal HPV among patients with ulcerative colitis did not differ from those without IBD. In multivariable analysis, factors associated with anal HPV infection included immunosuppressive treatment which may support the establishment of persistent infection;Citation17 however, another study of anal dysplasia among persons with IBD observed no increased dysplasia among immunosuppressed persons with IBD. In this last study, persons with CD had increased presence of abnormal anal cytology compared with non-CD patients with ID.Citation37 Two additional studies observed an increased risk for SCCA among persons with CD.Citation38,Citation39
As with anal cancer, Crohn’s Disease incidence has increased in western and developing countries since the mid-20th century and changes in diets (including the obesity epidemic) are one environmental factor hypothesized to be causal for the increase.Citation40,Citation41 Other changes in diets occurring or accelerating since the mid-20th century, like decreased fiber consumption and low water intake, may lead to constipation and the passage of hard stool which, in turn, may also cause microtears or fissures in the anal canal epithelium;Citation20–Citation22,Citation42 however, we are aware of no data establishing an association between fiber or water intake and anal HPV infection or sequelae.
Finally, cigarette smoking, a risk factor for anal cancer, has also seen dramatically increased use since the early twentieth centuryCitation43 and may be an attributable cause of some fraction of increased anal cancer incidence.
A primary limitation of this study is the lack of other measures of adiposity. Using only baseline BMI likely misclassifies the adiposity of some persons and would not reflect changes in adiposity over time.Citation44 In addition, although clinicians were well trained to collect exfoliated cells from the anal canal and avoid the perianal region, some collection of perianal exfoliated cells in possible. It is also possible that a definition of persistence of six months classifies some infections as persistent even though they may resolve or become undetectable in subsequent months.Citation45 It is possible that we may have misclassified some of the men’s sexual behavior. Anal sex and same-sex sexual behavior remain highly socially stigmatized behaviors, and some men avoid acknowledging them;Citation46 however, we have found the CASI instruments used in the HIM Study to be highly reliable with men in these 3 cities.Citation47
Among MSM, we observed that as BMI increased the number of male anal sex partners declined. Similarly, as BMI increased the prevalence of HPV declined even after controlling for number of male anal sexual partners. Such a situation might indicate residual uncontrolled confounding by number of sex partners among MSM. Number of sexual partners may also be considered a partial mediator of the association between BMI and HPV among MSM which supports its role in a causal network involving the association between BMI and HPV among MSM but not MSW.
The current study provides limited evidence of increasing prevalence and persistence of anal canal HPV by BMI among persons who report no receptive anal intercourse and no HIV infection. If obesity increases the risk of HPV persistence at the anal canal, then populations with high rates of obesity might see increased incidence of anal cancer unless HPV vaccination programs are broadly implemented and successful. Given the reach of the global obesity epidemic, the universal use of HPV vaccination among boys and girls would be one reasonable preventive strategy.
Materials and methods
Men were recruited in São Paulo, Brazil, Cuernavaca, Mexico, and Tampa, Florida from June 2005 to September 2009 for the prospective HPV Infection in Men (HIM) Study, a study of the natural history of genital HPV. Inclusion criteria included an age of 18–70 years, no plans to relocate during the 4-year study, no self-reported history of penile or anal cancer, genital warts, and no current sexually transmitted infection (STI) including HIV. Additional details of the study design have been previously described.Citation48,Citation49
Men were recruited in São Paulo from the general population through advertisements and from a genitourinary clinic that also tests for HIV and STIs. Men who went to the clinic because of STI symptoms or for treatment were excluded. In Cuernavaca, men were recruited through a health plan and from factories and the military. Men in Tampa, Florida were recruited from a university campus and the general public. MSM were not targeted for recruitment. All participants consented to the study and received a nominal incentive for participation. The study was approved by human subjects committees at each study site.
