ABSTRACT
The enteropathogen, Shigella, is highly virulent and remarkably adjusted to the intestinal environment of its almost exclusive human host. Key for Shigella pathogenicity is the injection of virulence effectors into the host cell via its type three secretion system (T3SS), initiating disease onset and progression by the vast diversity of the secreted T3SS effectors and their respective cellular targets. The multifaceted modulation of host signaling pathways exerted by Shigella T3SS effectors, which include the subversion of host innate immune defenses and the promotion of intracellular bacterial survival and dissemination, have been extensively reviewed in the recent past. This review focuses on the human species specificity of Shigella by discussing some possible evasion mechanisms towards the human, but not non-human or rodent gut innate defense barrier, leading to the lack of a relevant animal infection model. In addition, subversion mechanisms of the adaptive immune response are highlighted summarizing research advances of the recent years. In particular, the new paradigm of Shigella pathogenicity constituted of invasion-independent T3SS effector-mediated targeting of activated, human lymphocytes is discussed. Along with consequences on vaccine development, these findings offer new directions for future research endeavors towards a better understanding of immunity to Shigella infection.
Exploring Shigella specificity towards the human innate immune barrier in the gut
The striking selectivity of Shigella as a human-restricted pathogen relies on its evasion mechanisms of host innate defenses during gut transit and mucosal invasion, which are highly adapted to the human digestive tract. The significance of this species specificity becomes evident when considering that up-to-now there is no experimental animal model available, which convincingly mimics human infection, i.e. the onset of dysentery after oral inoculum.Citation1 As an exception, naturally acquired Shigella infection has been reported for some, non-human primates, including Rhesus macaques.Citation2–Citation4 Nevertheless, under laboratory conditions, the Rhesus macaque model requires an inoculum dose of about 108–109 bacteria,Citation5 whereas as few as 102–103 suffice to cause disease in humans.Citation6,Citation7 While sharing common features, there is increasing evidence suggesting for important species differences in the innate defense systems – in particular between primates and rodentsCitation8 – which may provide important leads as to understanding susceptibility versus resistance to infection observed in these species. For instance, after surviving the acidic environment of the stomach,Citation9 Shigella reaches the small intestine where it has to resist to degradation by bile salts that commonly vary in composition between humans, non-human primates, and rodents.Citation10 Interestingly, exposure to human-type bile salts was recently shown to increase Shigella virulence via the upregulation of survival genes as well as the induction of biofilm formation, hence facilitating better resistance to the challenging environmental conditions of the gut.Citation11 Upon entering the colon, Shigella is faced with the great abundance of resident microbes, the gut microbiota, which naturally varies significantly in their composition across species.Citation12,Citation13 How Shigella establishes its niche in such an adverse environment is subject of further investigations but of importance is its binding to the mucus layer, which is at its greatest thickness in the colon in comparison to other sections of the digestive tract.Citation14,Citation15 Immune properties of the mucus barrier present with marked differences between location and species and are associated with host selectivity. The absence of Shigella binding to the small intestinal mucusCitation16,Citation17 suggests selectivity of the bacterial/mucin interactions which is possibly related to the specific glycan composition present in the colonic mucosa. Shigella binds with high affinity to the heavily glycosylated mucins isolated from the human colon, as opposed to those from the guinea pig (less binding) and rat (no binding).Citation16 In addition, the inner mucus layer contains antimicrobial peptides (AMPs), which are important mediators in keeping the epithelial lining sterile. Interestingly, AMPs also underlie species-specificity presumably having adapted to the diverse ecological niches inhabited by mammals and the multitude of microbial challenges faced within.Citation18 Here, the rate of similarities and differences across species are also dependent on the type of AMPs. For instance, divergence exists for α-defensins when comparing mouse and human,Citation18 while in contrast Rhesus monkey β-defensins and cathelicidins are close homologs to the human molecules.Citation19 Considering this homology, the specific down-regulation of cathelicidin and human β-defensin 1 expression by Shigella during natural and experimental infection which contributes to bacterial survival in the intestineCitation20,Citation21 might also happen in Rhesus monkeys, thus contributing to their susceptibility to Shigella oral infection.
Another key point in the inter-species variation of innate immune system components includes the sensing of microbial associated molecular patterns (MAMPs) by host pattern recognition receptors (PRRs). PRR activation triggers the transcription of IL-8 (also known as CXCL-8), which serves as a potent chemoattractant responsible for the recruitment of polymorphonuclear neutrophils (PMNs), a hallmark of Shigella infection in humans. In contrast, mice, which show resistance to oral infection by Shigella fail to elicit PMN recruitment to the intestinal mucosa. However, if infection is performed together with recombinant human IL-8, PMN infiltration and subsequent mucosal inflammation and invasion occur.Citation22 This suggests that murine resistance to Shigella infection might be related to a defective pathogen sensing, needed for the initiation of an inflammatory response, which promotes the disruption of the epithelial integrity and further bacterial translocation and epithelial invasion.Citation23–Citation25 In other words, one might speculate that suboptimal MAMP/PRR interactions are involved in the lack of inflammation observed in response to Shigella infection in the mouse. Of note, in guinea pigs on the contrary, intrarectal administration of a dose of 109 Shigella induces rectocolitis accompanied by diarrhea similarly to human disease, suggesting for further differences between mice and guinea pigs in triggering gut inflammation.Citation26 Important is the notion of organ-specificity for such a differential bacterial sensing, since mice infected intranasally with Shigella develop an acute pulmonary infection characterized by a massive PMN infiltration with extensive tissular destruction.Citation27,Citation28
In summary, inter-species differences in key components of the human innate defense barrier present throughout the gastrointestinal tract including the colon are likely to reflect the discrepancies in the human susceptibility to Shigella infection versus the reduced sensitivity or resistance observed in non-human primates, guinea pig or mouse infection models.
