ABSTRACT
Respiratory syncytial virus (RSV) is a major respiratory pathogen in infants. The early formalin-inactivated RSV not only failed to protect infants against infection, but also was associated with enhanced pulmonary inflammatory disease upon natural infection. A safe and effective vaccine should prevent the inflammatory disease and provide protection. Immune memory is the cornerstone of vaccines. In this study, we evaluated three types of immune memory T cells, antibodies, and lung inflammation of a vaccine candidate G1F/M2, which includes a neutralizing epitope fragment of RSV G protein and a cytotoxic T lymphocyte epitope of M2 protein, with toll-like receptor 9 agonist CpG2006 as an adjuvant by intranasal (i.n.) and intraperitoneal (i.p.) immunization protocols. The results indicated that immunization of mice with G1F/M2 + CpG i.p. induced significantly higher level of CD4+ or CD8+ central memory (TCM), Th1-type effector memory (TEM), and balanced ratio of IgG1/IgG2a, but lower level of lung tissue-resident memory (TRM), compared with immunization with G1F/M2 + CpG i.n., G1F/M2 i.n., or G1F/M2 i.p. Following RSV challenge, the mice immunized with G1F/M2 + CpG i.p. showed higher level of Th1-type responses, remarkably suppressed inflammatory cytokines and histopathology in lungs, compared with mice immunized with G1F/M2 + CpG i.n., G1F/M2 i.n., or G1F/M2 i.p. These results suggested that high level of TCM and Th1 type of TEM in spleens may contribute to inhibition of lung inflammation, while high level of TRM in lungs and lack of or weak Th1-type immune memory in spleens may promote lung inflammation following RSV challenge.
1. Introduction
Human respiratory syncytial virus (RSV) is the most important cause of lower respiratory tract infection in infants and young children worldwide.Citation1 Reinfections occur frequently during life due to incomplete immunity after RSV infection. Development of RSV vaccines has been hampered by failed vaccine trials in which formalin-inactivated RSV (FI-RSV) not only failed to protect infants against infection, but also was associated with enhanced pulmonary disease in some vaccines upon natural infection.Citation2 Histology performed on the lungs of the deceased revealed extensive inflammatory cell infiltrating the lungs.Citation3 Investigations in mouse models have contributed to our understanding of the mechanism of the vaccines-enhanced disease. It is generally accepted that the disease enhancement is due to the induction of a Th2-biased immune response or unbalanced cellular immune responses and lack of protective antibody.Citation4,Citation5 A vaccine formulation that can avoid inflammatory pathology in lungs and protect from viral infection would therefore be desirable.
Memory T cells induced by vaccines may play a key role in the protection from infection and vaccine-associated inflammatory responses. More recently, memory T cells were subdivided into three main subsets: central memory (TCM), effector memory (TEM) T cells,Citation6 and tissue-resident memory (TRM) cells.Citation7,Citation8 TCM cells reside primarily in secondary lymphoid tissues, such as spleen, and TEM cells recirculate between blood and peripheral tissues. TRM cells are noncirculating and reside in nonlymphoid tissues. Because of TRM cells’ strategic location and rapid recall response, early activated memory T cell responses after natural infections in lung were largely mediated by the TRM cells, rather than TCM or TEM cells recruited from blood or secondary lymphoid tissues.Citation7 Recently, some studies suggested immune pathological TRM in inflammatory diseases.Citation9-Citation11
G protein of RSV was found to exhibit strong Th2 responses, as well as vaccine-enhanced disease following exposure to RSV, which may be linked to the failure of inducing a CD8+ cytotoxic T lymphocytes (CTLs).Citation12 We have engineered a recombinant vaccine candidate, G1F/M2,Citation13,Citation14 consisting of a domain of RSV G protein (amino acids [aa] 125 to 225; G1), which includes two identified neutralizing epitopes,Citation15 and a chimera F/M2, composed of a CTL epitope (aa 81 to 95; M2) of RSV M2 protein.Citation16 G1F/M2 immunization of mice with Al(OH)3 as an adjuvant induced a mixed Th1/Th2 response, CTLs, neutralizing antibodies,Citation13 and long-lasting protection.Citation17 However, the immunized mice developed vaccine-associated pulmonary inflammation and immunopathology after RSV challenge (unpublished data).
Toll-like receptor (TLR) recognizes various pathogen-associated molecular patterns and activates innate immune responses that serve as the first line of defense against invading pathogens and tailors the adaptive immune responses. CpG-containing oligodeoxynucleotides (CpG ODN), a TLR9 agonist, triggers the production of Th1-type cytokines, including IFN-γ and IL-12, via interaction with TLR9 on dendritic cells, macrophages, and B lymphocytes.Citation18,Citation19 As an adjuvant, CpG ODNs have been investigated in immunization of mice with purified RSV F or G,Citation20,Citation21 FI-RSV,Citation22 or killed bovine RSV,Citation23 which showed more Th1-associated immune responses and increases protection against RSV challenge. Therefore, we hypothesize that immunization of G1F/M2 with CpG (CpG2006, tcgtcgttttgtcgttttgtcgtt, Phosphorthioated) would boost the induction of type-І immune memory to minimize generation of Th2 memory cells and alleviate pulmonary inflammatory pathology during RSV challenge.