Study protocol
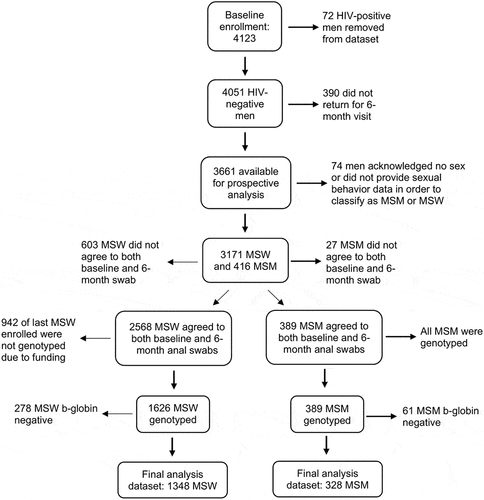
A total of 4123 men enrolled in the HIM Study. Follow-up occurred at 6-month intervals for a total of 4 years. A total of 72 men acknowledged HIV infection after enrollment and were removed from further analysis (). Of the remaining 4051 men, a total of 3661 (90.4%) men returned for at least the first 6-month follow-up visit. Approximately 93% of MSM and 81% of MSW consented to the optional collection of anal canal exfoliated cells at both visits. Those declining anal canal sampling at either visit were younger (18–30 years of age), single, never married, MSW, or a Florida resident (all P < .001) compared to those consenting to sampling. Only anal canal specimens were included for this analysis.
Men completed an 88-item computer-assisted self-interview (CASI) at enrollment written in the region’s primary language (Portuguese, Spanish, or English). The CASI elicited information about participant demographics, substance use, and sexual behaviors. At each follow-up visit, the CASI elicited information about a participant’s substance use and sexual behavior since the prior visit.
At each visit, a study clinician measured the weight (wearing indoor clothing) and height of participants before examining the men for STI symptoms. For HPV sampling, the clinician used 3 saline-wetted swabs to collect exfoliated cells from the penis (coronal sulcus, glans penis, and ventral and dorsal areas of the shaft) and scrotum. When uncircumcised men were swabbed for genital HPV, the prepuce was retracted by the clinician allowing swabbing of the glans penis and coronal sulcus with subsequent swabbing of the penile shaft. Then, the clinician used a fourth swab to sample the anal canal between the anal os and dentate line. Each swab was placed in its own vial of transport media (STM, Qiagen, Germany) and stored at – 80°C. First catch urine and blood were collected to test for Chlamydia trachomatis (Chlamydia LCx, Abbott Laboratories, Illinois and COBAS Amplicor CT/NG Test, Roche Diagnostics, England) and Herpes simplex virus-2 antibodies (HerpeSelect 2 ELISA IgG, Focus Diagnostics, California), respectively, at the enrollment visit and annually thereafter.
HPV analyses
DNA was extracted using the QIAamp Media MDx Kit (Qiagen, Inc.). The polymerase chain reaction (PCR) consensus primer system (PGMY 09/11) was used to amplify a fragment of the HPV L1 gene.Citation50 HPV genotyping was conducted using DNA probes labeled with biotin to detect 36 HPV types (including subtypes).Citation51 Accuracy and potential contamination were assessed using non-template negative controls and CaSki DNA positive controls.
In the current analysis, all MSM in the HIM Study who provided anal canal specimens at the time of consenting and the six–month visit had anal canal specimens genotyped and analyzed. Due to funding restrictions, only the first 1626 MSW consenting into the HIM Study who provided anal canal specimens at both the consenting and the six-month visits had their specimens genotyped and analyzed. A total of 83% of men had β-globin positive or HPV genotype positive specimens at both the consenting visit and the six-month visit and thus were included in analyses.
Statistical analyses
A specimen was considered positive for any anal HPV if it was positive for ≥1 of 36 genotypes. Specimens were labeled as high risk if ≥1 of 13 types were detected (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68)Citation52 regardless of the presence of other genotypes. Similarly, specimens were labeled as low risk if any of the remaining 23 types in the Linear Array were detected regardless of the presence of high-risk types; thus, prevalence estimates for high-risk and low-risk groups overlap.
Body mass index (BMI) was calculated from weight and height measurements at the consenting visit as weight in kilograms (k)/height (m2). Normal weight was defined as BMI < 25.0, overweight as 25.0– 29.9 BMI, and obese as ≥30.0 BMI.