T3SS-mediated subversion of innate immunity: a brief reminder
The main weaponry of Shigella to dampen host defenses, i.e. the type three secretion system (T3SS), has been extensively studied and its composition and structure, as well as regulation and mode of action have been recently reviewed.Citation29–Citation31 In brief, the T3SS mediates the delivery of virulence effectors from the bacterial cytoplasm into the targeted host cell. It is composed of a type three secretion apparatus (T3SA), the secreted effectors, translocators, chaperones and transcription regulators, which are all encoded on the Shigella virulence plasmid.Citation32 The syringe-like cylindrical T3SA spans both bacterial membranes and is composed of the protruding needle complex supported by the basal body.Citation33 The assembly of the T3SA is temperature-dependent and only triggered at 37°C,Citation34 the human body temperature, in order to keep the energetic costs related to its expression to a minimum. After its assembly, the T3SA remains inactive, i.e. translocators and several effectors remain associated to chaperons in the bacterial cytoplasm, until sensing of the host cell membrane occurs at the needle tip complex.Citation35 This acts as activation signalCitation36 and initiates the secretion of the stored translocators and effectors into the host cell cytoplasmCitation37-Citation39 and additionally triggers the transcription of genes encoding for a second set of bacterial effectors. After host cell invasion and vacuolar escape, cytosolic Shigella down-regulate the T3SA activityCitation40,Citation41 in order to replenish the bacterial effector pool required for its subsequent dissemination into neighboring cells.Citation41–Citation43
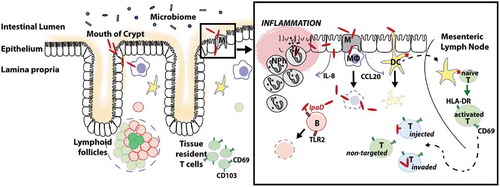
It is now well-established that Shigella T3SS effectors, similarly to those of other T3SS-carrying enteropathogens, target key cellular pathways of enterocytes and gut resident macrophages, leading to the modulation of important host cell functions.Citation44 Numerous Shigella T3SS effectors, listed in recent reviews,Citation44–Citation46 have been shown to affect signaling pathways involved in host cell actin cytoskeleton dynamics, trafficking, cell viability, and NF-κB-mediated inflammatory pathways. The system is amazingly efficient. On one hand, one given effector can ensure a diversity of actions towards different cell types while infection proceeds. On the other hand, different effectors can target multiple host proteins in a given pathway. In addition, effectors with antagonistic effects towards a given pathway ensure a timely efficient modulation of host innate immune responses as infection proceeds.Citation44–Citation46 In a nutshell, as depicted in , upon crossing the colonic mucosa, Shigella exerts a multitude of deregulatory mechanisms leading to the initiation of a pro-inflammatory milieu, accompanied by massive immune cell death, including macrophage pyroptosis, as well as facilitating its intra-epithelial survival and spreading along the colonic epithelium.
Figure 1. Model of Shigella pathogenicity.
Note. After its successful journey through the gut, passing a diversity of luminal innate immune components, Shigella eventually reaches the colonic epithelium. The apical pole of colonocytes is commonly regarded as resistant to invasion.Citation102 Instead, Shigella crosses the epithelial barrier via M cellsCitation103 or via colonocytes located at the mouth of the intestinal crypts, which has recently been described as alternative route of entry.Citation104 In case of the latter, it was hypothesized that physical forces employed by the gut peristalsis facilitate invasion by entrapping bacteria at the crypt opening and thus enforce entry of the colonocytes in close proximity. Independently of its mode of invasion, Shigella subsequently arrives in the subjacent lamina propria, which is densely populated with immune cells involved in the priming of both innate and adaptive immunity. The pro-inflammatory environment induced by Shigella in the colon is correlated with a massive cell death as observed in biopsies obtained from infected individuals.Citation49,Citation55,Citation105 Shigella evades from macrophage-induced killing via the induction of pyroptosis, a caspase-1 dependent cell death associated with the release of the pro-inflammatory cytokines IL-1β and IL-18.Citation106–Citation108 Escaping from the dying macrophage, Shigella invades enterocytes from their basolateral side and spreads throughout the epithelium.Citation42,Citation109–Citation111 Intracellular bacteria and their T3SS effectors, influence the innate inflammatory response by mobilizing a cascade of immunomodulatory processes in a time-dependent manner (reviewed in RefCitation44). A few hours post-infection, mucosal invasion progresses and is further exacerbated due to successive epithelial damage mediated by the arrival of PMNCitation25 following the chemoattractant, IL-8.Citation57,Citation112–Citation114 While PMN-induced tissue damage is thought to facilitate bacterial colonization, invasion and multiplication during the early phases of bacterial infection, Shigella is unable to counteract the antimicrobial activity of these professional phagocytes and is ultimately eliminated.Citation115,Citation116 Shigella impairs adaptive immune cell priming by hampering epithelial CCL20 secretion and the subsequent recruitment of dendritic cells (DCs).Citation21 Antigen-presenting DCs migrate to the mesenteric lymph nodes where they prime naïve T cells. These activated T lymphocytes then home back to the lamina propria where they render targetable by Shigella via injection-only or invasion mechanisms.Citation65 Finally, Shigella also inhibits B lymphocyte function by the induction of apoptosis via the T3SS needle-tip effector IpaD.Citation66
T3SS-mediated subversion of adaptive immunity: an as of yet underestimated implication
Upon crossing of the epithelial barrier, Shigella encounters lamina propria cells responsible for the priming of the adaptive immunity, which include dendritic cells (DCs), B and T lymphocytes. In addition, interactions with these cells may also occur in the lymphoid structures that are associated with the intestinal mucosa. In experimental infection, Shigella has been shown to reach the mesenteric lymph nodes (P. J. Sansonetti, personal communication), which constitutes the end of its journey since systemic dissemination is usually not observed, except for rare cases of immunocompromized, malnourished infants in endemic settings.Citation47
Immunity to Shigella is characterized by a humoral response mediated by mucosal sIgAs and systemic IgGs directed against the LPS O-antigen and some other bacterial molecules such as the Invasion plasmid Antigens (Ipa) proteins.Citation48–Citation50 Antibodies directed towards the LPS O-antigen are associated with protection in cohort studies.Citation50 The composition of the O-antigens differs greatly across strains and serotypes, accounting for the serotype-specificity observed in antibody-mediated protection.Citation28,Citation51 Shigella antigen-specific B memory cells can be primed and are associated with decreased disease severity in subjects challenged with Shigella. Citation52Nevertheless, the establishment of a protective adaptive immunity requires several rounds of infection and is only of short-term duration.Citation50,Citation53,Citation54 Interference with the innate and adaptive immune response during primary infection has been associated with the higher susceptibility of children to shigellosis, as compared to adults.Citation55,Citation56 Evidence indicates that both the induced pro-inflammatory response and a direct targeting of DCs, B and T lymphocytes might contribute to an inefficient priming of the adaptive immune response upon infection. Shigella reprogramming of gene expression in infected enterocytes towards a pro-inflammatory profile, includes the down-regulation of CCL20 production, a chemokine mediating DCs recruitment.Citation57 Accordingly, in an experimental model of infection, a reduced recruitment of DCs is observed towards the lamina propria of animals infected with invasive Shigella, as compared to those infected with non-invasive Shigella harboring a non-functional T3SS.Citation21 In addition, DC death occurs within a couple of hours of Shigella infection in vitro as a response to host cell caspase activation.Citation58 Whether recruited and gut resident DCs are susceptible to bacterial killing during the natural infection remains to be established, however the massive cell death observed in patient biopsies are supportive of this notion.Citation55 Another link between the host innate and adaptive immune responses is provided by the induction of Shigella-specific Th17 cells,Citation59 as a result of the pro-inflammatory environment induced upon infection in the mouse pulmonary model of infection.Citation60,Citation61 This might reflect what happens in the gut, since Th17 cells are a lineage of T helper cells abundantly present in the gut mucosa and defined by the secretion of IL-17, an important cytokine in the host immune response towards bacterial pathogens.Citation62 IL-6, which is significantly induced upon Shigella infection, contributes to the induction of Th17 cells while blocking the development and function of regulatory T cells (Treg).Citation63 Noteworthy, antigen-specific CD8+ T lymphocytes fail to be primed during Shigella infection in vivo,Citation59,Citation64 suggesting that specific CD8+ T cell-mediated killing of invaded enterocytes is likely to be impaired.