In this study, two delivery route (1) nasal delivery that induces mucosal immune response and (2) intraperitoneal (i.p.) delivery that induces systemic immune response were used in mice. We examined the Th1/Th2 TEM, CD4+ or CD8+ TCM, TRM, and antibodies induced by G1F/M2 + CpG, as well as lung inflammatory responses, histopathology, and RSV-N gene expression as a surrogate for RSV infection after RSV challenge. The results showed the i.p. immunization with G1F/M2 or G1F/M2 + CpG i.p. induced significantly more IFN-γ-producing TEM and CD4+ or CD8+ TCM. In contrast, remarkably less TRM was induced. Mice immunized with G1F/M2 + CpG i.p. showed remarkably inhibited pulmonary inflammatory responses, histopathology, and RSV-N expression following RSV challenge. These results suggested that Th1-type of TEM and TCM may contribute to inhibition of lung inflammation following RSV challenge. TRM in lungs and lack of Th1-type immune memory in spleens may be important pro-inflammatory factors that enhance lung inflammation following RSV challenge.
2. Results
2.1. G1F/M2 + CpG immunization i.p. induced markedly high levels of CD4+/CD8+ TCM cells
CD44+CD62L+ is a phenotype that generally typifies TCM cells.Citation24 We monitored the expression of CD44 and CD62L in total CD4+ or CD8+ splenocytes of immunized mice by flow cytometric assay.Citation25 Mice immunized with G1F/M2 + CpG or G1F/M2 intranasal (i.n.) showed few CD4+/CD8+CD44+CD62L+ memory cells (). In contrast, G1F/M2 + CpG immunization i.p. induced markedly higher levels of CD4+/CD8+CD44+CD62L+ memory cells than G1F/M2 + CpG or G1F/M2 immunization i.n. and G1F/M2 immunization i.p. ()). These data suggest that G1F/M2 + CpG delivered by i.p. route, but not by i.n. route, could promote induction of CD4+/CD8+ TCM cells.
Figure 1. Percentage of CD44+CD62L+ cells of CD4+ or CD8+ spleen cells in immunized mice. Mice were immunized as described in Section 4. Spleens from immunized mice were removed after the last immunization. Splenocytes were incubated with anti-CD4-FITC/anti-CD8-FITC, anti-CD44-PE, and anti-CD62L-Pecy5, then detected by flow cytometry. (a) CD4+CD44+CD62L+ T cells in the splenocytes of immunized mice. (b) CD8+CD44+CD62L+ T cells in the splenocytes of immunized mice. (c) Percentage of CD4+CD44+CD62L+ T cells in the splenocytes of immunized mice. (d) Percentage of CD8+CD44+CD62L+ T cells in the splenocytes of immunized mice. Results are presented as mean ± SD of five mice per group. *P < 0.05 represents significant difference.
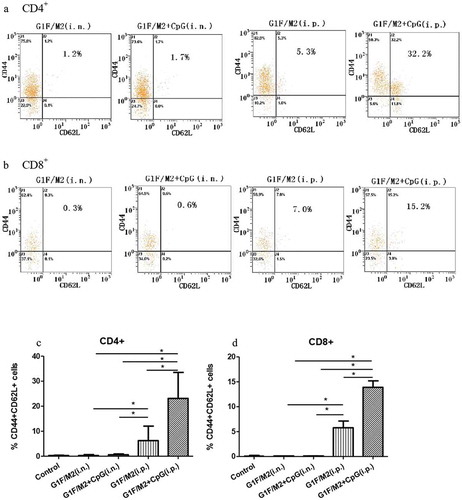
2.2. G1F/M2 + CpG immunization i.p. induced significant high frequency of IFN-γ-secreting TEM
Since Th1-type responses are characterized by the production of IFN-γ, while Th2 responses are characterized by the production of IL-4, we compared the frequency of IFN-γ- or IL-4-secreting TEMs in splenocytes of immunized mice by standard enzyme-linked immunospot (ELISPOT) assay, which measures cytokine-secreting TEM T cells.Citation26 G1F/M2 + CpG- or G1F/M2-immunization i.p. induced significantly higher frequency of IFN-γ-secreting cells than G1F/M2 + CpG- or G1F/M2-immunization i.n., respectively (), p < 0.05). IFN-γ-secreting cells were induced more by G1F/M2 + CpG i.p. than G1F/M2. (), p < 0.05). No difference was observed in the frequency of IL-4-secreting cells between different experimental groups. The results indicated that i.p. delivery route of G1F/M2 + CpG is a more effective for induction of Th1-type in TEM.
Figure 2. Frequency of IFN-γ- or IL-4-secreting effector memory cells in immunized mice. Mice were immunized as described in Section 4. Spleens from immunized mice were removed 3 weeks after the last immunization. Splenocytes were restimulated for 48 h with 20 μg G1F/M2. Number of specific IFN-γ-secreting T cells and IL-4-secreting T cells was evaluated using an ELISPOT assay as described in Section 4 . (a) Number of IFN-γ producing T cells. (b) Number of IL-4 producing T cells. Results are presented as mean ± SD of the number of spots observed for 106 spleen cells of five mice per group, obtained from triplicate wells. *P < 0.05 represents significant difference.
