ABSTRACT
We assessed how an awareness of influenza vaccination might influence both the willingness of pregnant women to be vaccinated and the readiness of obstetricians to recommend antenatal influenza vaccination in Beijing, China. From March to April 2016, we surveyed pregnant women who were attending antenatal clinics at eight hospitals in Beijing, along with obstetricians at the same clinics. Demographic, attitudinal, and behavioral information regarding influenza vaccination were collected using structured questionnaires. Consent and completed questionnaires were obtained from 988 of 1009 pregnant women and 165 of 173 obstetricians. Only 113 (11.4%) pregnant women reported being willing to receive an influenza vaccine during their pregnancies. Willingness to receive an influenza vaccination was positively associated with ever having a history of vaccination or influenza (aOR=6.74, 95%CI: 1.72-26.4, P=0.006), perceiving benefits of vaccination (aOR=1.67, 95%CI: 1.00-2.79, P=0.050), and having a higher level of influenza knowledge (aOR=82.2, 95%CI: 21.7-311.1, P<0.001). Among obstetricians, only 19.4% reported being willing to recommend influenza vaccination to their pregnant patients and 15.2% reported knowledge that influenza vaccination during pregnancy was recommended by China’s National Health Commission. Neither pregnant women nor their obstetricians were aware of Chinese government recommendations that antenatal influenza vaccination should be encouraged and provided. Pregnant women and their obstetricians were ill-informed of the relevant evidence. It is in emergent need to train and disseminate the updated evidence on influenza vaccination to obstetricians. It also warranted more high-quality trials regarding influenza vaccination during pregnancy to address public concern.
Introduction
Pregnant women infected with influenza virus experience excess morbidity and mortality when compared with other groups at high risk.Citation1 Influenza vaccination is an effective way to protect pregnant women from influenza, and it is safe at all stages of pregnancy.Citation2-Citation4 Therefore, since 2005, the World Health Organization (WHO) has recommended influenza vaccination for pregnant women in the influenza season.Citation5,Citation6
Influenza vaccination rates are extremely low in China.Citation7,Citation8 In fact, influenza vaccination was prohibited during pregnancy before 2005 in ChinaCitation9 but it is now recommended for all pregnant women.Citation10 It should be noted that pregnancy is still described as a contraindication in some package inserts of influenza vaccine productions on sale in China (national medicine permission number: S20030072) and the latest edition of Chinese Pharmacopoeia.Citation11 A recent Cochrane Review also questioned the effectiveness of influenza vaccination during pregnancy.Citation12 Unless informed well, pregnant women tend to make decision blindly facing such complex information. Aside from policy barriers over a decade earlier, it is plausible that there are additional reasons for low vaccination rates during pregnancy. The knowledge-attitude-belief-practice model suggests that knowledge and attitudes can affect willingness of obstetricians to offer, and pregnant women to accept influenza vaccine.Citation13 According to the health belief model, factors associated with vaccination willingness during pregnancy include barriers, cues to action, perceived benefit, perceived severity, and perceived susceptibility.Citation14-Citation16 Other factors commonly associated with vaccination willingness include older age, higher educational attainment, and a history of prior influenza vaccination.Citation17 Not surprisingly, obstetricians’ recommendations are positive determinants for pregnant women to accept influenza vaccination.Citation18,Citation19 Few studies have explored the attitudes of pregnant women regarding influenza vaccination in the Chinese mainland.Citation7,Citation8 Prior studies in China have not done contemporaneous surveys of both pregnant women and obstetricians, including vaccine knowledge, willingness, and attitudes.
China’s capital, Beijing, is among the nation’s best-capacitated cities with some of the nation’s highest quality of medical services. Attitudes of pregnant women and obstetricians in Beijing may reflect a “best case” scenario for influenza vaccination elsewhere in China. We conducted a survey in antenatal clinics of both pregnant women and obstetricians in Beijing to explore factors that might influence the willingness of pregnant women to accept influenza vaccine and the willingness of obstetricians to recommend these vaccines.
Results
Pregnant women
Of 1,009 pregnant women interviewed (72.3% of the 1396 women approached), 988 (97.9%) completed the questionnaires, excluding 21 participants due to missing key information. Women’s mean age was 30.5 (±4.1 SD) years, 76.4% lived in urban areas, and 75.3% reported an educational attainment of college or above. Other participant characteristics are shown in .