Evidence for the direct targeting of human lymphocytes by Shigella has been provided in the last years, predominantly conducted by our laboratory. Historically, Shigella is regarded as primarily intracellular bacterium able to invade a large diversity of cells following the injection of T3SS effectors in vitro. We recently showed that the injection of T3SS effectors does not inevitably result in cell invasion.Citation65 Indeed, using an optimized T3SS injection reporter, we demonstrated that effector injection without subsequent cell invasion, termed the “injection-only” mechanism, is the main route of lymphocyte targeting utilized by Shigella.Citation65 In vitro-activated human peripheral blood B, CD4+ T, and CD8+ T lymphocytes, as well as switched memory B cells, are primarily targeted by the injection-only mechanism, as are B and T lymphocytes extracted from human colonic tissue, i.e. representing the lamina propria-residing cells encountered by Shigella in the gut. These findings reveal that through this mechanism, Shigella targeting can be extended to a large diversity of host cells, including those refractory to invasion.Citation65 Furthermore, B cells were shown to undergo apoptosis after the activation of a TLR2-dependent signaling pathway triggered by the T3SS needle-tip protein, revealing a Shigella targeting mechanism that is independent of both invasion and T3SS effector injection.Citation66 Consequently, we propose an additional mode of Shigella pathogenicity, termed the “kiss-and-run” strategy, describing the ability of Shigella to target cells via a T3SS-dependent contact, which results in either effector interaction with cell-surface receptors or effector delivery into the host cells not followed by cell invasion. These findings highlight an as of yet underestimated facet of Shigella’s extracellular mode of action.
Another interesting aspect is the observation that only activated human CD4+ T lymphocytes are found targeted by Shigella, as opposed to non-activated cells. Investigating the underlying molecular mechanism, we demonstrated that non-activated human CD4+ T lymphocytes, which are refractory to Shigella invasion and effector-injection, become susceptible to targeting upon loading of their plasma membrane with sialylated glycosphingolipids (gangliosides) that are abundantly present in activated cells.Citation67 Interactions between the sugar polar part of gangliosides and the polysaccharide moiety of Shigella lipopolysaccharide (LPS) promote bacterial binding resulting in the injection of T3SS effectors. While interactions of surface glycans has been previously reported,Citation68 these findings suggest that bacterial-host glycan interactions are important for Shigella pathogenesis by driving selective interactions with host immune cells. This study also provides a new cell adherence mechanism available to Shigella and maybe other pathogens, which are devoid of adhesins and therefore rely on alternative strategies to effectively bind to the host cells. In this context, various molecules promoting Shigella binding to enterocytes have been identified including the human enteric α-defensin 5 (HD5),Citation69 the virulence T3SS factors OspE1/E2 and IcsA acting as adhesin-like molecule following bile salt exposure.Citation70,Citation71 Therefore, beyond its potential importance in driving host specificity, Shigella binding to specific cell surface glycans is likely to direct selective targeting of the different T lymphocyte subsets and, more globally, the wide-range of mucosal immune cells with their specific membrane glycosylation patterns.Citation72
The so far only example of the outcomes of direct targeting of human T lymphocytes by Shigella is the demonstration of the impairment of activated CD4+ T cell dynamics and migration, both in vitroCitation73 and in vivo,Citation74 which is mediated by the T3SS effector IpgD.Citation73 IpgD is a phosphoinositide 4-phosphatase mediating hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). In T lymphocytes, the IpgD-mediated reduction of the pool of PIP2 at the plasma membrane leads to dephosphorylation of the ERM proteins and their inability to relocalize at one T cell pole upon chemokine stimulus, likely affecting the formation of the polarized edge required for cell migration.Citation73 The impact on other key T cell functions such as synapse formation and subsequent activation are currently under investigation. An updated model of Shigella physiopathology comprising the recent findings on B and T lymphocyte targeting is schematized in .
Future perspectives
The molecular mechanisms downstream of Shigella lymphocyte targeting and the global role of the injected T3SS effectors on adaptive immunity remain to be deciphered. While the impact of specific immune priming on infection can be accomplished by in vitro studies on human lamina propria cells, further in-depth investigations of the effector phase and in vivo implications are compromized by the previously mentioned lack of a suitable animal model. The use of the controlled human infection model (CHIM) can present an important alternative, as recently exemplified by studying human immune response to Salmonella infection.Citation75–Citation77 However, so far CHIM for Shigella are restricted to efficacy tests for vaccine candidates but may present an opportunity to be further exploited.Citation78,Citation79 Another important point lays in the establishment of adult and infant cohort studies in endemic regions of shigellosis, which especially in the light of now available high throughput analysis tools would certainly provide important insights into human immune responses to natural infection.
