ABSTRACT
Typhax is an investigational typhoid fever vaccine candidate that was GMP manufactured applying Protein Capsular Matrix Vaccine (PCMV) technology. It consists of Vi polysaccharide antigen, derived from S. Typhi, non-covalently entrapped in a glutaraldehyde catalyzed cross-linked α-poly-L-lysine and CRM197 protein matrix. Analysis of Typhax determined the average molecular weight of the vaccine particles was approximately 6 x 106 Daltons, corresponding to particles containing 1–2 molecules of Vi polysaccharide and 10–20 molecules of CRM197 protein. The ratio of the concentration of Vi to CRM197 protein in Typhax is 2.4:1. Preclinical immunogenicity studies in mice demonstrated that Typhax was immunogenic and elicited a significant increase in anti-Vi IgG antibody titers following each immunization. The anti-Vi IgG antibody response elicited by Typhax in rabbits increased as the dose increased from 0.1 µg to 2.5 µg. Further, at the 2.5 and 10 µg dose levels, the anti-Vi IgG antibody titers increased after the second and third immunizations. At the 10 µg dose level, 100% of rabbits seroconverted. In the non-human primate (NHP) study, 100% seroconversion was observed at both 2.5 µg and 10 µg dose levels after the first immunization. A murine in vivo immunopotency study demonstrated that Typhax stored at 4°C was stable for at least 30 months. Collectively, the Typhax in vitro profile, preclinical immunogenicity studies, and rabbit toxicology study indicate that Typhax is a viable typhoid fever vaccine candidate for Phase 1 clinical trial evaluation.
Introduction
Typhoid fever, commonly referred to as typhoid, is a human disease caused by Salmonella enterica serovar Typhi (S. Typhi) that is transmitted primarily via contaminated food and water. Although the disease has largely been eliminated from developed countries, typhoid fever remains widespread in developing countries, particularly in children and primarily in rural regions lacking modern sewage treatment facilities.Citation1 WHO estimates that typhoid fever is one of the main causes of foodborne deaths.Citation2 Epidemiological data from a 2010 study estimated that there were 216,000 deaths from 21 million cases of typhoid fever worldwide.Citation3-Citation5 Although originally thought to be a disease of school-age children, these studies report a higher incidence rate for children under the age of 5.
Until recently, two vaccine modalities were available for the prevention of typhoid fever. Parenteral, Vi polysaccharide subunit vaccines that include Typhim Vi® (Sanofi), Typherix® (GSK), and Typbar® (Bharat Biotech) and Vivotif (Emergent Biosolutions.), a live attenuated vaccine that is less widely used.Citation6 These vaccines range in protective efficacy from 55% to 95%.Citation1,Citation4,Citation5,Citation7 Typhim Vi®, Typherix®, and Typbar® are single-dose, intramuscularly administered vaccines approved for use in adults and children >2 y of age that confer protection for 2–3 y. Vivotif® is a four-dose orally administered vaccine indicated for use in adults and children >6 y of age that provides protection for 5 y.
Although the widely available Vi polysaccharide subunit vaccines confer a significant degree of protection against typhoid in older children and adults, they are poorly immunogenic in both infants and toddlers due to their inability to elicit a T-cell dependent immune response and immunological memory.Citation8,Citation9 Thus, several conjugates vaccines are in development with the aim to improve the immunogenicity of Vi polysaccharide in both adults as well as in infants and toddlers. A Vi polysaccharide conjugate vaccine, in which the Vi polysaccharide is covalently coupled to a protein antigen, generated higher anti-Vi polysaccharide IgG antibody titers than the unconjugated polysaccharide in a clinical studyCitation10 and a Vi-rEPA candidate, elicited an increase in Vi specific IgG titers following a second immunization when evaluated in a clinical trial.Citation11 Two Vi polysaccharide conjugate vaccines, PedaTyph® (BioMed Pvt. Ltd.) and Typbar-TCV® (Bharat Biotech), have been approved for use in children in India.Citation12 PedaTyph® is indicated for children over 3 months of age and Typbar-TCV® is indicated for children at least 6 months of age and adults.Citation13 In January 2018, Typbar-TCV® received WHO prequalification for administration to subjects 6 months and older (www.bharatbiotech.com). Other conjugate vaccine candidates are being evaluated in clinical trials.Citation12