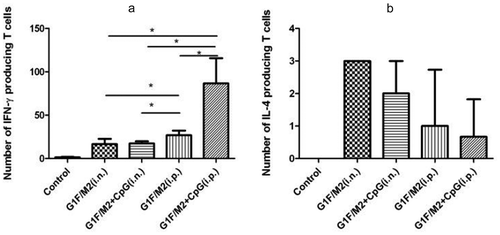
2.3. G1F/M2 + CpG or G1F/M2 immunization i.p. induced lower level of lung TRM cells
Several studies have highlighted the role of TRM in infections and inflammatory diseases.Citation9-Citation11,Citation27 TRM cells may play a role in vaccine-enhanced inflammatory disease.Citation9-Citation11 CD69 is one of cardinal TRM markers. As shown in , both G1F/M2 + CpG and G1F/M2 immunization i.n. induced higher level of TRM, compared with G1F/M2 + CpG and G1F/M2 immunization i.p. (p < 0.05). No difference was observed between G1F/M2 + CpG and G1F/M2 immunization i.n. or i.p. (), p > 0.05). The results indicated that G1F/M2 + CpG or G1F/M2 immunization i.p., unlikely G1F/M2 + CpG or G1F/M2 immunization i.n., induced low level of TRM cells.
Figure 3. TRM cells in lungs of immunized mice. Mice were injected intravenously with anti-CD3-FITC. After 10 mins, lung cells were isolated and stained in vitro with anti-CD69-PE and anti-CD3-PerCP-Cy5. Stained cells were analyzed by using flow cytometry (BD). (a), (b), (c), (d), (e), and (f) represent pictures of TRM in lung cells. (g) The percent of TRM cells in total lung T cells. Results are presented as mean ± SD of five mice per group. *P < 0.05 represents significant difference.
2.4. G1F/M2 + CpG immunization i.p. induced high titer of antibody IgG2a
We investigated the titers of the IgG, IgG1, and IgG2a antibodies, and evaluated the IgG1/IgG2a ratio. G1F/M2 + CpG or G1F/M2 alone induced high titer of specific IgG, IgG1, and IgG2a antibodies in mice immunized by i.n. or i.p. route, compared with phosphate buffered saline (PBS) (). The titer of IgG induced by G1F/M2 + CpG i.p. was lower than those by G1F/M2 i.p. (p < 0.05). No difference was observed among other groups. The titer of IgG1 induced by G1F/M2 + CpG i.p. was lower than those by G1F/M2 i.p., G1F/M2 + CpG i.n., or G1F/M2 i.n. (, p < 0.05). The titer of IgG2a was lower than the titer of IgG1 induced by G1F/M2 i.n. or i.p. (p < 0.05). In contrast, no difference between IgG1 and IgG2a was observed in G1F/M2 + CpG i.n. or i.p. group (p > 0.05). The ratio of IgG1/IgG2a in G1F/M2 + CpG i.n. or G1F/M2 + CpG i.p. group was 1.05 or 0.97, while that in G1F/M2 i.n. or G1F/M2 i.p. group was 1.25 or 1.30 in G1F/M2 i.n. group, respectively. These results indicated that the CpG delivered by i.p. route could promote specific IgG2a, and inhibit IgG1.
Table 1. Mean specific IgG, IgG1, and IgG2a antibody titers and IgG1/IgG2a ratio after immunization.
2.5. G1F/M2 + CpG immunization i.p. remarkably inhibited pro-inflammatory cytokines and promoted anti-inflammatory cytokines after RSV challenge
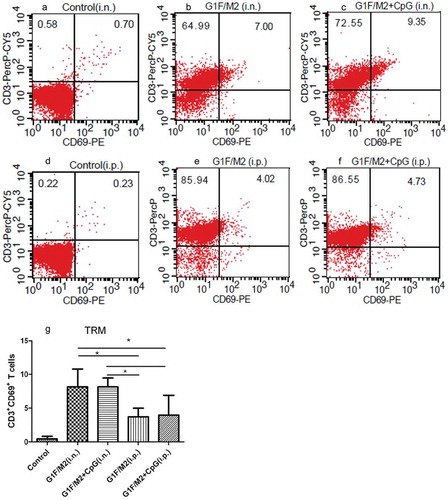
The inflammatory cytokines and chemokines in lungs of immunized mice after RSV challenge were detected by real-time Polymerase Chain Reaction (PCR). The relative expression of TNF-α in G1F/M2 + CpG i.p. group was lower than that in PBS, G1F/M2 i.n., or G1F/M2 i.p. group (), p < 0.05). There was no difference between G1F/M2 + CpG i.n. and G1F/M2 + CpG i.p. groups (), p > 0.05). The relative expression of IL-1β in G1F/M2 i.n. group was lower than that in PBS group, but was significantly higher than those in control, G1F/M2 + CpG i.n., G1F/M2 i.p., or G1F/M2 + CpG i.p. group after RSV challenge (), p < 0.05). The relative expression of IL-1β in G1F/M2 + CpG i.p. group was lower than those in the other groups (), p < 0.05). No difference on the relative expression of IL-1β was observed between G1F/M2 + CpG i.n. and G1F/M2 i.p. (), p > 0.05). The relative expression of Gro-α in G1F/M2 + CpG i.p. group was lower than those in other experimental groups (), p < 0.05). G1F/M2 + CpG i.n. and G1F/M2 i.p. groups showed inhibited expression of Gro-α, compared with G1F/M2 i.n. group (), p < 0.05), and no difference was observed between the two groups (), p > 0.05). The relative expression of Eotaxin in G1F/M2 + CpG i.p. group was lower than those in other groups (), p < 0.05). There was no difference among G1F/M2 i.n., G1F/M2 + CpG i.n., and G1F/M2 i.p. groups (), p > 0.05). In addition, the protein level of anti-inflammatory cytokine IL-10 by ELISA in G1F/M2 + CpG i.n. group was significantly lower than those in PBS/RSV and G1F/M2 + CpG i.p. group (), p < 0.05), and also lower than those in G1F/M2 i.n. and i.p. groups (p > 0.05%). These results indicated that G1F/M2 + CpG immunization i.p. remarkably inhibited lung pro-inflammatory cytokine and promoted anti-inflammatory cytokine after RSV challenge.