Table 1. Characteristics of pregnant women (N = 988).
Only 113 (11.4%) pregnant women reported having a willingness to receive an influenza vaccine during their pregnancy. The characteristics of two groups holding vaccine-favorable or unfavorable attitudes were similar in the univariate analysis except for occupation (health-care workers were more favorable, P = 0.027) and educational attainment (more educated were less favorable, P = 0.032). If the obstetrician recommended influenza vaccine, 44.3% stated that they would agree to take it.
Questions used to assess pregnant women’s attitudes and associated factors are in supplementary Table S1. Multivariable logistic regression analysis revealed that the willingness of receiving an influenza vaccination among pregnant women was positively associated with ever having a history of any vaccination or influenza (aOR = 6.74, 95%CI: 1.72–26.4, P = 0.006), perceiving great benefits of vaccination (aOR = 1.67, 95%CI: 1.00–2.79, P = 0.050), and having higher level of knowledge about influenza (aOR = 82.2, 95%CI: 21.7–311.1, P < 0.001) (). Besides, higher educational attainment (aOR = 0.72, 95%CI: 0.55–0.94, P = 0.015) was negatively related to pregnant women’s willingness to receive an influenza vaccine. Noting the strong effect of knowledge about influenza, sensitivity analysis was conducted by excluding the factor. Details of the results were described in Supplementary Table S2.
Table 2. Univariate and multivariable logistic analyses on willingness to receiving an influenza vaccine during pregnancy (Pregnant women N = 988).
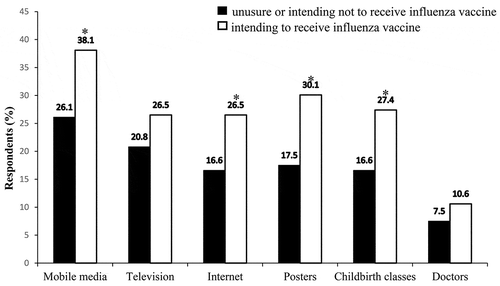
Participants’ knowledge about influenza vaccine was obtained from multiple sources (). Participants who were willing to be vaccinated were more likely to be exposed to relevant information from the mobile media, the internet, public service announcements in the hospital, and childbirth classes, compared with those who were unsure or had no desire to be vaccinated.
Obstetricians
The overall survey response rate for obstetricians was 95.4% (165/173), excluding seven obstetricians who declined to participate and one with extensive missing information. Of the total, 98.8% of obstetricians were women, 71.5% worked in the secondary hospitals, 60.6% had what in China is termed a bachelor’s degree of medicine, and 59.4% reported having >10 years of experience working as obstetricians ().
Table 3. Characteristics of obstetricians (N = 165).
Fewer than one in five (19.4%) obstetricians were willing to recommend the influenza vaccine to pregnant women during influenza season. No significant differences were found in characteristics between obstetricians who would or would not recommend vaccinations (). Unwilling obstetricians cited safety concerns about the fetuses and/or the pregnant women and concerns about their responsibility/liability for adverse events following immunization during pregnancy (supplementary Table S3).
Only 15.2% of obstetricians knew that China’s National Health Commission pregnant women recommended influenza vaccination for pregnant women and one in five knew that the WHO had advised pregnant women to be vaccinated. According to the multivariable logistic regression analysis (), obstetricians with higher professional titles were more inclined to recommend influenza vaccination (P for trend = 0.018). However, older obstetricians were less likely to recommend influenza vaccination (P for trend = 0.047).
Table 4. Univariate and multivariable logistic analyses on obstetrician’s willingness to recommend influenza vaccine (N = 165).
Discussion
About one tenth of pregnant women in Beijing was likely to want influenza vaccine (11.4%) and one fifth of their obstetricians would like to recommend it (19.4%). Among clients and physicians, there was a low awareness of the antenatal benefits of influenza vaccination that likely translate into the low influenza vaccination rates during pregnancy in China.