While conjugate vaccines have been highly effective in the prevention of infectious disease, Citation14-Citation17 they are often difficult and expensive to manufacture. Protein Capsular Matrix Vaccine (PCMV) technology, a ‘virtual conjugate’, is a proprietary vaccine platform technology developed by Matrivax as an alternative to traditional conjugate technology. In the PCMV process, polysaccharides (PS) are entrapped in a cross-linked protein matrix as opposed to conjugate vaccine technology in which the PS is covalently coupled to a protein carrier molecule. The PCMV process is a technologically simpler alternative to conjugate vaccine technology and renders simplified multivalent PS vaccines possible. Initial studies exploring PCMV technology utilized poly-glutamic acid (PGA) capsular polymer from Bacillus anthracis or serotype 14 capsular polysaccharide from Streptococcus pneumoniae as antigens and a mutant B. anthracis Protective Antigen protein, DNI, as the matrix protein.Citation18 These PCMVs were highly immunogenic in mice, elicited an anamnestic response, and elicited functional antibodies when assayed in an opsonophagocytosis assay.Citation18
Although PCMV technology is most suitably applied to the production of multivalent polysaccharide vaccines, Matrivax strategized to initially apply PCMV technology to a monovalent polysaccharide vaccine targeting typhoid fever and to demonstrate proof-of-concept by advancing this vaccine candidate to a Phase 1 clinical trial. Towards this end, here, we describe the manufacture, release/characterization, and preclinical studies evaluating the safety and immunogenicity of Typhax, an investigational Vi antigen-based typhoid fever vaccine synthesized using PCMV technology. In Typhax, Vi polysaccharide antigen purified from S. Typhi is entrapped in a glutaraldehyde catalyzed matrix of cross-linked α-poly-L-lysine (α-PLL) and CRM197 protein, a genetic toxoid of diphtheria toxin and a common carrier protein of conjugate vaccine.Citation19,Citation20 Murine and rabbit preclinical immunogenicity studies indicated that Typhax immunogenicity was greatly enhanced with an aluminum phosphate adjuvant and that Typhax was well tolerated and highly immunogenic in mice, rabbits, and non-human primates (NHPs). These preclinical immunogenicity data and results from a rabbit toxicology study were submitted in an IND application and Typhax advanced to a Phase 1 clinical trial evaluation (manuscript in preparation).
Results
Synthesis and characterization of Typhax
Typhax, or Vi PCMV, was manufactured with purified Vi polysaccharide, chemically synthesized α-poly-L-lysine (α-PLL), and CRM197 protein, a genetic toxoid of diphtheria toxin. α-PLL is a polycation that facilitates the formation of Vi PCMV particles by ionically interacting with the negatively charged Vi polysaccharide and acting as an additional source of primary amine groups for matrix formation by glutaraldehyde. The addition of α-PLL to the PCMV reaction increased the amount of Vi entrapment and enhanced particle immunogenicity (data not shown). All components were manufactured in compliance with GMP guidelines.
Vi PCMV particles were separated from reactants by size exclusion chromatography (SEC) using Sephacryl® S1000 resin. Fractions were collected and analyzed for Vi polysaccharide and protein content using the Stains-All and Micro BCA assays, respectively (). High molecular weight material that contained a co-eluting peak of Vi polysaccharide and CRM197 protein (fractions 3–11) was pooled, sterile filtered, and vialed to make the Typhax drug substance (DS).