Figure 4. The inflammatory cytokines and chemokines in lungs of immunized mice after RSV challenge. Three weeks after the final immunization, mice were challenged i.n. with 105 TCID50/100 µl RSV-A. Five days after post-challenge, lungs were removed. The cytokines in lungs were measured by qRT-PCR or ELISA. Naïve mice neither immunized nor challenged were used as controls. The results are represented as the expression fold of each gene in experimental mice relative to control mice. The relative expression or concentration of (a) TNF-α, (b) IL-1β, (c) GRO-α, (d) EOTAXIN, and (e) IL-10. Results are means ± SD of five or six mice per group. *P < 0.05 represents significant difference.
2.6. G1F/M2 + CpG immunization i.p. inhibited pulmonary inflammatory pathology following RSV challenge
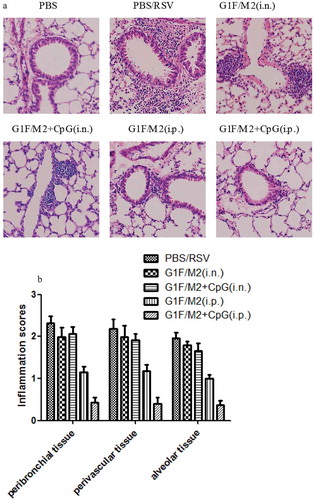
To further characterize the pulmonary inflammatory pathology, lungs of pre-immunized mice after challenge were stained with hematoxylin and eosin (H&E; )). Mice in PBS group following RSV challenge showed the abundant inflammatory cell infiltration in the peribronchial and perivascular spaces, and multifocal alveolitis. Mice pre-immunized with G1F/M2 + CpG i.n. showed also severe pulmonary inflammatory cell infiltration, which was similar to those with G1F/M2 i.n. Mice in G1F/M2 i.p. group showed suppressed inflammatory cell infiltration. It is worth noting that mice in G1F/M2 + CpG i.p. group show little inflammatory pathology in lungs. As shown in ), mice pre-immunized with G1F/M2 + CpG i.p. had significantly lower level of inflammation score than those with PBS, G1F/M2 i.n., G1F/M2 + CpG i.n., or G1F/M2 i.p. (p < 0.05). These results suggested that G1F/M2 + CpG immunization i.p. remarkably inhibited pulmonary inflammatory pathology following RSV challenge
Figure 5. Pulmonary pathology following RSV challenge. Tissue sections were obtained from each mouse after 5 d of post-challenge. Lung histology was stained by H&E. (a) A image for a representative animal of each group. (b) Scores for pulmonary inflammation as described in Section 4. Results are means ± SD of five or six mice per group.
2.7. G1F/M2 + CpG immunization i.p. promoted TH1-type response after RSV challenge
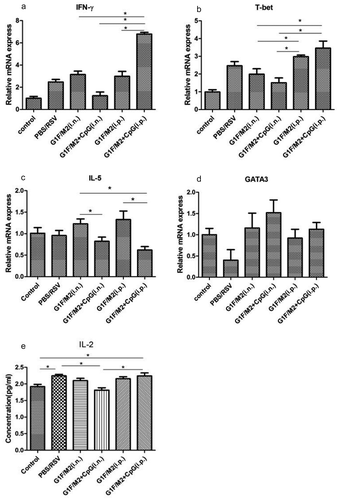
It is generally believed that vaccine-enhanced disease is characterized by Th2-biased memory response activated by RSV challenge. T-bet and GATA3 are the Th1 and Th2 cell lineage-specific transcription factors, respectively.Citation28 We tested the relative expression of T-bet, GATA3, Th1/Th2-type cytokine IFN-γ, and IL-5 by PCR and concentration of IL-2 by ELISA in the lungs following RSV challenge. As shown in ), the relative expression of IFN-γ in G1F/M2 + CpG i.p. group was significantly higher than those in other groups (p < 0.05). The relative expression of T-bet in mice immunized with G1F/M2 + CpG or G1F/M2 i.p. was higher than that in mice immunized with G1F/M2 + CpG or G1F/M2 i.n. (), p < 0.05). G1F/M2 + CpG i.p. and PBS/RSV groups showed significantly higher level of IL-2, compared with G1F/M2 + CpG i.n. or control group (), p < 0.05). In contrast, the relative expression of IL-5 in G1F/M2 + CpG immunized i.n. or i.p. mice after RSV challenge showed lower, compared with that of G1F/M2 immunized i.n. or i.p. mice (), p < 0.05). No difference on expression of GATA3 was observed between each vaccine group and PBS group (), p > 0.05). These data suggested that the G1F/M2 + CpG i.p. immunization could promote the pulmonary Th1-type response after RSV challenge, compared with G1F/M2 i.p., G1F/M2 + CpG i.n., or G1F/M2 i.n.