Men enrolled in the study were classified as MSM, MSW, and men having no sexCitation53 solely based upon their answers to over 20 questions about recent and lifetime penetrative sexual behavior (vaginal, anal, and oral sex). Recent sexual behavior was assessed by questions about behavior in the prior 3 months or 6 months, or since the prior study visit. A man was classified as MSW if he acknowledged sex only with women at all study visits. A man was classified as MSM if he acknowledged sex with men at any study visit without regard to sex with women. A total of 1348 MSW and 328 MSM were available for analysis. Given substantial differences in the prevalence of HPV infection,Citation9 all analyses were stratified by MSM and MSW status.
Characteristics of men were assessed at the first consenting visit and stratified by BMI. Fisher exact tests and tests were used to assess associations between characteristics and BMI category for both MSM and MSW. To assess the association between BMI and anal HPV prevalence among both MSW and MSM we estimated both unadjusted and adjusted prevalence ratios (and 95% confidence intervals [CI]) for high-risk HPV, 9vHPV types, 4vHPV types, and HPV-16 infection using Poisson regression with a robust sandwich estimator of the variance. We conducted mediation analysis to better understand the role of number of sexual partners in the association between BMI and HPV.Citation54
Persistent infection was defined as having the same HPV genotype at both the consenting visit and the 6-month visit.
Data were analyzed using SAS 9.4 (TS1M5) (SAS Institute Inc., Cary, North Carolina, USA).
Disclosure of potential conflicts of interest
Dr. Giuliano is a member of several scientific advisory boards of Merck & Co, Inc, and her institution has received funding to conduct investigator-initiated studies as well as Merck sponsored vaccine clinical trials.
Acknowledgments
Special thanks to the men who provided personal information and biological specimens for the study. Thanks to the HPV Infection in Men (HIM) Study Team in São Paulo, Cuernavaca, and Tampa: Lenice Galan, Elimar Gomes, Elisa Brito, Filomena Cernicchiaro, Rubens Matsuo, Vera Souza, Ricardo Cintra, Ricardo Cunha, Birgit Fietzek, Raquel Hessel, Viviane Relvas, Fernanda Silva, Juliana Antunes, Graças Ribeiro, Roberta Bocalon, Rosária Otero, Rossana Terreri, Sandra Araujo, Meire Ishibashi, the Centro de Referência e Treinamento em Doenças Sexualmente Transmissíveis/AIDS nursing team, Aurelio Cruz, Pilar Hernandez, Griselda Diaz Garcia, Oscar Rojas Juarez, Rossane del Carmen Gonzales Sosa, Rene de Jesus Alvear Vazquez, Christine Gage, Kathy Cabrera, Nadia Lambermont, Kayoko Kennedy, Kim Isaacs-Soriano, Andrea Bobanic, Michael O’Keefe, Bradley Sirak, and Ray Viscidi (HIM Study Coinvestigator, Johns Hopkins).
Additional information
Funding
References
- Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004101(2):270–80. doi:10.1002/cncr.20365. PubMed PMID: 15241823.
- Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc H. 201046(4,Supplement 1):S20–S6. doi:10.1016/j.jadohealth.2010.01.016.
- van der Zee RP, Richel O, de Vries HJ, Prins JM. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med. 201371(8):401–11. Epub 2013/ 10/16
- Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2016. doi:10.1093/ije/dyw276. Epub 2016/ 10/30.
- Halperin DT. Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, Part I. AIDS Patient Care STDS. 199913(12):717–30. doi:10.1089/apc.1999.13.717. Epub 2000/ 04/01. PubMed PMID: 10743535
- Daling JR, Weiss NS, Hislop TG, Maden C, Coates RJ, Sherman KJ, Ashley RL, Beagrie M, Ryan JA, Corey L. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987317(16):973–77. doi:10.1056/NEJM198710153171601. PubMed PMID: 2821396.
- Frisch M, Glimelius B, van Den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, Goldman S, Svensson C, Adami HO, Melbye M. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997337(19):1350–58. doi:10.1056/NEJM199711063371904. PubMed PMID: 9358129.