Figure 1. Separation of Typhax reaction products by size exclusion chromatography. The GMP Typhax (Vi PCMV) reaction mixture was applied to a 6 L Sephacryl® S1000 (9 cm x 90 cm) column equilibrated with 10 mM sodium phosphate buffer (pH 7.4) containing 5 mM NaCl. Fractions were collected and the amount of Vi PS (solid line) and CRM197 protein (dashed line) determined by the Stains-All® and Micro BCA™ assays, respectively. Fractions containing co-eluting peaks of Vi PS and CRM197 protein (gray box) were pooled to make the Typhax drug substance. Un-entrapped CRM197 protein eluted after fraction 20 thus its position is not shown on the chromatogram
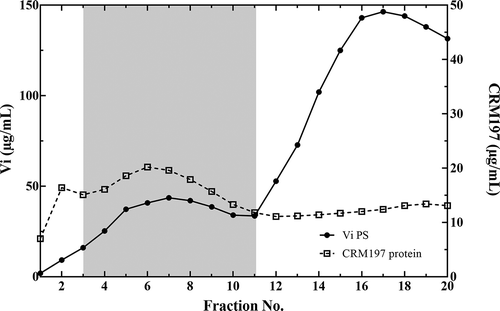
Typhax DS contained 25.2 µg/mL Vi polysaccharide and 10.4 µg/mL CRM197 as determined by the Stains-All assay and micro BCA assay, respectively (). Quantitation of Vi polysaccharide by the Stains-All assay was not impacted by the presence of CRM197 or α-PLL (data not shown). The amount of Vi in Typhax DS was also determined by O-acetylation content (Hestrin Assay). This assay is specific for Vi polysaccharide since it is the only source of O-acetyl groups in Typhax. Furthermore, since O-acetylation of Vi polysaccharide is critical for its immunogenicity, Citation21 this assay provided a method to assess the stability of the vaccine. The disparity in the measurements of Vi content as determined by the Hestrin and Stains-All assay (20.4 µg/mL versus 25.2 µg/mL, respectively) is likely because only 60–90% of the monosaccharide residues of Vi polysaccharide isolated from S. Typhi are O-acetylated.Citation14 The average molecular size of the PCMV particles in Typhax was 6 × 106 Da as determined by SEC-MALS (). Since Vi polysaccharide has an average molecular weight of 1–2 × 106 Da (data not shown and Citation22), it was deduced that Typhax particles contained 1–2 chains of polysaccharide with 10–20 molecules of CRM197 protein.
A capture ELISA was used to demonstrate that Vi polysaccharide was entrapped in the PCMV matrix. Further characterization demonstrated approximately 3% of the Vi polysaccharide and 14% of the CRM197 protein in Typhax were not contained in the PCMV matrix (). The impact of these amounts of free Vi polysaccharide and CRM197 protein on the immunopotency of Typhax is expected to be small. These values for free Vi polysaccharide and CRM197 protein provide a baseline for assessing the stability of the vaccine.
Typhax murine immunogenicity
In exploratory studies, a non-GMP Vi PCMV, synthesized in an engineering run at Walter Reed Army Institute of Research-Bioproduction Facility (WRAIR-BPF, Silver Spring, MD), was used in murine immunogenicity experiments to determine the effective dose level. These studies revealed that the anti-Vi antibody titer increased as the dose level decreased from 2 µg with maximal anti-Vi antibody levels observed at a 50 ng dose of Vi PCMV (data not shown). In studies evaluating adjuvanticity, Vi PCMV adjuvanted with Adju-Phos® elicited higher anti-Vi IgG antibody titers than Vi PCMV adjuvanted with Alhydrogel® (data not shown). Based on these studies, Typhax adjuvanted with Adju-Phos® (50 ng dose) was incorporated into a murine immunopotency stability study at the 9-month time point.
shows data from the immunopotency evaluation of Typhax stored for 9 months at 4ºC. Analysis of Day 42 sera showed that 50 ng of Typhax adjuvanted with Adju-Phos® elicited an anti-Vi IgG antibody GMT of 40,290 which was 73-fold higher than that elicited from an equivalent dose of Typhim Vi®. As shown in , Typhax retained its immunopotency after 30 months storage at 4ºC.
Table 1. Characterization of Typhax drug substance
Table 2. Mouse anti-Vi IgG GMTs elicited by Typhax immunization
Typhax rabbit immunogenicity
The immunogenicity and rate of seroconversion of rabbits immunized with Typhax were determined as part of a GLP repeat-dose toxicology study conducted in collaboration with SNBL USA, Ltd. (Mukilteo, WA) to support an IND application. Typhax elicited no local or systemic reactogenicity in rabbits (data not shown) and the immunogenicity results from this study are shown in . Unlike in mice, a dose-dependent antibody response was observed in rabbits immunized with Typhax adjuvanted with Adju-Phos® as the dose increased from 0.1 µg. At Day 42 rabbits immunized with 0.1 µg of Typhax adjuvanted with Adju-Phos® elicited a ~ 2.4-fold greater GMT compared to GMTs of pre-immune sera or the adjuvant control sera and 63% of the rabbits had seroconverted by the end of the study. At the next higher dose of 2.5 µg of Typhax, the GMT was 11-fold and ~27-fold higher than pre-immune sera at Days 28 and 42, respectively. At this dose, 50% of rabbits seroconverted at the Day 14 timepoint (2 weeks following the first immunization) and 88% of the rabbits had seroconverted by the end of the study. Of note, although the anti-Vi IgG antibody GMTs of rabbits immunized with 10 µg Typhax adjuvanted with Adju-Phos® were comparable to those elicited from the 2.5 µg dose at all time points, higher rates of seroconversion were observed with the 10 µg Typhax dose regimen at Day 28 (80%) and Day 42 (100%).