Figure 6. Lung Th1/Th2-type responses after RSV challenge. Mice were immunized as described in Section 4 . Three weeks after the final immunization, mice were challenged i.n. with 105 TCID50/100 µl RSV-A. Five days after post-challenge, lungs were removed. The transcription factors and cytokines in lungs were measured by qRT-PCR or ELISA. Naïve mice neither immunized nor challenged were used as controls. The results are represented as the relative expression of (a) IFN-γ, (b) T-bet, (c) IL-5, (d) GATA3, and concentration of (e) IL-12. Results are mean ± SD of five or six mice. *P < 0.05 represents significant difference.
2.8. G1F/M2 + CpG immunization i.p. suppressed RSV-N expression in lungs
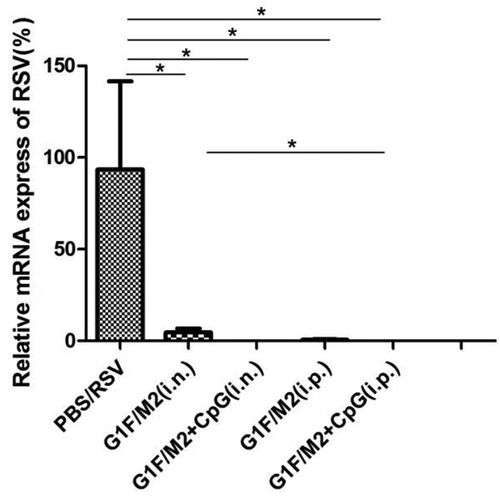
Mice were challenged i.n. with RSV 3 weeks after final immunization. Five days after challenge, the expression of RSV-N in the lungs was examined by real-time quantitative PCR (qRT-PCR). The N-gene expression of PBS-treated mice after RSV challenge served as the control (100%). As shown in , PBS-treated mice after RSV challenge showed obvious RSV-N gene expression in the lungs. In contrast, no or little RSV-N was observed in mice immunized with G1F/M2 + CpG i.n., G1F/M2 i.p., or G1F/M2 + CpG i.p. G1F/M2 immunization i.n. also significantly inhibited the expression of RSV-N, compared with PBS treatment after RSV challenge (, p < 0.05). These results indicated that immunization with G1F/M2 + CpG i.p., G1F/M2 + CpG i.n., or G1F/M2 i.p. inhibited RSV gene expression in lungs after RSV infection.
Figure 7. The expression of RSV-N in lungs after RSV challenge. Mice were challenged i.n. with RSV-A 3 weeks after the final immunization. Lungs were removed after 5 d post-challenge. The relative expression of RSV-N gene in lung was tested by real-time qRT-PCR. Results are presented as mean ± SD of five mice per group. *P < 0.05 represents significant difference.
3. Discussion
Vaccine-associated lung inflammatory disease is an important obstacle for RSV vaccine development. Immune memory is the conceptual basis for how vaccines work. Unbalanced cellular immune memory, including excessive or lack of CD8+ T, Th1 or Th2 memory cells in mice previously immunized with FI-RSV or subunit vaccine candidates contribute to vaccine-enhanced disease.Citation4,Citation29,Citation30 A recent study demonstrated that CpG pre-exposure was protective against enhanced disease during secondary adult RSV challenge, with a reduction in viral load and an increase in Th1 responses.Citation31 In this study, we found that immunization of mice with G1F/M2 + CpG i.p. induced significantly more CD4+ or CD8+ TCM cells, which would be activated and secrete IFN-γ to promote Th1 differentiation, and IFN-γ-secreting TEM cells, compared to that with G1F/M2 i.n., G1F/M2 + CpG i.n., or G1F/M2 i.p. Since the type-cellular immune memory was induced by immunization with G1F/M2 + CpG i.p., RSV challenge should active type-cellular memory response. The results after RSV challenge demonstrated the hypothesis. Increased Th1-type response after RSV challenge was detected in mice pre-immunized with G1F/M2 + CpG i.p., which would contribute to relieve pulmonary inflammatory responses, compared with mice pre-immunized with G1F/M2 i.n., G1F/M2 + CpG i.n., or G1F/M2 i.p. ()). Expectedly, we found suppressed inflammatory cytokines and histopathology in lungs of mice pre-immunized with G1F/M2 + CpG i.p. after RSV challenge, while stronger inflammatory responses and histopathology in lungs of mice pre-immunized with G1F/M2 i.n., G1F/M2 + CpG i.n., or G1F/M2 i.p. ( and ). Consistent with our data, Johnson et al. (2009) demonstrated that the administration of CpG during FI-RSV immunization increased production of Th1-type cytokines, and decreased inflammatory responses following RSV challenge.Citation32 Castilow et al. reported that in the absence of IFN-γ, vacv-F-immunized mice developed pulmonary eosinophilia after RSV challenge.Citation33 Durrant et al. also found that absence of T-bet led to increased pulmonary histological inflammation and numbers of eosinophils and neutrophils.Citation34 All the above results indicated that lack of or impaired Th1 memory response could be an important factor for vaccine-enhanced inflammatory disease after RSV infection.