In the context of the adaptive immunomodulation and recurrent infections frequently observed in Shigella infected individuals, further insights into the underlying mechanisms are needed which include a better understanding of the adaptive immune cell subsets that are targeted as well as the specific T3SS effectors involved and the thereby affected host signaling pathways. Of particular interest here is the analysis of the vast diversity of immune cells present in the human colonic mucosa at a single-cell level. It is important to note that most experiments conducted so far are based on either T cell-lines or peripheral blood lymphocytes, which do not represent the entire spectrum of lymphocyte subsets encountered by Shigella in the colonic lamina propria. Based on human peripheral blood mononuclear cells (PBMCs), T lymphocytes are classified into CD45RA+ naïve and CD45RO+ memory T cells, the latter being further subdivided into central memory T cells (TCM) or effector memory T cells (TEM) according to the presence or absence of the lymphoid homing factor CCR7 and CD62L on the cell surface, respectively.Citation80 However, more recent publications highlight the existence of a non-circulating, tissue resident memory T cell (TRM) population that constitutively express the integrin CD103, while being devoid of lymphoid homing factors.Citation80,Citation81 Analysis of various tissue samples obtained from healthy individuals confirmed the presence of these specific TRM cells in all mucosal sites and in particular in the colon, while being completely absent in circulating PBMCs.Citation81 Colonic TRM cells are predominantly of the CD8+ resident effector type as identified by the constitutive expression of CD69+ in the absence of CCR7, and play an important role in the local protective immunity to infection.Citation80,Citation82 Of particular interest in the light of our previous findings highlighting Shigella preferential targeting of activated T cells, is the “active state” of colonic TRM cells, as judged by the constitutive surface expression of the activation marker CD69.Citation80 Therefore, it would be meaningful to investigate Shigella interactions with this specific non-circulating TRM subset and to assess its implication on the local immunity. Another interesting point to be considered for further investigation is the observed effect of Shigella on the host cell metabolism by rerouting the metabolic output of intestinal epithelial cells after invasion to facilitate its energetic demands.Citation83 Interestingly, metabolic programming is also implicated in the switch from effector to memory T cell phenotype and therefore play an important role in the establishment of long-term immunity.Citation84
Implications for vaccine development
The new paradigm of Shigella pathogenicity including the novel targeting mechanisms of human lymphocytes has implications on the different Shigella vaccine strategies that are currently under investigation. One strategy relies on the use of orally-administered, live, rationally attenuated vaccine candidates for which T3SS activity toward B and T lymphocytes has been so-far-underestimated. The latest developed candidates of this typeCitation85 have yet been rationally attenuated on the bases of virulence knowledge (i.e. alteration of metabolic functions essential for in vivo growth, of dissemination capacities in invaded tissues) that preceded recent deciphering of Shigella immunosuppressive capacities. In consequence, these candidates are likely to express a functional T3SS and secrete its dedicated effectors, thus retaining their ability to subvert human lymphocyte functions. As a matter of fact, although some of these candidates have shown decent mucosal and systemic immunogenicity in phase I-II studies, including significant protection against dysentery in a challenge study carried out in Western volunteers,Citation86 the performance of these candidates were far less promising in phase I-II studies carried out in endemic areas.Citation87 These latter studies pointed to the issue that colonization by these candidates was insufficient in level and duration in order to achieve sufficient and reproducible protective mucosal immunogenicity. This poor colonization capacity in endemic zones may be linked to several factors including a colonization barrier effect of the resident intestinal microbiota keeping attenuated candidates at bay and leading to minimal engagement with the epithelial surface and its associated immune system. Hence the joined action of persisting immunosuppressive properties and deficient colonization performance possibly prevents efficacy of current live-attenuated candidates. Trying to address these combined issues and rationally design a new generation of candidates is not impossible but sets substantial challenges and must address two main questions: (i) the mutation, both individually and collectively, of several immunosuppressive effectors and their global assessment, (ii) the yet poorly identified parameters involved in colonization and interaction with the microbiota barrier. All this has to be achieved in the context of a human-specific disease and under consideration of the low predictive value of animal models. Considering the limited resources available for research of neglected infectious diseases such as shigellosis, the time and funding scale to achieve the goal of a tetravalent vaccine on such basis seems rather challenging.
Besides orally administered, live, rationally attenuated vaccine strains, a different approach relies on the development of parenterally delivered subunit vaccines. This strategy is based on the notion that specific bacterial surface polysaccharides, i.e. the O-antigen of membrane lipopolysaccharide (LPS), are the primary targets of the antibody response associated to protection against homologous re-infection.Citation50 Among the different vaccine candidates,Citation85 only the glycoconjugate vaccine approach incorporating detoxified LPS, i.e. the O-antigen conjugated to a carrier protein, has reached phase III clinical trial testing. In particular, along with safety, protective efficacy was shown with a S. sonnei glycoconjugate in adults and children above, but not below three years of age.Citation88 Based on these promising results, several candidates are now under development. Bioconjugate vaccines, sourced from gene-edited E. coli, have the benefit of an easy and large-scale production and have been shown to be safe and immunogenic.Citation89,Citation90 On the other hand, synthetic molecular glycovaccines also bear advantages for they are rationally designed and fully defined. Such a glycoconjugate vaccine incorporating a synthetic oligosaccharide mimicking the O-antigen of S. flexneri 2a has been shown to be safe, well tolerated and immunogenic in healthy adultsCitation91 (Cohen D. et al., in preparation). Along with the GMMA (Generalized Module for Membrane Antigens) approach based on the use of bacterial outer membrane particles,Citation92,Citation93 these three strategies are currently supported for further clinical development by the Bill and Melinda Gates FoundationCitation94 and the Wellcome Trust.Citation95 The aim is to develop a tetravalent vaccine against S. flexneri 2a, 3a and 6, and S. sonnei to cover, with some expected cross-protection, about 90–95% of the circulating Shigella strains. Hopefully, with the current support from funding agencies in advancing clinical studies on the already available tetravalent bioconjugates and GMMA candidates,Citation94–Citation97 a tetravalent Shigella vaccine might be available at the horizon 2028. Preventing shigellosis remains more than ever a public health priority.Citation98–Citation100 Indeed, as recently reviewed,Citation101 there is a growing appreciation for the role of vaccines in confronting the problem of antimicrobial resistance, which includes the alarming emergence of multi-drug resistant Shigella strains.Citation98
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
References
- Marteyn BS. Shigella vaccine development : the model matters. JSM Trop Med Res. 2016;1:1011–14.