Table 3. Rabbit anti-Vi IgG GMTs and percent (%) seroconversion elicited by Typhax immunization
The anti-Vi IgG antibody GMTs for rabbits immunized with 10 µg of Typhax without adjuvant were about 10-fold lower than GMTs for the rabbits immunized with the equivalent adjuvanted dose at the Day 28 and Day 42 time points and was comparable to that observed with 0.1 µg Typhax plus Adju-Phos® at Day 42 (). These data support the use of Adju-Phos® as an adjuvant for Typhax. About 50% of the rabbits immunized with 10 µg of unadjuvanted Typhax seroconverted by the Day 14 and the seroconversion rate remained essentially at this level even after the second and third immunizations.
Typhax non-human primate immunogenicity
Four non-human primates (NHP) were immunized intramuscularly (IM) on Day 0 and 28 with 2.5 µg or 10 µg dose regimens of Typhax adjuvanted with Adju-Phos® to further evaluate Typhax implementing a dosing regimen like that proposed for the Phase 1 clinical trial. Typhax administered at a 2.5 µg dose level elicited a 60-fold (NHP#1) and 5-fold (NHP#2) increase in anti-Vi IgG antibody titer 10 d following the first immunization compared to pre-immunization titers (). At 28 d post-immunization, anti-Vi IgG antibody titers remained similar or decreased compared to Day 10. However, two weeks following the second immunization (Day 45), anti-Vi IgG antibody titers increased 2- to 4-fold compared to Day 28 titers (). At study termination (Day 45), the anti-Vi IgG antibody titer for NHP#1 and NHP#2 was 30-fold and 10-fold above their pre-immunization titer, respectively (, Day 0 vs. Day 45 titers).
Table 4. Non-human primate (NHP) anti-Vi IgG antibody titers elicited by Typhax immunization
Non-human primates immunized with a 10 µg Typhax dose developed a 100-fold (NHP#3) and 10-fold (NHP#4) increase in anti-Vi IgG antibody titers over pre-immune titers 10 d following the first immunization (). Anti-Vi IgG antibody titers remained similar or decreased by the Day 28 timepoint but increased 2- to 4-fold two weeks following the second immunization (, Day 28 vs, Day 45 titers). By study termination on Day 45 (~2 weeks after the second and final immunization), NHP#3 developed an anti-Vi IgG antibody titer 200-fold above its pre-immunization titer while NHP#4 developed an anti-Vi IgG antibody titer 10-fold above its pre-immunization titer (, Day 0 vs. Day 45 titers). All NHPs immunized with Typhax adjuvanted with Adju-Phos® at either the 2.5 µg or the 10 µg dose levels seroconverted (≥4-fold increase of anti-Vi IgG titer over pre-immune controls) after the primary immunization.
Discussion
PCMV technology is a vaccine platform delivery system that is a simpler alternative to the traditional conjugate vaccine technology. A PCMV, like a conjugate vaccine, is designed to increase the immunogenicity of a polysaccharide antigen such that it can elicit higher antibody titers not typically observed from polysaccharide antigen. The PCMV process is highly applicable to the development of multivalent polysaccharide-based vaccines. In the PCMV process, polysaccharide antigens are non-covalently entrapped in a protein matrix which differs from conjugate technology in which the PS antigen is covalently bonded to the carrier protein. The entrapment aspect of the PCMV process has the potential to enable the manufacture of multivalent polysaccharide vaccines in a single reaction as opposed to the multiple reactions required for multivalent conjugate vaccines. The creation of the PCMV matrix, or ‘virtual conjugate’, requires only a single crosslinking chemistry and is independent of PS structure. Another advantage of the PCMV process over typical conjugate technology is that the polysaccharide antigens do not need to be reduced in size to make them suitable reaction products.