Over the past decade, it has become clear that there is another important subset of memory T cells: TRM cells. TRM cells are critical to immune-mediated protection,Citation7,Citation8,Citation27 due to their rapid response to pathogen challenge at the lung and other mucosal sites independently of recruitment of T cells from the blood.Citation7,Citation8 In contrast to protective TRM, increased studies demonstrated pathological TRM cells in skin and mucosal disease, which influence tissue inflammation. Established psoriasis has been shown to be mediated largely by TRM cells.Citation9 The gastrointestinal tract is another site where certain diseases exhibit the behavior of TRM cell-mediated inflammatory diseases.Citation10 Recently, CD4+ TRMs were demonstrated persisting long term following cessation of house dust mite administration. Lung CD4+ TRMs are localized around airways and are rapidly reactivated upon allergen re-exposure accompanied by the rapid induction of airway hyperresponsiveness and increased recruitment and activation of dendritic cells in the lungs independent of circulating T cells.Citation35 Lung TRMs can be generated to intranasally administered RSV vaccines.Citation11 In this study, immunization with G1F/M2 or G1F/M2 + CpG i.n. induced significantly more TRM cells, compared with immunization with G1F/M2 or G1F/M2 + CpG i.p. (). Consistently, mice immunized with G1F/M2 or G1F/M2 + CpG i.n. exhibited more severe lung inflammatory pathology, compared with mice immunized with G1F/M2 or G1F/M2 + CpG i.p. (). The results suggested that TRM cells could contribute to lung inflammatory pathology following RSV.
Appropriate humoral immune memory is also important for protection and vaccine-associated lung inflammatory disease, since the candidate vaccine induced both cellular and humoral immune memory. In mice immunized with G1F/M2 + CpG i.p., the induction of IgG1 was significantly inhibited, and IgG1/IgG2a ratio was the lowest. This is consistent with the general view that Th1-type cytokine IFN-γ drives B cell to switch to IgG2a isotype,Citation36 and Th2-type cytokine IL-4 promotes IgG1 switching,Citation37 since immunization with G1F/M2 + CpG i.p. induced significant Th1-type memory responses ( and ). However, G1F/M2 + CpG (i.n.) groups also showed low IgG1/IgG2a ratio, although they suppressed Th1 immune memory and response. Some studies provide evidence to explain such a result. It has been shown that IFN-γ-independent IgG2a class switching can be induced directly by stimulation of B cells with CpG rather than indirectly by inducing Th1 responses.Citation38 Jegerlehner et al. demonstrated that the critical factor for the induction of IgG2a antibody was not Th1 cytokines, but rather TLR expression in B cells.Citation39
Our study has several limitations. The injection routes only included i.n and i.p. and the effect of the candidate vaccine with other injection routes, for example intramuscular injection route and subcutaneous injection route, was therefore not demonstrated. The study did not detect CD4+ or CD8+ TRM in lungs, which could play different effect on protection or lung inflammation.Citation40 In addition, only real-time PCR of RSV-N gene was used to determine viral loads, and plaque titers or TCID50 (tissue culture infective dose 50) titers were not used.
In sum, there were no body weight losses in mice immunized with G1F/M2 or G1F/M2 + CpG by i.p. or i.n. route (data not shown). Administration of G1F/M2 + CpG by i.p. route, but not i.n. route, prevented vaccine-enhanced inflammatory disease in lungs. High level of TCM and TEM in spleens may contribute to inhibition of lung inflammation, while high level of TRM in lungs may promote lung inflammation following RSV challenge. In addition, lack of or weak Th1-type immune response, which was showed in mice immunized with G1F/M2 and G1F/M2 + CpG i.n., or G1F/M2 i.p., but not Th2-biased response, could be a critical factor for vaccine-enhanced inflammatory disease. Thus, a safe and efficient RSV vaccine would require innovative solutions through optimized combination of antigen, adjuvant, and delivery route.
4. Materials and methods
4.1. Preparation of G1F/M2 protein, virus, and mice
Gene assembly, vector constructions, protein expression in E. coli, purification by affinity chromatography, renaturation by dialysis, and analyzation of G1F/M2 were undertaken as previously described.Citation13 RSV-A (Long strain, from ATCC) was propagated in HEp-2 cells and purified by a discontinuous sucrose gradient as previously described.Citation41 The cell pellet was used to prepare viral antigen for ELISA coating. RSV was stored at −70°C until use.
4.2. Virus titer assay
Virus is titered on Hep-2 cells using twofold serial dilution steps. After incubation at 37°C, 5% CO2 for 3 up to a maximum of 6 d, the Cytopathic Effect (CPE) was assessed for each dilution to calculate the virus titer. The TCID50 was calculated using the Spearman–Karber formula.
4.3. Immunizations and RSV challenge procedures
Female BALB/c mice, aged 6–8 weeks, were purchased from Hebei Laboratory Animal Center (Hebei, China), housed and manipulated according to the Care and Use of Laboratory Animals under specific pathogen-free conditions. Mice were immunized i.n. using 20 μg G1F/M2 with or without 20 μg CpG in 100 μl PBS, or i.p. using 20 μg G1F/M2 with or without 20 μg CpG ODN in 200 μl PBS. An interval of 2 weeks was invariably employed for the second and third immunizations. Except where indicated, half of mice was bled 3 weeks after the last immunization for antibodies and cellular immune memory studies. The other half of mice was challenged by i.n. instillation of 105 TCID50/100 µl RSV 3 weeks after the last immunization, and sacrificed 5 d later for cytokines, histopathology, and protection studies.