Beijing, where the study was conducted, has the highest per capita gross domestic product in the Chinese mainland. It is also the capital of China with a life expectancy of 81.95 years in 2015, even higher than the average of high-income countries (80.8 years).Citation20 However, vaccine-in-pregnancy awareness for influenza vaccine has been far below the high-income countries. The present findings in relatively prosperous Beijing were consistent with the few previous studies conducted in other mainland Chinese cities that showed a low level of pregnant women’s acceptance and low obstetricians’ willingness to recommend influenza vaccination. In an earlier five-city study,Citation8 108 pregnant women were surveyed and none reported having received influenza vaccine during pregnancy. In another earlier study in three eastern Chinese cities, only 8% health-care workers reported always recommending influenza vaccination to pregnant patients during the influenza season.Citation21 The level of pregnant women’s willingness to receive influenza vaccine in this study (11.4%) was lower compared to that of a survey in prosperous Zhejiang province.Citation7 In the survey conducted Zhejiang province, fully 76.3% of pregnant women were willing to receive seasonal influenza vaccination during their pregnancy. This high patient willingness may have been influenced by a severe influenza outbreak just before the January 2014 survey; the number of reported influenza cases in Zhejiang province was the highest in China in 2013.Citation22 In contrast, the present survey was conducted in 2016 flu season when the prevalence was lower compared with previous seasons.Citation23 Pregnant women in Zhejiang province in 2014 perceived more risk with a more severe flu season, and they had more knowledge than did Beijing women in 2016. This contrast suggests that local lessons-learned are not being translated nationwide.
Most components of the theory framework in our questionnaire were significantly associated with a pregnant women’s willingness to vaccinate except for perceived severity of influenza and perceived susceptibility. Others report that the effect size of perceiving influenza severity and susceptibility on motivation for vaccination to be relatively small;Citation24,Citation25 perhaps the sample size of this survey was not large enough to detect a small association. As with previous studies,Citation13,Citation26,Citation27 the acceptance of influenza vaccination was positively associated with ever having a history of any vaccination or influenza, perceiving great benefits of vaccination, and a high level of knowledge about influenza. However, higher educational attainment was negatively associated with pregnant women’s willingness to receive the influenza vaccine in contrast with earlier studies.Citation13,Citation28 In March 2016, a vaccine scandal (vaccine transportation without cold chain) happened in Shandong province (just to the south of Beijing), just prior to our survey, causing a public panic and chaos about the safety and effectivity of vaccination in China.Citation29 Pregnant women with higher educational attainment may be more exposed to social media, resulting in mistrust in the safety of vaccination. This is reminiscent of a survey in 2003 when a controversy regarding the association of autism and the combined measles, mumps, and rubella (MMR) vaccine appeared; parents at that time with higher educational attainment were less likely to get their children vaccinated.Citation30
In the present study, knowledge was assessed by questions about influenza and vaccination separately. More than two-thirds of pregnant women gave the correct response to the four questions about influenza (92.8%, 81.7%, 66.6%, 69.9%), while far fewer knew the correct responses on the two influenza vaccination questions (13.9%, 27.5%). It is evident that while pregnant women had a reasonably high level of knowledge about influenza, their awareness of influenza vaccination in pregnancy is low. We found that only a fraction of obstetricians (19.4%) was willing to recommend influenza vaccine in pregnancy. Even obstetricians seem to be unaware of the overall benefits of influenza vaccination in pregnant women. Higher perceived susceptibility to and seriousness of influenza, and lower vaccine safety concerns are positively associated with the likelihood of obstetricians recommending vaccination for their pregnant patients.Citation31 Recommendations from obstetricians have been key promoters for pregnant women to get vaccinated.Citation19,Citation32,Citation33 Similar to previous studies,Citation21 while more pregnant women (44.3%) would be willing to get vaccinated with a recommendation from obstetricians, presumably based on their trust in their doctor’s advice, it still was not a majority.Citation31
Although official recommendations are generally clear,Citation6,Citation10 an updated Cochrane Review raised doubts over the effectiveness of influenza vaccination during pregnancy.Citation12 The available evidence may be insufficient to a prompt scale-up to universal vaccination among pregnant women, especially considering the lack of high-quality trials.Citation34 Various views are undoubtedly valuable for pregnant women to weigh benefits and risks of vaccination. The ideal scene is that pregnant women are well-informed and their obstetricians could communicate the uncertainties with caution. Unfortunately, according to this survey, it was common that both pregnant women and their obstetricians were exposed to sparse information on which to base decisions.