Herein, we describe the GMP manufacture, characterization, and preclinical immunogenicity of Typhax, a Vi PCMV, intended for use in the prevention of typhoid fever. In earlier work, PCMV candidates synthesized using capsular antigens from B. anthracis and S. pneumoniae were highly immunogenic and elicited conjugate-like responses in mice.Citation18 These PCMV candidates were synthesized on a small scale and were not fully characterized. With Typhax, we have demonstrated that PCMV technology can be scaled and manufactured in compliance with GMP guidelines and produce a vaccine that is safe, immunogenic and suitable for testing in a Phase 1 clinical trial.
Characterization of Typhax demonstrated that it consists of high molecular weight particles (average MW 6 × 106 Daltons) that likely contain 1–2 molecules of Vi polysaccharide and 10–20 molecules of CRM197 protein. About 3.1% of the Vi polysaccharide and 14% of the CRM197 detected in Typhax is not incorporated in the particle. In vivo murine immunogenicity studies showed that Typhax was immunopotent and retained its immunopotency at least 30 months post-manufacture.
In mouse and rabbit immunogenicity studies, Typhax required an adjuvant to elicit high anti-Vi IgG antibody titers. In unpublished studies, we determined that aluminum phosphate (Adju-Phos®) was the preferred form of aluminum adjuvant. Conversely, Vi conjugate vaccines did not require an adjuvant to elicit a high anti-Vi IgG antibody response.Citation14 We also observed that anti-Vi IgG antibody responses elicited by Typhax in mice decreased as the dose level increased above 50 ng (data not shown). This surprising result differs from that observed with mice immunized with a Vi-CRM197 conjugate in which the anti-Vi IgG antibody titers were similar or increased slightly as the dose levels were increased from 0.125 µg up to 16 µg.Citation23 The requirement for an adjuvant with Typhax and the observed optimal murine dose of Typhax at 50 ng is suggestive that PCMV interacts with the immune system in a manner different than conjugate vaccines.
Rabbits, in contrast to mice, developed a dose-dependent response when the dose was increased from 0.1 µg to 2.5 µg. Although the anti-Vi IgG antibody titers did not increase further with the 10 µg dose, a greater number of rabbits seroconverted with the 10 µg dose regimen compared to the 2.5 µg dose regimen after the second and third immunizations. These data indicate that the higher dose resulted in seroconversion even though it did not elicit higher anti-Vi IgG antibody titers. All NHPs immunized with both dose levels had seroconverted following the first immunization even though the anti-Vi IgG antibody titers varied greatly. Although these studies demonstrate that Typhax elicits anti-Vi IgG antibody in mice, rabbits, and NHPs, they do not predict whether these antibodies are functional and would protect people from S. Typhi infection. Furthermore, the immune response observed in animals may not predict what will be observed in humans. However, the preclinical data strongly suggest that Typhax is likely to be safe and immunogenic in a Phase 1 clinical trial.
In summary, we have demonstrated that PCMV technology can be used in a GMP process to synthesize a Vi polysaccharide-based vaccine candidate that elicits high anti-Vi IgG antibody titers in preclinical models and that titers increase following each immunization. Although we observed some differences in the immune response elicited by Typhax compared to that reported for Vi conjugates, the ability of Typhax to elicit a conjugate-like immune response suggests that it is a viable alternative to conjugate vaccine technology that may allow for more cost-effective production of polysaccharide-based vaccines.
Materials and methods
Bacterial strain and CRM197 protein
Vi polysaccharide was derived from an S. Typhi strain Ty2 strain obtained from the Salmonella Genetic Stock Center, University of Calgary, Alberta, Canada. GMP grade CRM197 protein used for the synthesis of Typhax was purchased from Pfenex, Inc. (San Diego, CA).
GMP processes
Vi GMP polysaccharide purification
Vi polysaccharide was purified in compliance with GMP guidelines at Walter Reed Army Institute of Research-Bioproduction Facility (WRAIR-BPF, Silver Spring, MD). The growth of S. Typhi strain Ty2 was done with minor modifications, as described by Jang et al. Vi polysaccharide, which is shed into the culture supernatant during glucose and oxygen starvationCitation24, was isolated by removing cells and cellular debris by centrifugation then 0.2 µm filtration of the culture supernatant. Clarified culture supernatant was concentrated by ultrafiltration using a 100 kDa ultrafilter (Watersep, Marlborough, MA) and then diafiltered using 10 mM phosphate buffer (pH 7.4) containing 150 mM NaCl.