4.4. Standard ELISPOT assay
Spleens were removed 3 weeks after the last immunization. Splenocytes were prepared by passing the organs through a 74-μm stainless steel sieves. Cytokines IFN-γ- and IL-4-secreting splenocytes were quantified using an ELISPOT kit (eBioscience TM) according to the manufacturer’s instructions. Briefly, 96-well filtration plates were coated with anti-IFN-γ or IL-4 antibody. Splenocytes (1 × 106/well) were co-cultured with 20 μg/ml G1F/M2 for 48 h at 37°C in triplicate wells. After removing cells, plates were incubated with biotinylated anti-IFN-γ or anti-IL-4 antibodies for 2 h, streptavidin-horseradish peroxidase for 45 min, then freshly substrate solution. Spots were then counted using an immunospot image analyzer. Results are shown as the mean value obtained for triplicate wells.
4.5. In vivo antibody labeling and flow cytometric assay for TRM cells
For in vivo antibody labeling, mice were injected intravenously with 2.5 μg of the fluorochrome-conjugated antibody anti-CD3-FITC. After 10 min, mice were exsanguinated, and peripheral leukocytes were obtained by lysing erythrocytes; lungs were rinsed free of blood, and cells were isolated. Peripheral leukocytes were stained in vitro with anti-CD69-PE, and lung cells were stained in vitro with anti-CD69-PE and anti-CD3-PerCP-Cy5. Stained cells were analyzed using flow cytometry (BD), and fluorescence-activated cell sorting (FACS) data were analyzed using CellQuest software.
4.6. Flow cytometric assay
Percentages of CD4+ T and CD8+ T cells in the spleen cells of mice were analyzed with flow cytometry. Ten microliters of anti-CD4-FITC or anti-CD8-FITC, anti-CD44-PE, and anti-CD62L-Pecy5 were incubated with 1.0 × 106 spleen cells in a 100 μl staining volume for 20 min at room temperature. The cells were run on flow cytometry (Beckman Coulter, USA), and FACS data were analyzed using Cellquest software.
4.7. ELISA assays
IgG, IgG1, and IgG2a antibody titers in sera from vaccinated mice were assessed by standard ELISA protocol. Antibody titers were expressed as the reciprocal of the last sample dilution giving an absorbance of at least twofold that of the pre-immune sample and with an OD ≥0.15.
4.8. Real-time PCR
RNA was isolated from the lungs using TRIzol (Invitrogen, Carlsbad, CA). M-MLV and Oligo(dT) (MBI Fermentas) were used for synthesis of the first strand of cDNA. The expression of the RSV-N gene, cytokines, and chemokines in lung tissue was assessed by qRT-PCR with SYBR green to detect amplified products. Gene expression by qRT-PCR was normalized to β-actin before the fold change was calculated. The fold change of RSV-N gene in each group was calculated via the comparison to that in control mice following RSV challenge. The fold change of cytokines and chemokines in each group was calculated via the comparison to that of naïve mice without any treatment.
4.9. Histology
The lungs were isolated and immediately fixed in 4% paraformaldehyde. Lung samples were subsequently processed, embedded in paraffin, thin sectioned, and placed on L-lysine-coated slides. Sections were stained with H&E. Five sections per mouse were obtained. Sections from each mouse were scored blindly for the degree of inflammation in the peribronchial and perivascular spaces on a scale of 0–3, and in the alveolar tissue on scale of 0–4, as previously described.Citation42 Mean scores were calculated for each mouse.
4.10. Statistical analysis
Experiments using more than two groups with normal Gaussian distribution of the data were analyzed using an analysis of variance. Data that did not have normal Gaussian distributions were analyzed using Kruskal–Wallis test. Experiments using two groups were analyzed using an unpaired t-test or a Mann–Whitney U test in which Gaussian distribution was not observed. P-value of <0.05 was defined as a statistically significant difference.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr Infect Dis J. 2004;23:S11–8. doi:10.1097/01.inf.0000108188.37237.48.
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34.
- Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82:2881–88. doi:10.1099/0022-1317-82-12-2881.
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–60.
- Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–25.
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi:10.1038/44385.
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–57. doi:10.1016/j.immuni.2014.03.007.
- Schluns KS, Klonowski KD. Protecting the borders: tissue-resident memory T cells on the front line. Front Immunol. 2015;6:90. doi:10.3389/fimmu.2015.00090.
- Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol. 2011;131:391–400. doi:10.1038/jid.2010.280.
- Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–34. doi:10.1084/jem.20081712.
- Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10:545–54. doi:10.1038/mi.2016.48.
- Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–32.
- Zeng RH, Gong W, Fan CF, Wang YF, Mei XG. Induction of balanced immunity in BALB/c mice by vaccination with a recombinant fusion protein containing a respiratory syncytial virus G protein fragment and a CTL epitope. Vaccine. 2006;24:941–47. doi:10.1016/j.vaccine.2005.08.064.
- Zeng R, Gong W, Mei X, Diao Z, Hao L. Preparation of Recombinant Protein D sbA2G1F/M 2 of Respiratory Syncytial Virus and its Immunogenicity. Chin Pharm J. 2007;142:90–93.
- Murata Y, Lightfoote PM, Falsey AR, Walsh EE. Identification of and human serum reactogenicity to neutralizing epitopes within the central unglycosylated region of the respiratory syncytial virus attachment protein. Clin Vaccine Immunol. 2010;17:695–97. doi:10.1128/CVI.00432-09.
- Hsu SC, Chargelegue D, Steward MW. Reduction of respiratory syncytial virus titer in the lungs of mice after intranasal immunization with a chimeric peptide consisting of a single CTL epitope linked to a fusion peptide. Virology. 1998;240:376–81. doi:10.1006/viro.1997.8923.