A strength of our study is its large sample sizes compared to previous work and our high participation rates, improving the generalizability of our findings. Some limitations are also apparent: 1) The high educational attainment and comparatively high income suggest that the findings are not generalizable to all of China. However, it is hard to imagine that things are better elsewhere, with the exception of Zhejiang province as described above. 2) Given previous studies,Citation35 there is still a long way from “intention to action”, i.e., to successful vaccination; intention is not a reliable predictor of vaccine uptake. Too few women who actually got vaccinated to use this as an outcome.Citation8 3) As data were collected retrospectively, some recall bias may be extant. However, given how seriously Chinese women in the one-child policy era took their pregnancies, recall bias during pregnancy might be limited.
In conclusion, the findings underscore the critical need for training and dissemination of the updated evidence on influenza vaccination to doctors and patients alike. While further studies are needed to investigate the awareness of influenza vaccination in various regions of China, we believe the scenario in Beijing is an epitome. Information is available to balance potential benefits and risks for pregnant women,Citation2,Citation12,Citation36-Citation38 but such information is not simple enough and not reaching pregnant women and their obstetricians. Physicians working at the forefront need to keep their knowledge up to date and inform pregnant women objectively about potential risks and benefits. We believe that it is time to integrate a health education campaign into nationwide influenza preparedness for information dissemination on risks and benefits of influenza vaccination for obstetricians, midwife, and the public. Meanwhile, the average national coverage of influenza vaccination was just 1.5–2.2% in China.Citation39 Given the fact that the uptake of influenza vaccine in other high-risk groups is also low,Citation40,Citation41 it is meaningful to extend this survey to those populations.
Methods
In 2016, there were 122 hospitals offering obstetrics in Beijing, distributed into 16 districts. Based on their levels of health services delivery, medical education, and scientific research, those hospitals are graded into primary, secondary or tertiary hospitals, among which tertiary hospitals are at the top in the three-tier system. From March–April 2016, a random stratified sampling scheme was used to recruit the participants for the study. First, four districts were randomly selected and from each district, one secondary hospital and one tertiary hospital were randomly selected within the sampling frame (Supplementary Figure S1). The eight enrolled hospitals were Beijing Luhe Hospital, Tongzhou Maternal and Child Health Hospital, Beijing Tongren Hospital, Beijing Daxing Maternal, and Child Health Hospital, Beijing Obstetrics and Gynecology Hospital, Beijing Chaoyang Maternal and Child Health Hospital, Beijing Electric Power Hospital, and the Fengtai Maternal and Child Health Hospital. All obstetricians working in these eight hospitals were approached to participate in the survey in that period, along with a proportion of pregnant women attending the hospitals’ out-patient antenatal care programs.
Survey of pregnant women
This clinic-based survey was conducted among pregnant women who were visiting the hospitals during a 2-h survey period, 1 h each in the morning and the afternoon, and who agreed to converse with the interviewers. The only exclusion criterion was an inability or lack of willingness to be interviewed. The target survey sample size was 384 women to estimate a proportional frequency of 50% with a two-tailed confidence level of 95% (95%CI) and a ± 5% error rate. Trained study interviewers administered the <20-min questionnaire survey.
The questionnaire was developed based on the health belief model, which consists of following constructs: perceived severity of influenza infection, perceived susceptibility, perceived benefits of vaccination, barriers to vaccination and cues to action. According to previous literatures,Citation16 barriers to vaccination in pregnant women mainly ascribe to low awareness of influenza, misconception about influenza vaccines and difficult access to vaccination services. Since our participants were limited in Beijing, where policies and vaccination service conditions are applied equally. To be more specific and easier to interpret, influenza knowledge was measured to account for barriers to vaccination acceptance. Besides, considering the effect of personal habitual behaviors and experience on health-related decision-making process,Citation42 personal history of influenza or any vaccination was assessed. The main outcome indicator was whether the women were willing to receive the influenza vaccine during the current pregnancy. Influenza knowledge was determined by asking questions about differences between the common cold and influenza, the influenza season in China, how influenza virus is transmitted, and eligibility of influenza vaccination during pregnancy. Those questions were measured in three ways: correct, incorrect, or “do not know”. Since both incorrect answers and “I do not know” reflected a lack of knowledge, responses were collapsed into two categories “correct” and “incorrect/did not know” as had other studies.Citation43,Citation44 Other domains were assessed by items listed in the supplementary table 1. Components were scored by the proportion of correct/agree answers by the total number of questions. The sociodemographic information was also collected including age, educational attainment, occupation, income, and parity history.