- Hernandez BY, McDuffie K, Zhu X, Wilkens LR, Killeen J, Kessel B, Wakabayashi MT, Bertram CC, Easa D, Ning L, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev. 200514(11 Pt 1):2550–56. doi:10.1158/1055-9965.EPI-05-0460. Epub 2005/11/15. PubMed PMID: 16284377; PMCID: PMC1475824
- Nyitray AG, Carvalho Da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, Papenfuss M, Villa LL, Lazcano-Ponce E, Giuliano AR. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in Men (HIM) study. J Infect Dis. 2011203(1):49–57. doi:10.1093/infdis/jiq021. Epub 2010/12/15. PubMed PMID: 21148496
- Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis. 201818(2):198–206. doi:10.1016/S1473-3099(17)30653-9. Epub 2017/ 11/22. PubMed PMID: 29158102
- Nyitray AG, Carvalho Da Silva RJ, Baggio ML, Smith D, Abrahamsen M, Papenfuss M, Lin HY, Quiterio M, Salmeron J, Lazcano-Ponce E, et al. Six-month incidence, persistence, and factors associated with persistence of anal human papillomavirus in men: the HPV in men study. J Infect Dis. 2011204(11):1711–22. doi:10.1093/infdis/jir637. Epub 2011/10/04. PubMed PMID: 21964400; PMCID: 3203231
- Centers for Disease Control & Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males – advisory Committee on Immunization Practices (ACIP), 2011. MMWR-Morb Mortal Wkly Rep. 201160(50):1705–08. Epub 2011/12/23. mm6050a3 [pii]. PubMed PMID: 22189893.
- Garland SM, Kjaer SK, Munoz N, Block SL, Brown DR, DiNubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 201663(4):519–27. doi:10.1093/cid/ciw354. Epub 2016/ 05/28. PubMed PMID: 27230391; PMCID: PMC4967609
- Petrosky E, Bocchini JA Jr., Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. Centers for disease C, prevention. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 201564(11):300–04. PubMed PMID: 25811679
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 201230(Suppl 5):F123–38. doi:10.1016/j.vaccine.2012.04.108. PubMed PMID: 23199956
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 200713:857–61. doi:10.1038/nm1598. PubMed PMID: WOS:000247902800033.
- Vuitton L, Jacquin E, Parmentier AL, Crochet E, Fein F, Dupont-Gossart AC, Plastaras L, Bretagne CH, Mauny F, Koch S, et al. High prevalence of anal canal high-risk human papillomavirus infection in patients with Crohn’s disease. Clin gastroenterol Hepatol. 201816(11):1768–76 e5. doi:10.1016/j.cgh.2018.03.008. Epub 2018/03/20. PubMed PMID: 29551740
- Chutkan R, Fahey G, Wright WL, McRorie J. Viscous versus nonviscous soluble fiber supplements: mechanisms and evidence for fiber-specific health benefits. J Am Acad Nurse Pract. 201224(8):476–87. doi:10.1111/j.1745-7599.2012.00758.x. Epub 2012/ 08/01. PubMed PMID: 22845031
- Markland AD, Palsson O, Goode PS, Burgio KL, Busby-Whitehead J, Whitehead WE. Association of low dietary intake of fiber and liquids with constipation: evidence from the national health and nutrition examination survey. Am J Gastroenterol. 2013108:796–803. doi:10.1038/ajg.2013.73. Epub 2013/ 04/10. PubMed PMID: 23567352.
- Jensen SL. Diet and other risk factors for fissure-in-ano. Prospective case control study. Dis Colon Rectum. 198831(10):770–73. Epub 1988/10/01.PubMed PMID: 3168663
- Madalinski MH. Identifying the best therapy for chronic anal fissure. World J Gastrointest Pharmacol Ther. 20112(2):9–16. doi:10.4292/wjgpt.v2.i2.9. Epub 2011/ 05/18. PMCID: PMC3091162
- Beck D, Roberts P, Rombeau J, Stamos M, Wexner S. Benign Anorectal: anal Fissure. In: Wexner SD, Stamos MJ, Rombeau J, Roberts PL, Beck DE, editors. The ASCRS manual of colon and rectal surgery. New York, NY: Springer 2009. p. 259–72.