Vi polysaccharide was purified using a cetyltrimethylammonium bromide (CTAB) precipitation procedure followed by sequential ethanol extractions to remove impurities and solubilize Vi. The bulk Vi solution was sterile filtered, aliquoted, frozen at −80°C, and then freeze-dried. The lyophilized material was stored at −20°C until use. Purified Vi polysaccharide contained <1% protein contamination, <2% nucleic acid contamination, and met WHO standards for molecular size and degree of O-acetylation for Vi polysaccharide as a vaccine.Citation2
α-Poly L-lysine hydrochloride (α-PLL) GMP manufacture
The α-PLL used for the synthesis of the Vi PCMV particles was manufactured by Dalton Pharma Services, Inc. (Toronto, ON, Canada) in compliance with GMP guidelines. Briefly, α-PLL was synthesized in a two-step process. In the first step, Nε-carbobenzoxy-L-lysine was treated with triphosgene which reacts with the unprotected α-amine group to form Nε-carbobenzoxy-L-lysine N-carboxy anhydride (L-Z-lysine-NCA). In the second step, the L-Z-lysine-NCA precursor was polymerized into α-PLL by addition of sodium methoxide. The degree of polymerization was controlled by the ratio of L-Z-lysine-NCA to sodium methoxide. The terminal NCA group and the benzyloxycarbonyl groups on the ε-amines were removed by hydrolysis in hydrogen bromide (HBr) solution to form α-PLL HBr. The solution was neutralized with sodium carbonate, diafiltered against water to remove unreacted components, and the bromide ions associated with α-PLL replaced with chloride ions by treatment with dilute HCl. After synthesis was complete, α-PLL HCl was lyophilized, aliquoted into amber glass bottles, and stored at −20°C.
GMP Vi PCMV manufacture
Typhax (Lot 1860) was manufactured in compliance with GMP guidelines at WRAIR-BPF in a 100 mL reaction volume that contained 4 mg/mL Vi polysaccharide, 2 mg/mL CRM197, 0.2 mg/mL α-PLL, 50 mM borate buffer pH 8.5, 20% glycerol and 0.25% glutaraldehyde. PCMV particles were separated from unreacted reaction components by size exclusion chromatography (SEC) using Sephacryl® S-1000 resin (GE Healthcare, Pittsburgh, PA). Fractions were collected and analyzed for polysaccharide and protein content by the Stains-All assay and Micro BCA™ assay, respectively. The pooled fractions were filter sterilized and stored at 2–8°C prior to being aliquoted into vials.
Release and characterization assays
Stains all® assay
The assay procedure described by Schrager et al.Citation25 was modified to be performed in a microtiter plate.
Protein quantitation
Protein concentrations were determined with a Micro BCA™ assay kit following manufacturer’s instructions (Thermo Scientific, Waltham, MA).
Vi determination by O-acetylation content
Vi polysaccharide is variably O-acetylated on the 3-carbon of the repeating monosaccharide. The degree of O-acetylation correlates with the immunopotency of the Vi polysaccharide.Citation21 O-acetyl groups of Vi polysaccharide were quantified by the Hestrin assay which uses the reactivity of the O-acetyl groups with hydroxylamine and detection using ferric chloride.Citation26
Endotoxin content
The endotoxin level in Vi PCMVs was determined by a Limulus Ameobocyte Lysate (LAL) gel clot procedure using a kit from Lonza (Allendale, NJ).
Molecular size distribution of Vi PCMV and unincorporated CRM197 protein
The molecular size of Typhax and the amount of unincorporated CRM197 was determined using a 7.8 mm x 30 cm TOSOH TSKgel G6000 PWXL SEC column (Tokyo, Japan) using a mobile phase of 50 mM phosphate buffer pH 7.4 containing 150 mM NaCl at a flow rate of 0.3 mL/min. Column eluate was monitored for absorbance at 280 nm, refractive index, and light scattering using a DAWN® HELEOS®-II multi-angle laser light scattering (MALS) detector (Wyatt Technologies, Santa Barbara, CA). The molecular weight of the Vi PCMV was determined using ASTRA® software associated with the MALS detector.