- Banish LD, Sims R, Sack D, Montali RJ, Phillips L, Bush M. Prevalence of shigellosis and other enteric pathogens in a zoologic collection of primates. J Am Vet Med Assoc. 1993;203:126–32.
- Black-Schultz L, Coatney RW, Warnick CL, Swif B. Lack of reactivation of shigellosis in naturally infected enrofloxacin-treated cynomolgus monkeys after exogenous immunosuppression. Lab Anim Sci. 1997;47:602–05.
- Vickers JH. Infectious diseases of primates related to capture and transportation. Am J Phys Anthropol. 1973;38:511–13. doi:https://doi.org/10.1002/(ISSN)1096-8644.
- Collins TA, Barnoy S, Baqar S, Ranallo RT, Nemelka KW, Venkatesan MM. Safety and colonization of two novel virG(icsA)-based live Shigella sonnei vaccine strains in Rhesus macaques (Macaca mulatta). Comp Med. 2008;58:88–94.
- Dupont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–28.
- Islam MS, Hasan MK, Khan SI. Growth and survival of Shigella flexneri in common bangladeshi foods under various conditions of time and temperature. Appl Environ Microbiol. 1993;59:652–54.
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;175:2731–38. doi:https://doi.org/10.4049/jimmunol.172.5.2731.
- Gorden J, Small PLC. Acid resistance in enteric bacteria. Infect Immun. 1993;61:364–67.
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–80. doi:https://doi.org/10.1002/(ISSN)1099-081X.
- Nickerson KP, Chanin RB, Sistrunk JR, Rasko DA, Fink PJ, Barry EM, Nataro JP, Faherty CS. Analysis of Shigella flexneri resistance, biofilm formation, and transcriptional profile in response to bile salts. Infect Immun. 2017;85:e01067–16. doi:https://doi.org/10.1128/IAI.01067-16.
- Lagkouvardos I, Pukall R, Abt B, Foesel BU, Meier-Kolthoff JP, Kumar N, Bresciani A, Martínez I, Just S, Ziegler C, et al. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 2016;1:16131. doi:https://doi.org/10.1038/nmicrobiol.2016.131.
- Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9:279–90. doi:https://doi.org/10.1038/nrmicro2540.
- Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–85. doi:https://doi.org/10.1038/nri3738.
- McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–78. doi:https://doi.org/10.1038/nrmicro2538.
- Sai Sudha P, Devaraj H, Devaraj N. Adherence of Shigella dysenteriae 1 to human colonic mucin. Curr Microbiol. 2001;42:381–87. doi:https://doi.org/10.1007/s002840010234.
- Prakash R, Raja S, Devaraj H, Devaraj SN. Up-regulation of MUC2 and IL-1β expression in human colonic epithelial cells by Shigella and its interaction with mucins. PLoS One. 2011;6:e27046. doi:https://doi.org/10.1371/journal.pone.0027046.
- Patil A, Hughes AL, Zhang G. Rapid evolution and diversification of mammalian α-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genomics. 2004;20:1–11. doi:https://doi.org/10.1152/physiolgenomics.00150.2004.
- Bals R, Lang C, Weiner DJ, Vogelmeier C, Welsch U, Wilson JM. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin Diagnostic Lab Immunol. 2001;8:370–75.
- Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson GH. Downregulation of bactericidal peptides in enteric infections: A novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–85. doi:https://doi.org/10.1038/84627.
- Sperandio B, Regnault B, Guo J, Zhang Z, Stanley SL, Sansonetti PJ, Pédron T. Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J Exp Med. 2008;205:1121–32. doi:https://doi.org/10.1084/jem.20071698.
- Singer M, Sansonetti PJ. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol. 2004;173:4197–206. doi:https://doi.org/10.4049/jimmunol.173.6.4197.
- Perdomo JJ, Gounon P, Sansonetti PJ. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest. 1994;93:633–43. doi:https://doi.org/10.1172/JCI117015.
- Sansonetti PJ, Arondel J, Cavaillon JM, Huerre M. Role of interleukin-1 in the pathogenesis of experimental shigellosis. J Clin Invest. 1995;96:884–92. doi:https://doi.org/10.1172/JCI118135.
- Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun. 1999;67:1471–80.
- Shim D-H, Suzuki T, Chang S-Y, Park S-M, Sansonetti PJ, Sasakawa C, Kweon M-N. New animal model of shigellosis in the guinea pig: its usefulness for protective efficacy studies. J Immunol. 2007;178:2476–82. doi:https://doi.org/10.4049/jimmunol.178.4.2476.
- Voino-Yasenetsky MV, Voino-Yasenetskaya MK. Experimental pneunomia caused by bacteria of the Shigella group. Acta Morphol Acad Sci Hung. 1962;11:439–54.
- Phalipon A, Kaufmann M, Michetti P, Cavaillon J-M, Huerre M, Sansonetti PJ, Kraehenbuhlll J-P. Monoclonal immunoglobulin a antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–78. doi:https://doi.org/10.1084/jem.182.3.769.
- Galán JE, Waksman G. Protein-injection machines in bacteria. Cell. 2018;172:P1306–1318. doi:https://doi.org/10.1016/j.cell.2018.01.034.
- de Nisco NJ, Rivera-Cancel G, Orth K. The biochemistry of sensing: enteric pathogens regulate type III secretion in response to environmental and host cues. MBio. 2018;9:e02122–17. doi:https://doi.org/10.1128/mBio.02122-17.
- Gunasinghe SD, Webb CT, Elgass KD, Hay ID, Lithgow T. Super-resolution imaging of protein secretion systems and the cell surface of gram-negative bacteria. Front Cell Infect Microbiol. 2017;7:220. doi:https://doi.org/10.3389/fcimb.2017.00517.
- Sansonetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–60.
- Galán JE, Lara-Tejero M, Marlovits TC, Wagner S. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol. 2014;68:415–38. doi:https://doi.org/10.1146/annurev-micro-092412-155725.