Survey of obstetricians
All obstetricians working in the sampled hospitals were included, except for those who refused to participate in the survey. Trained study interviewers administered the <20-min questionnaire survey. The questionnaire for obstetricians consisted of three parts: demographic data; knowledge of the impact of influenza and of influenza vaccine on pregnancy; and their willingness to recommend the vaccine to pregnant women. Interviewers also probed into reasons for participants’ unwillingness to recommend influenza vaccine with a multiple-choice question.
Ethical considerations and statistical analysis
Ethics approval for this study was granted by the Ethics Committee of the Center for Disease Prevention and Control (CDC) of Chaoyang District, Beijing, China. All participants provided a written informed consent.
Categorical variables were described as proportions, and continuous data were expressed as mean values and standard deviations (SD). Baseline characteristics were compared with analysis of variance, Kruskal–Wallis, or χ2 tests, as appropriate. Considering the multistage stratified cluster sampling method, a multilevel logistic model was performed at the preliminary stage. The model building started with an empty model with no predictors to determine variations caused by inter-hospital variation. Variations between hospitals were not significant (z = 0.54, P = 0.29). Therefore, the logistic regression analysis adjusted for sociodemographic characteristics was used to investigate the association between the willingness to receive the influenza vaccine during pregnancy and knowledge about the influenza, cues to action, history of vaccination or influenza, perceiving susceptibility, perceiving severity as well as perceiving the benefit of influenza vaccine. Additional analysis was conducted to test the robustness of the results by excluding “knowledge” from the model. Crude odds ratios (cOR) and adjusted odds ratios (aOR) with 95%CI were calculated. Logistic regression analysis was also used for analysis of factors associated with obstetricians’ willingness to provide recommendations for pregnant women to be vaccinated. And then, to test for trend, the ordered categories that included age group, educational attainment, professional title and years of working were modeled as a one degree-of-freedom linear term. Statistical analyses were conducted by SAS 9.2 (SAS Institute, Cary, NC, USA). Two-tailed P values <0.05 were considered statistically significant.
Author Contributions
J. Wang and D. Sun designed this study; D. Li and Y. Hu provided guidance on study implementation, data analysis, and manuscript writing; J. Wang, D. Sun, and X. Abudusaimaiti conducted questionnaire interviews; D. Sun and X. Abudusaimaiti analyzed the data; J. Wang, D. Sun, X. Abudusaimaiti, S.H. Vermund and Y Hu interpreted the data and drafted the manuscript. All authors read and approved the final manuscript. All authors attest they meet the ICMJE criteria for authorship.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Statement of Ethics
The study protocol was approved by the Ethics Committee of the Center for Disease Prevention and Control of Chaoyang District, Beijing. All participants provided a written informed consent.
Supplemental Material
Download PDF (1,023.6 KB)Acknowledgments
We thank the study participants for their contribution.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Sokolow LZ, Naleway AL, Li DK, Shifflett P, Reynolds S, Henninger ML, Ferber JR, Odouli R, Irving SA, Thompson MG. Severity of influenza and noninfluenza acute respiratory illness among pregnant women, 2010–2012. Am J Obstet Gynecol. 2015;212(2):202.e1–11. doi:10.1016/j.ajog.2014.08.004.
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, Adrian PV, van Niekerk N, Treurnicht F, Ortiz JR, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–31. doi:10.1056/NEJMoa1401480.
- Sperling RS, Riley LE. Influenza vaccination, pregnancy safety, and risk of early pregnancy loss. Obstet Gynecol. 2018;131(5):799–802. doi:10.1097/aog.0000000000002573.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 732 summary: Influenza vaccination during pregnancy. Obstet Gynecol. 2018;131(4):752–53. doi: 10.1097/aog.0000000000002586.
- World Health Organization. Vaccines against influenza WHO position paper. Wkly. Epidemiol. Rec. 2005;80(33):279–87.
- World Health Organization. Vaccines against influenza WHO position paper. Wkly. Epidemiol. Rec. 2012;87(47):461–76.
- Hu Y, Wang Y, Liang H, Chen Y. Seasonal influenza vaccine acceptance among pregnant women in Zhejiang Province, China: evidence based on health belief model. Int J Environ Res Public Health. 2017;14(12):1551. doi:10.3390/ijerph14121551.
- Li R, Xie R, Yang C, Rainey J, Song Y, Greene C. Identifying ways to increase seasonal influenza vaccine uptake among pregnant women in China: A qualitative investigation of pregnant women and their obstetricians. Vaccine. 2018;36(23):3315–22. doi:10.1016/j.vaccine.2018.04.060.