- Nyitray AG, Nielson CM, Harris RB, Flores R, Abrahamsen M, Dunne EF, Giuliano AR. Prevalence of and risk factors for anal human papillomavirus infection in heterosexual men. J Infect Dis. 2008197(12):1676–84. doi:10.1086/588145. PubMed PMID: 18426367.
- Nyitray AG, Smith D, Villa L, Lazcano-Ponce E, Abrahamsen M, Papenfuss M, Giuliano AR. Prevalence of and risk factors for anal human papillomavirus infection in men who have sex with women: a cross-national study. J Infect Dis. 2010201(10):1498–508. doi:10.1086/652187. Epub 2010/04/07. PubMed PMID: 20367457; PMCID: 2856726
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 201230(Suppl 5):F55–70. doi:10.1016/j.vaccine.2012.06.083. Epub 2012/12/05. PubMed PMID: 23199966
- Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 200540(4):451–55. PubMed PMID: 16280701
- Cress RD, Holly EA. Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973-1999. Prev Med. 200336(5):555–60. PubMed PMID: 12689800
- Liu G, Hariri S, Bradley H, Gottlieb SL, Leichliter JS, Markowitz LE. Trends and patterns of sexual behaviors among adolescents and adults aged 14 to 59 Years, United States. Sex Transm Dis. 201542(1):20–26. doi:10.1097/olq.0000000000000231. PubMed PMID: 00007435-201501000-00006
- Piketty C, Darragh TM, Da Costa M, Bruneval P, Heard I, Kazatchkine MD, Palefsky JM. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med. 2003138(6):453–59. PubMed PMID: 12639077
- Holly EA, Whittemore AS, Aston DA, Ahn DK, Nickoloff BJ, Kristiansen JJ. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst. 198981(22):1726–31. PubMed PMID: 2810388
- Tseng HF, Morgenstern H, Mack TM, Peters RK. Risk factors for anal cancer: results of a population-based case-control study. Can Cause Control. 200314(9):837–46. doi:10.1023/B:CACO.0000003837.10664.7f. PubMed PMID: 14682441.
- Ruel J, Ko HM, Roda G, Patil N, Zhang D, Jharap B, Harpaz N, Colombel JF. Anal Neoplasia in inflammatory bowel disease is associated with HPV and perianal disease. Clin Transl Gastroenterol. 20167:e148. doi:10.1038/ctg.2016.8. Epub 2016/03/05. PubMed PMID: 26938479; PMCID: PMC4822100.
- Hanauer SB, Sandborn W. Practice parameters committee of the American college of G. management of Crohn’s disease in adults. Am J Gastroenterol. 200196(3):635–43. doi:10.1111/j.1572-0241.2001.3671_c.x. Epub 2001/03/31. PubMed PMID: 11280528
- Wisniewski A, Flejou JF, Siproudhis L, Abramowitz L, Svrcek M, Beaugerie L. Anal Neoplasia in inflammatory bowel disease: classification proposal, epidemiology, carcinogenesis, and risk management perspectives. J Crohn’s colitis. 201711(8):1011–18. doi:10.1093/ecco-jcc/jjx035. Epub 2017/ 04/06. PubMed PMID: 28379306
- Holmes F, Borek D, Owen-Kummer M, Hassanein R, Fishback J, Behbehani A, Baker A, Holmes G. Anal cancer in women. Gastroenterology. 198895(1):107–11. PubMed PMID: 2836255
- Frisch M, Glimelius B, Van Den Brule AJC, Wohlfahrt J, Meijer CJLM, Walboomers JMM, Adami HO, Melbye M. Benign anal lesions, inflammatory bowel disease and risk for high-risk human papillomavirus-positive and -negative anal carcinoma. Br J Cancer. 199878:1534–38.