- Maurelli AT, Blackmon B, Curtis R III. Temperature-Dependent Expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201.
- Veenendaal AKJ, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol. 2007;63:1719–30. doi:https://doi.org/10.1111/j.1365-2958.2006.05509.x.
- Ménard R, Sansonetti PJ, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. Embo J. 1994;13:5293–302. doi:https://doi.org/10.1002/j.1460-2075.1994.tb06863.x.
- Enninga J, Mounier J, Sansonetti P. Tran Van Nhieu G. Secretion of type III effectors into host cells in real time. Nat Methods. 2005;2:959–65. doi:https://doi.org/10.1038/nmeth738.
- Marlovits TC, Kubori T, Sukhan A, Galán JE, Unger VM. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–42. doi:https://doi.org/10.1126/science.1102610.
- Martinez-Argudo I, Blocker AJ. The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol Microbiol. 2010;78:1365–78. doi:https://doi.org/10.1111/j.1365-2958.2010.07413.x.
- Demers B, Sansonetti PJ, Parsot C. Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. Embo J. 1998;17:2894–903. doi:https://doi.org/10.1093/emboj/17.11.3016.
- Campbell-Valois FX, Schnupf P, Nigro G, Sachse M, Sansonetti PJ, Parsot C. A fluorescent reporter reveals on/off regulation of the Shigella type III secretion apparatus during entry and cell-to-cell spread. Cell Host Microbe. 2014;15:177–89. doi:https://doi.org/10.1016/j.chom.2014.01.005.
- Page AL, Ohayon H, Sansonetti PJ, Parsot C. The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell Microbiol. 1999;1:183–93.
- Schuch R, Sandlin RC, Maurelli AT. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol. 1999;34:675–89. doi:https://doi.org/10.1046/j.1365-2958.1999.01627.x.
- Pinaud L, Sansonetti PJ, Phalipon A. Host cell targeting by enteropathogenic bacteria T3SS effectors. Trends Microbiol. 2018;26:266–83. doi:https://doi.org/10.1016/j.tim.2018.01.010.
- de Jong MF, Alto NM. Cooperative immune suppression by Escherichia coli and Shigella effector proteins. Infect Immun. 2018;86:e00560–17. doi:https://doi.org/10.1128/IAI.00560-17.
- Ashida H, Mimuro H, Sasakawa C. Shigella manipulates host immune responses by delivering effector proteins with specific roles. Front Immunol. 2015;6:219. doi:https://doi.org/10.3389/fimmu.2015.00219.
- Jain S, Sharma M, Gupta R, Shree N, Kumar M. Multidrug resistant Shigella flexneri: A rare case of septicemia in an infant. J Clin Diagnostic Res. 2014;8:DD03–DD04.
- Islam D, Wretlind B, Hammarström L, Christensson B, Lindberg AA. Semiquantitative estimation of Shigella antigen-specific antibodies: correlation with disease severity during shigellosis. Apmis. 1996;104:563–74. doi:https://doi.org/10.1111/j.1699-0463.1996.tb04912.x.
- Islam D, Veress B, Bardhan PK, Lindberg AA, Christensson B. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect Immun. 1997;65:739–49.
- Barry EM, Pasetti MF, Sztein MB, Fasano A, Kotloff KL, Levine MM. Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroenterol Hepatol. 2013;10:245–55. doi:https://doi.org/10.1038/nrgastro.2013.12.
- Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164:533–37. doi:https://doi.org/10.1093/infdis/164.3.533.
- Wahid R, Simon JK, Picking WL, Kotloff KL, Levine MM, Sztein MB. Shigella antigen-specific B memory cells are associated with decreased disease severity in subjects challenged with wild-type Shigella flexneri 2a. Clin Immunol. 2013;148:35–43. doi:https://doi.org/10.1016/j.clim.2013.03.009.
- Dupont HL, Hornick RB, Snyder MJ, Libonati JP, Formal SB, Gangarosa EJ. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972;125:12–16. doi:https://doi.org/10.1093/infdis/125.1.12.
- Raqib R, Qadri F, Sarker P, Mia SMS, Sansonnetti PJ, Albert MJ, Andersson J. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol. 2002;55:414–23. doi:https://doi.org/10.1046/j.1365-3083.2002.01079.x.
- Raqib R, Ekberg C, Sharkar P, Bardhan PK, Zychlinsky A, Sansonetti PJ, Andersson J. Apoptosis in acute shigellosis is associated with increased production of fas/fas ligand, perforin, caspase-1, and caspase-3 but reduced production of Bcl-2 and interleukin-2. Infect Immun. 2002;70:3199–207. doi:https://doi.org/10.1128/IAI.70.6.3199-3207.2002.
- Raqib R, Mia SMS, Qadri F, Alam TI, Alam NH, Chowdhury AK, Mathan MM, Andersson J. Innate immune responses in children and adults with shigellosis. Infect Immun. 2000;68:3620–29. doi:https://doi.org/10.1128/IAI.68.6.3620-3629.2000.
- Pédron T, Thibault C, Sansonetti PJ. The invasive phenotype of Shigella flexneri directs a distinct gene expression pattern in the human intestinal epithelial cell line Caco-2. J Biol Chem. 2003;278:33878–86. doi:https://doi.org/10.1074/jbc.M303749200.
- Edgeworth JD, Spencer J, Phalipon A, Griffin GE, Sansonetti PJ. Cytotoxicity and interleukin-1β processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur J Immunol. 2002;32:1464–71. doi:https://doi.org/10.1002/1521-4141(200205)32:5<1464::AID-IMMU1464>3.0.CO;2-G.
- Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J Immunol. 2010;184:2076–85. doi:https://doi.org/10.4049/jimmunol.0902016.
- Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–96.
- Raqib R, Lindberg AA, Bjork L, Bardhan PK, Wretlind B, Andersson U, Andersson J. Down-regulation of gamma interferon, tumor necrosis factor type I, interleukin 1 (IL-1) type I, IL-3, IL-4, and transforming growth factor beta type I receptors at the local site during the acute phase of Shigella infection. Infect Immun. 1995;63:3079–87.
- Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–85. doi:https://doi.org/10.1111/imm.2008.126.issue-2.
- Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi:https://doi.org/10.1016/j.immuni.2009.05.001.
- Jehl SP, Doling AM, Giddings KS, Phalipon A, Sansonetti PJ, Goldberg MB, Starnbach MN. Antigen-specific CD8+ T cells fail to respond to Shigella flexneri. Infect Immun. 2011;79:2021–30. doi:https://doi.org/10.1128/IAI.00040-11.
- Pinaud L, Samassa F, Porat Z, Ferrari ML, Belotserkovsky I, Parsot C, Sansonetti PJ, Campbell-Valois F-X, Phalipon A. Injection of T3SS effectors not resulting in invasion is the main targeting mechanism of Shigella toward human lymphocytes. Proc Natl Acad Sci. 2017;114:9954–59. doi:https://doi.org/10.1073/pnas.1707098114.
- Nothelfer K, Arena ET, Pinaud L, Neunlist M, Mozeleski B, Belotserkovsky I, Parsot C, Dinadayala P, Burger-Kentischer A, Raqib R, et al. B lymphocytes undergo TLR2-dependent apoptosis upon Shigella infection. J Exp Med. 2014;211:1215–29. doi:https://doi.org/10.1084/jem.20130914.
- Belotserkovsky I, Brunner K, Pinaud L, Rouvinski A, Dellarole M, Baron B, Dubey G, Samassa F, Parsot C, Sansonetti P, et al. Glycan-glycan interaction determines Shigella tropism toward human T lymphocytes. MBio. 2018;9:e02309–17. doi:https://doi.org/10.1128/mBio.02309-17.
- Shanker S, Hu L, Ramani S, Atmar RL, Estes MK. Venkataram Prasad B V. Structural features of glycan recognition among viral pathogens. Curr Opin Struct Biol. 2017;44:211–18. doi:https://doi.org/10.1016/j.sbi.2017.05.007.
- Xu D, Liao C, Zhang B, Tolbert WD, He W, Dai Z, Zhang W, Yuan W, Pazgier M, Liu J, et al. Human enteric α-defensin 5 promotes Shigella Infection by enhancing bacterial adhesion and invasion. Immunity. 2018;48:1233–43. doi:https://doi.org/10.1016/j.immuni.2018.04.005.
- Faherty CS, Redman JC, Rasko DA, Barry EM, Nataro JP. Shigella flexneri effectors OspE1 and OspE2 mediate induced adherence to the colonic epithelium following bile salts exposure. Mol Microbiol. 2012;85:107–21. doi:https://doi.org/10.1111/j.1365-2958.2012.08092.x.
- Brotcke Zumsteg A, Goosmann C, Brinkmann V, Morona R, Zychlinsky A. IcsA is a Shigella flexneri adhesion regulated by the type III secretion system and required for pathogenesis. Cell Host Microbe. 2014;15:435–45. doi:https://doi.org/10.1016/j.chom.2014.03.001.
- Baum LG, Crocker PR. Glycoimmunology: ignore at your peril!. Immunol Rev. 2009;230:5–8.
- Konradt C, Frigimelica E, Nothelfer K, Puhar A, Salgado-Pabon W, Bartolo V, Scott-Algara D, Rodrigues CD, Sansonetti PJ, Phalipon A. The Shigella flexneri type three secretion system effector IpgD inhibits T cell migration by manipulating host phosphoinositide metabolism. Cell Host Microbe. 2011;9:263–72. doi:https://doi.org/10.1016/j.chom.2011.03.010.
- Salgado-Pabon W, Celli S, Arena ET, Nothelfer K, Roux P, Sellge G, Frigimelica E, Bousso P, Sansonetti PJ, Phalipon A. Shigella impairs T lymphocyte dynamics in vivo. Proc Natl Acad Sci. 2013;110:4458–63. doi:https://doi.org/10.1073/pnas.1300981110.
- Toapanta FR, Bernal PJ, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, Blohmke CJ, Angus B, Levine MM, et al. Oral challenge with wild-type Salmonella typhi induces distinct changes in B cell subsets in individuals who develop typhoid disease. PLoS Negl Trop Dis. 2016;10:e0004766. doi:https://doi.org/10.1371/journal.pntd.0004766.
- Fresnay S, McArthur MA, Magder L, Darton TC, Jones C, Waddington CS, Blohmke CJ, Angus B, Levine MM, Pollard AJ, et al. Salmonella typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J Transl Med. 2016;14:62. doi:https://doi.org/10.1186/s12967-016-0867-z.
- Juel HB, Thomaides-Brears HB, Darton TC, Jones C, Jones E, Shrestha S, Sie R, Eustace A, Galal U, Kurupati P, et al. Salmonella typhi bactericidal antibodies reduce disease severity but do not protect against typhoid fever in a controlled human infection model. Front Immunol. 2018;8:1916. doi:https://doi.org/10.3389/fimmu.2017.01960.
- Porter CK, Louis Bourgeois A, Frenck RW, Prouty M, Maier N, Riddle MS. Developing and utilizing controlled human models of infection. Vaccine. 2017;35:6813–18. doi:https://doi.org/10.1016/j.vaccine.2017.05.068.
- Porter CK, Lynen A, Riddle MS, Talaat K, Sack D, Gutiérrez RL, McKenzie R, DeNearing B, Feijoo B, Kaminski RW, et al. Clinical endpoints in the controlled human challenge model for Shigella: A call for standardization and the development of a disease severity score. PLoS One. 2018;13:e0194325. doi:https://doi.org/10.1371/journal.pone.0194325.
- Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8+ memory T cells. Front Immunol. 2012;3:340. doi:https://doi.org/10.3389/fimmu.2012.00198.
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJC, Bickham KL, Lerner H, Goldstein M, Sykes M, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–97. doi:https://doi.org/10.1016/j.immuni.2012.09.020.
- Bergsbaken T, Bevan MJ, Fink PJ. Local inflammatory cues regulate differentiation and persistence of CD8+ tissue-resident memory T cells. Cell Rep. 2017;19:114–24. doi:https://doi.org/10.1016/j.celrep.2017.03.031.
- Kentner D, Martano G, Callon M, Chiquet P, Brodmann M, Burton O. Shigella reroutes host cell central metabolism to obtain high-flux nutrient supply for vigorous intracellular growth. Proc Natl Acad Sci USA. 2014;111:9929–34.