- Chinese Center for Disease Control and Prevention. Guidance on Influenza Vaccination in China. Beijing (China): China CDC; 2005. [accessed 2018 Oct 20] http://www.chinacdc.cn/jkzt/crb/lxxgm/ymjz/200509/t20050908_24123.htm
- Chinese Center for Disease Control and Prevention. Guidance on Influenza Vaccination in China. Beijing (China): China CDC; 2010. [accessed 2018 Oct 20] http://www.chinacdc.cn/tzgg/201011/t20101124_40513.htm
- Chinese Pharmacopoeia Commission.Chinese Pharmacopoeia. Beijing (China): China Medical Science Press; 2015.
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018;2:Cd001269. doi:10.1002/14651858.CD001269.pub6.
- Mayet AY, Al-Shaikh GK, Al-Mandeel HM, Alsaleh NA, Hamad AF. Knowledge, attitudes, beliefs, and barriers associated with the uptake of influenza vaccine among pregnant women. Saudi Pharm J. 2017;25(1):76–82. doi:10.1016/j.jsps.2015.12.001.
- Fridman D, Steinberg E, Azhar E, Weedon J, Wilson TE, Minkoff H. Predictors of H1N1 vaccination in pregnancy. Am J Obstet Gynecol. 2011;204(6Suppl 1):S124–7. doi:10.1016/j.ajog.2011.04.011.
- Goldfarb I, Panda B, Wylie B, Riley L. Uptake of influenza vaccine in pregnant women during the 2009 H1N1 influenza pandemic. Am J Obstet Gynecol. 2011;204(6Suppl 1):S112–5. doi:10.1016/j.ajog.2011.01.007.
- Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014;32(36):4602–13. doi:10.1016/j.vaccine.2014.06.067.
- Bish A, Yardley L, Nicoll A, Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29(38):6472–84. doi:10.1016/j.vaccine.2011.06.107.
- Regan AK, Mak DB, Hauck YL, Gibbs R, Tracey L, Effler PV. Trends in seasonal influenza vaccine uptake during pregnancy in Western Australia: implications for midwives. Women Birth. 2016;29(5):423–29. doi:10.1016/j.wombi.2016.01.009.
- Bonville CA, Cibula DA, Domachowske JB, Suryadevara M. Vaccine attitudes and practices among obstetric providers in New York State following the recommendation for pertussis vaccination during pregnancy. Hum Vaccin Immunother. 2015;11(3):713–18. doi:10.1080/21645515.2015.1011999.
- Zhou MG, Li YC, Wang HD, Zeng XY, Wang LJ, Liu SW, Liu YN, Liang XF. [Analysis on life expectancy and healthy life expectancy in China, 1990–2015]. Zhonghua liuxingbingxue zazhi. 2016;37(11):1439–43. doi:10.3760/cma.j.issn.0254-6450.2016.11.001.
- Song Y, Zhang T, Chen L, Yi B, Hao X, Zhou S, Zhang R, Greene C. Increasing seasonal influenza vaccination among high risk groups in China: do community healthcare workers have a role to play? Vaccine. 2017;35(33):4060–63. doi:10.1016/j.vaccine.2017.06.054.
- Gong Z, Lv H, Ding H, Han J, Sun J, Chai C, Cai J, Yu Z, Chen E. Epidemiology of the avian influenza A (H7N9) outbreak in Zhejiang Province, China. BMC Infect Dis. 2014;14:244. doi:10.1186/1471-2334-14-244.
- Chinese National Influenza Center. China seasonal influenza weekly report. Beijing (China): China CDC; 2016. [accessed 2018 Oct 20] http://www.chinaivdc.cn/cnic/zyzx/lgzb/201606/t20160602_130503.htm
- Floyd DL, Prentice-Dunn S, Rogers RW. A meta‐analysis of research on protection motivation theory. J Appl Soc Psychol. 2010;30(2):407–29. doi:10.1111/j.1559-1816.2000.tb02323.x.
- Maher L, Hope K, Torvaldsen S, Lawrence G, Dawson A, Wiley K, Thomson D, Hayen A, Conaty S. Influenza vaccination during pregnancy: coverage rates and influencing factors in two urban districts in Sydney. Vaccine. 2013;31(47):5557–64. doi:10.1016/j.vaccine.2013.08.081.