- Shah SB, Pickham D, Araya H, Kamal A, Pineda CE, Ghole S, Shih L, Kong C, Pai R, Welton M. Prevalence of anal dysplasia in patients with inflammatory bowel disease. Clin gastroenterol Hepatol. 201513(11):1955–61.e1. doi:10.1016/j.cgh.2015.05.031. Epub 2015/ 06/06. PubMed PMID: 24131389
- Beaugerie L, Carrat F, Nahon S, Zeitoun JD, Sabate JM, Peyrin-Biroulet L, Colombel JF, Allez M, Flejou JF, Kirchgesner J, et al. High risk of anal and rectal cancer in patients with anal and/or perianal Crohn’s disease. Clin gastroenterol Hepatol. 201760(12):1307–13. doi:10.1016/j.cgh.2017.11.041. Epub 2017/ 12/05. PubMed PMID: 29112567
- Slesser AA, Bhangu A, Bower M, Goldin R, Tekkis PP. A systematic review of anal squamous cell carcinoma in inflammatory bowel disease. Surg Oncol. 2013. doi:10.1016/j.suronc.2013.08.002. Epub 2013/09/21. PubMed PMID: 24050823.
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012142(1):46–54.e42; quiz e30. doi:10.1053/j.gastro.2011.10.001. Epub 2011/ 10/18. PubMed PMID: 22001864
- Kreuter R, Wankell M, Ahlenstiel G, Hebbard L. The role of obesity in inflammatory bowel disease. Biochimica Et Biophysica Acta Molecular Basis of Disease. 20191865(1):63–72. doi:10.1016/j.bbadis.2018.10.020. Epub 2018/10/24. PubMed PMID: 30209778
- Steele SR, Madoff RD. Systematic review: the treatment of anal fissure. Aliment Pharmacol Ther. 200624(2):247–57. doi:10.1111/j.1365-2036.2006.02990.x. Epub 2006/ 07/18. PubMed PMID: 16842451
- Proctor RN. The global smoking epidemic: a history and status report. Clin Lung Cancer. 20045(6):371–76. doi:10.3816/CLC.2004.n.016. Epub 2004/ 06/26
- Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. 200832(Suppl 3):S56–9. doi:10.1038/ijo.2008.87. Epub 2008/ 08/21
- Nyitray AG, Carvalho Da Silva RJ, Chang M, Baggio ML, Ingles DJ, Abrahamsen M, Papenfuss M, Lin HY, Salmeron J, Quiterio M, et al. Incidence, duration, persistence, and factors associated with high-risk anal human papillomavirus persistence among HIV-negative men who have sex with men: a multinational study. Clin Infect Dis. 201662(11):1367–74. doi:10.1093/cid/ciw140. PubMed PMID: 26962079; PMCID: 4872291
- Weinhardt LS, Forsyth AD, Carey MP, Jaworski BC, Durant LE. Reliability and validity of self-report measures of HIV-related sexual behavior: progress since 1990 and recommendations for research and practice. Arch Sex Behav. 199827(2):155–80. PubMed PMID: 9562899
- Nyitray AG, Kim J, Hsu CH, Papenfuss M, Villa L, Lazcano-Ponce E, Giuliano AR. Test-retest reliability of a sexual behavior interview for men residing in Brazil, Mexico, and the United States: the HPV in Men (HIM) Study. Am J Epidemiol. 2009170(8):965–74. doi:10.1093/aje/kwp225. Epub 2009/09/11.PubMed PMID: 19741044
- Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, Papenfuss MR, Abrahamsen M, Jolles E, Nielson CM, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 200817(8):2036–43. doi:10.1158/1055-9965.EPI-08-0151. Epub 2008/ 08/19. PubMed PMID: 18708396
- Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011377(9769):932–40. S0140-6736(10)62342-2 [pii]. 10.1016/S0140-6736(10)62342-2. Epub 2011/ 03/04. PubMed PMID: 21367446.
- Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 200038(1):357–61. PubMed PMID: 10618116
- Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 199836(10):3020–27. PubMed PMID: 9738060
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 200910(4):321–22. doi:10.1016/S1470-2045(09)70234-7. Epub 2009/ 04/08.PubMed PMID: 19350698.
- Savin-Williams RC, Ream GL. Prevalence and stability of sexual orientation components during adolescence and young adulthood. Arch Sex Behav. 200736(3):385–94. doi:10.1007/s10508-006-9088-5. Epub 2006/ 12/30. PubMed PMID: 17195103
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 198651(6):1173–82. Epub 1986/ 12/01.PubMed PMID: 3806354