- Buck MDD, O’Sullivan D, Klein Geltink RII, Curtis JDD, Chang CH, Sanin DEE, Qiu J, Kretz O, Braas D, van der Windt GJJW, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi:https://doi.org/10.1016/j.cell.2016.05.035.
- Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34:2887–94. doi:https://doi.org/10.1016/j.vaccine.2016.10.045.
- Coster TS, Hoge CW, VanDeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–43.
- Rahman KM, Arifeen SE, Zaman K, Rahman M, Raqib R, Yunus M, Begum N, Islam MS, Sohel BM, Rahman M, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29:1347–54. doi:https://doi.org/10.1016/j.vaccine.2010.10.035.
- Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine. 2010;28:2231–35. doi:https://doi.org/10.1016/j.vaccine.2009.12.050.
- Riddle MS, Kaminski RW, Di Paolo C, Porter CK, Gutierrez RL, Clarkson KA, Weerts HE, Duplessis C, Castellano A, Alaimo C, et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: A single-blind, randomized phase i study. Clin Vaccine Immunol. 2016;23:908–17. doi:https://doi.org/10.1128/CVI.00224-16.
- Hatz CFR, Bally B, Rohrer S, Steffen R, Kramme S, Siegrist CA, Wacker M, Alaimo C, Fonck VG. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: A single blind, partially randomized Phase I study. Vaccine. 2015;33:4594–601. doi:https://doi.org/10.1016/j.vaccine.2015.06.102.
- Phalipon A, Tanguy M, Grandjean C, Guerreiro C, Belot F, Cohen D, Sansonetti PJ, Mulard LA. A synthetic carbohydrate-protein conjugate vaccine candidate against Shigella flexneri 2a infection. J Immunol. 2009;182:2241–47. doi:https://doi.org/10.4049/jimmunol.0802775.
- Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Sciré AS, Maugard A, Marchetti E, Zancan S, Huo Z, et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBio Med. 2017;22:164–72. doi:https://doi.org/10.1016/j.ebiom.2017.07.013.
- Gerke C, Colucci AM, Giannelli C, Sanzone S, Vitali CG, Sollai L, Rossi O, Martin LB, Auerbach J, Di Cioccio V, et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One. 2015;10:e0134478. doi:https://doi.org/10.1371/journal.pone.0134478.
- Bill & Melinda Gates Foundation. Enteric and diarrheal diseases strategy overview [Internet]; [accessed 2018 Dec 10]. https://www.gatesfoundation.org/What-We-Do/Global-Health/Enteric-and-Diarrheal-Diseases.
- Global Biodefences. Wellcome trust to finance development of Shigella vaccine [Internet]. [accessed 2019 Feb 11]. https://globalbiodefense.com/2013/07/30/wellcome-trust-to-finance-development-of-shigella-vaccine/.
- The European & Developing Countries Clinical Trials Partnership (EDCTP). Vaccines for diarrhoeal diseases or lower respiratory tract infections: Research and Innovation Action (RIA) call budget [Internet]; [accessed 2018 Dec 10]. http://www.edctp.org/call/vaccines-for-diarrhoeal-diseases-or-lower-respiratory-tract-infections/.
- PATH. Diarrheal disease [Internet]; [accessed 2018 Dec 10]. https://www.path.org/diarrheal-disease/.
- Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. Lancet. 2017;17:33296–98. doi:https://doi.org/10.1016/S1473-3099(17)30242-6.
- Williams PCM, Berkley JA. Paediatrics and International Child Health Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr Int Child Health. 2018;9047:1–16.
- Chen WH, Kotloff L, Riddle MS, Kaminski RW, Di Paolo C, Porter CK, Gutierrez RL, Immu CV. Shigella vaccine development: finding the path of least resistance. Clin Vaccine Immunol. 2016;23:904–07.
- Lipsitch M, Siber GR. How can vaccines contribute to solving the antimicrobial resistance problem? MBio. 2016;7:e00428–16. doi:https://doi.org/10.1128/mBio.00428-16.
- Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti PJ. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–48.
- Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin Immunol. 1999;11:193–203. doi:https://doi.org/10.1006/smim.1999.0175.
- Arena ET, Campbell-Valois F-X, Tinevez J-Y, Nigro G, Sachse M, Moya-Nilges M, Nothelfer K, Marteyn B, Shorte SL, Sansonetti PJ. Bioimage analysis of Shigella infection reveals targeting of colonic crypts. Proc Natl Acad Sci. 2015;112:E3282–90. doi:https://doi.org/10.1073/pnas.1509091112.
- Mathan MM, Mathan VI. Morphology of rectal mucosa of patients with shigellosis. Rev Infect Dis. 1991;13:314–18.
- Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–900. doi:https://doi.org/10.1074/jbc.273.49.32895.
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–69. doi:https://doi.org/10.1038/358167a0.
- Arizmendi O, Picking WD, Picking WL. Macrophage apoptosis triggered by IpaD from Shigella flexneri. Infect Immun. 2016;84:1857–65. doi:https://doi.org/10.1128/IAI.01483-15.
- Sansonetti PJ, Ryter A, Clerc P, Maurelli AT, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–69.
- Goldberg MB, Barzu O, Parsot C, Sansonettil PJ, De Biochimie U. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–96.
- Bernardini ML, Mounier J, Dwhauteville HNE, Coquis-Rondont M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–71.
- Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi:https://doi.org/10.1074/jbc.C200651200.
- Philpott DJ, Yamaoka S, Israel A, Sansonetti PJ. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J Immunol. 2000;165:903–14. doi:https://doi.org/10.4049/jimmunol.165.2.903.
- Kasper CA, Sorg I, Schmutz C, Tschon T, Wischnewski H, Kim ML, Arrieumerlou C. Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity. 2010;33:804–16. doi:https://doi.org/10.1016/j.immuni.2010.10.015.
- Lowell GH, MacDermott RP, Summers PL, Reeder AA, Bertovich MJ, Formal SB. Antibody-dependent cell-mediated antibacterial activity: K lymphocytes, monocytes, and granulocytes are effective against Shigella. J Immunol. 1980;125:2778–84.
- Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. Neutrophil elastase targets virulence factors of enterobacteria. Nature. 2002;417:91–94. doi:https://doi.org/10.1038/417091a.