- Zeng R, Qi X, Gong W, Mei X, Wei L, Ma C, Yin X. Long-lasting balanced immunity and protective efficacy against respiratory syncytial virus in mice induced by a recombinant protein G1F/M2. Vaccine. 2007;25:7422–28. doi:10.1016/j.vaccine.2007.08.007.
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi:10.1038/nrd2059.
- Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–83.
- Hancock GE, Heers KM, Smith JD, Scheuer CA, Ibraghimov AR, Pryharski KS. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV). Vaccine. 2001;19:4874–82.
- Hancock GE, Heers KM, Pryharski KS, Smith JD, Tiberio L. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine. 2003;21:4348–58.
- Oumouna M, Mapletoft JW, Karvonen BC, Babiuk LA, van Drunen Littel-van Den Hurk S. Formulation with CpG oligodeoxynucleotides prevents induction of pulmonary immunopathology following priming with formalin-inactivated or commercial killed bovine respiratory syncytial virus vaccine. J Virol. 2005;79:2024–32. doi:10.1128/JVI.79.4.2024-2032.2005.
- Mapletoft JW, Oumouna M, Townsend HG, Gomis S, Babiuk LA, van Drunen Littel-van Den Hurk S. Formulation with CpG oligodeoxynucleotides increases cellular immunity and protection induced by vaccination of calves with formalin-inactivated bovine respiratory syncytial virus. Virology. 2006;353:316–23. doi:10.1016/j.virol.2006.06.001.
- Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–78. doi:10.1172/JCI13296.
- Laouar A, Manocha M, Haridas V, Manjunath N. Concurrent generation of effector and central memory CD8 T cells during vaccinia virus infection. PLoS One. 2008;3:e4089. doi:10.1371/journal.pone.0004089.
- Calarota SA, Dai A, Trocio JN, Weiner DB, Lori F, Lisziewicz J. IL-15 as memory T-cell adjuvant for topical HIV-1 DermaVir vaccine. Vaccine. 2008;26:5188–95. doi:10.1016/j.vaccine.2008.03.067.
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–14. doi:10.4049/jimmunol.1102243.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69.
- Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156–60.
- Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, Varga SM, Walker CM. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 2018;14:e1006810. doi:10.1371/journal.ppat.1006810.
- Yamaguchi Y, Harker JA, Wang B, Openshaw PJ, Tregoning JS, Culley FJ. Preexposure to CpG protects against the delayed effects of neonatal respiratory syncytial virus infection. J Virol. 2012;86:10456–61. doi:10.1128/JVI.01082-12.
- Johnson TR, Rao S, Seder RA, Chen M, Graham BS. TLR9 agonist, but not TLR7/8, functions as an adjuvant to diminish FI-RSV vaccine-enhanced disease, while either agonist used as therapy during primary RSV infection increases disease severity. Vaccine. 2009;27:3045–52. doi:10.1016/j.vaccine.2009.03.026.
- Castilow EM, Olson MR, Meyerholz DK, Varga SM. Differential role of gamma interferon in inhibiting pulmonary eosinophilia and exacerbating systemic disease in fusion protein-immunized mice undergoing challenge infection with respiratory syncytial virus. J Virol. 2008;82:2196–207. doi:10.1128/JVI.01949-07.
- Durrant DM, Gaffen SL, Riesenfeld EP, Irvin CG, Metzger DW. Development of allergen-induced airway inflammation in the absence of T-bet regulation is dependent on IL-17. J Immunol. 2009;183:5293–300. doi:10.4049/jimmunol.0803109.
- Turner DL, Goldklang M, Cvetkovski F, Paik D, Trischler J, Barahona J, Cao M, Dave R, Tanna N, D’Armiento JM, et al. Biased generation and in Situ Activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic Asthma. J Immunol. 2018;200:1561–69. doi:10.4049/jimmunol.1700257.
- Snapper CM, Paul WE. B cell stimulatory factor-1 (interleukin 4) prepares resting murine B cells to secrete IgG1 upon subsequent stimulation with bacterial lipopolysaccharide. J Immunol. 1987;139:10–17.
- Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–30. doi:10.1038/380627a0.
- Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–87. doi:10.1002/eji.200324736.
- Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–20.
- Zhang L, Li H, Hai Y, Yin W, Li W, Zheng B, Du X, Li N, Zhang Z, Deng Y, et al. CpG in combination with an inhibitor of notch signaling suppresses formalin-inactivated respiratory syncytial virus-enhanced airway hyperresponsiveness and inflammation by inhibiting Th17 memory responses and promoting tissue-resident memory cells in lungs. J Virol. 2017;91:e02111–16. dio:10.1128/JVI.02111-16.
- Zeng R, Zhang Z, Mei X, Gong W, Wei L. Protective effect of a RSV subunit vaccine candidate G1F/M2 was enhanced by a HSP70-Like protein in mice. Biochem Biophys Res Commun. 2008;377:495–99. doi:10.1016/j.bbrc.2008.10.002.
- Mok H, Lee S, Utley TJ, Shepherd BE, Polosukhin VV, Collier ML, Davis NL, Johnston RE, Crowe JE. Venezuelan equine encephalitis virus replicon particles encoding respiratory syncytial virus surface glycoproteins induce protective mucosal responses in mice and cotton rats. J Virol. 2007;81:13710–22. doi:10.1128/JVI.01351-07.