- Bodeker B, Walter D, Reiter S, Wichmann O. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine. 2014;32(33):4131–39. doi:10.1016/j.vaccine.2014.06.007.
- Vila-Candel R, Navarro-Illana P, Navarro-Illana E, Castro-Sanchez E, Duke K, Soriano-Vidal FJ, Tuells J, Diez-Domingo J. Determinants of seasonal influenza vaccination in pregnant women in Valencia, Spain. BMC Public Health. 2016;16(1):1173. doi:10.1186/s12889-016-3823-1.
- Napolitano F, Napolitano P, Angelillo IF. Seasonal influenza vaccination in pregnant women: knowledge, attitudes, and behaviors in Italy. BMC Infect Dis. 2017;17(1):48. doi:10.1186/s12879-016-2138-2.
- Cao L, Zheng J, Cao L, Cui J, Xiao Q. Evaluation of the impact of Shandong illegal vaccine sales incident on immunizations in China. Hum Vaccin Immunother. 2018;14(7):1672–78. doi:10.1080/21645515.2018.1473697.
- Smith PJ, Chu SY, Barker LE. Children who have received no vaccines: who are they and where do they live? Pediatrics. 2004;114:187–95.
- Henninger M, Naleway A, Crane B, Donahue J, Irving S. Predictors of seasonal influenza vaccination during pregnancy. Obstet Gynecol. 2013;121(4):741–49. doi:10.1097/AOG.0b013e3182878a5a.
- Laenen J, Roelants M, Devlieger R, Vandermeulen C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine. 2015;33(18):2125–31. doi:10.1016/j.vaccine.2015.03.020.
- MacDougall DM, Halperin SA. Improving rates of maternal immunization: challenges and opportunities. Hum Vaccin Immunother. 2016;12(4):857–65. doi:10.1080/21645515.2015.1101524.
- Donzelli A. Influenza vaccinations for all pregnant women? Better evidence is needed. Int J Environ Res Public Health. 2018;15(9). doi:10.3390/ijerph15092034.
- Liao Q, Cowling BJ, Lam WW, Fielding R. Factors affecting intention to receive and self-reported receipt of 2009 pandemic (H1N1) vaccine in Hong Kong: a longitudinal study. PLoS One. 2011;6(3):e17713. doi:10.1371/journal.pone.0017713.
- Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, Stewart L, Mullany LC, Chu HY, LeClerq SC, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17(9):981–89. doi:10.1016/s1473-3099(17)30252-9.
- Tapia MD, Sow SO, Tamboura B, Teguete I, Pasetti MF, Kodio M, Onwuchekwa U, Tennant SM, Blackwelder WC, Coulibaly F, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16(9):1026–35. doi:10.1016/s1473-3099(16)30054-8.
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–64. doi:10.1056/NEJMoa0708630.
- Yang J, Atkins KE, Feng L, Pang M, Zheng Y, Liu X, Cowling BJ, Yu H. Seasonal influenza vaccination in China: landscape of diverse regional reimbursement policy, and budget impact analysis. Vaccine. 2016;34(47):5724–35. doi:10.1016/j.vaccine.2016.10.013.
- Hu Y, Chen Y, Zhang B. Two-dose seasonal influenza vaccine coverage and timeliness among children aged 6 months through 3 years: an evidence from the 2010–11 to the 2014–15 seasons in Zhejiang province, east China. Hum Vaccin Immunother. 2017;13(1):75–80. doi:10.1080/21645515.2016.1225640.
- Ye C, Zhu W, Yu J, Li Z, Hu W, Hao L, Wang Y, Xu H, Sun Q, Zhao G. Low coverage rate and awareness of influenza vaccine among older people in Shanghai, China: A cross-sectional study. Hum Vaccin Immunother. 2018;14(11):1–7. doi:10.1080/21645515.2018.1491246.
- Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q. 1984;11(1):1–47. doi:10.1177/109019818401100101.
- Muhwezi WW, Banura C, Turiho AK, Mirembe F. Parents’ knowledge, risk perception and willingness to allow young males to receive human papillomavirus (HPV) vaccines in Uganda. PLoS One. 2014;9(9):e106686. doi:10.1371/journal.pone.0106686.
- Eppes C, Wu A, You W, Cameron KA, Garcia P, Grobman W. Barriers to influenza vaccination among pregnant women. Vaccine. 2013;31(27):2874–78. doi:10.1016/j.vaccine.2013.04.031.