Unincorporated CRM197 and CRM197 degradation products in the Vi PCMV sample were determined by calculating the amount of A280 signal that eluted as full-length CRM197 protein and lower molecular weight material relative to the total A280 signal present in chromatogram peaks. The amount of unincorporated CRM197 and degraded CRM197 protein products are presented as a percent of total A280 signal in all three peaks.
Free Vi polysaccharide determination
An adaptation of the rocket immunoelectrophoresis procedure described by Axelsen and BockCitation27 was used to determine the amount of un-entrapped (free) Vi polysaccharide in the Typhax DS.
Demonstration of entrapment of Vi in PCMV matrices via capture ELISA
A capture ELISA was used to demonstrate the presence of entrapped Vi polysaccharide in Vi PCMV (Typhax) matrix. In this assay, Vi polysaccharide entrapped in PCMV particles bound to the microtiter plate via a mouse monoclonal antibody to CRM197 generated a positive signal when probed with either anti-diphtheria toxin and anti-Vi rabbit polyclonal antisera. An admixed control containing Vi polysaccharide and CRM197 generated a positive signal with anti-diphtheria toxin antisera but a negative signal with anti-Vi antisera.
Analysis of Typhax immunogenicity
Murine immunization
Three to 5-week-old female BALB/c mice were obtained from Charles River Laboratory (Wilmington, MA). The methods for animal experimentation were approved by Boston University Medical Center Institutional Care and Use Committees (IACUC). For most experiments, groups of five BALB/c mice were immunized intramuscularly at biweekly intervals with 50 ng Typhax adjuvanted with Adju-Phos® (Aluminum phosphate, Al-PO4; Brenntag Biosector, Ballerup, Denmark).
Rabbit immunization
To assess the nonclinical safety and immunogenicity of Typhax, a repeat-dose study evaluating Typhax (Vi PCMV) in rabbits was conducted at SNBL USA, Ltd. (Everett, WA) in compliance with the Nonclinical Laboratory Studies Good Laboratory Practice (GLP) guidelines using a protocol approved by their IACUC.
Non-human primate (NHP) immunization
Four recycled Rhesus monkeys (Macaca mulatta) were obtained from the Bioqual, Inc. (Rockville, MD) and were maintained according to the guidelines of the National Institutes of Health (NIH) Guide to the Care and Use of Laboratory Animals and the approval of the Institutional Animal Care and Use Committee (IACUC) of Bioqual, Inc.
Vi-specific IgG antibody quantification by ELISA
Serum samples from groups of immunized animals (mice, rabbits, or non-human primates) were analyzed for anti-Vi specific IgG antibodies by ELISA adapted from methods described previously.Citation11
Disclosure of Potential Conflicts of Interest
TG, AT, RC, and KK are employees of Matrivax Research & Development Corporation. JM is a founder of Matrivax.
References
- Amicizia D, Arata L, Zangrillo F, Panatto D, Gasparini R. Overview of the impact of typhoid and paratyphoid fever. Utility of Ty21a vaccine (Vivotif(R). J Prev Med Hyg. 2017;58:E1–E8.
- Anonymous. WHO expert committee on biological standardization. World Health Organ Tech Rep Ser 1994;840:1–218.
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53.
- Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis. 2010;50:241–46. doi:https://doi.org/10.1086/648977.
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001921. doi:https://doi.org/10.1371/journal.pmed.1001809.
- Lopez-Gigosos R, Segura-Moreno M, Diez-Diaz R, Plaza E, Mariscal A. Commercializing diarrhea vaccines for travelers. Hum Vaccin Immunother. 2014;10:1557–67. doi:https://doi.org/10.4161/hv.27737.
- Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2:010401. doi:https://doi.org/10.7189/jogh.01.010401.
- Keitel WA, Bond NL, Zahradnik JM, Cramton TA, Robbins JB. Clinical and serological responses following primary and booster immunization with Salmonella typhi Vi capsular polysaccharide vaccines. Vaccine. 1994;12:195–99.
- Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984;150:436–49. doi:https://doi.org/10.1093/infdis/150.3.436.
- Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, Juvekar S, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis. 2014;14:119–29. doi:https://doi.org/10.1016/S1473-3099(14)70940-5.
- Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, Khiem HB, Trach DD, Karpas A, Hunt S, et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun. 1999;67:5806–10.
- Szu SC. Development of Vi conjugate - a new generation of typhoid vaccine. Expert Rev Vaccines. 2013;12:1273–86. doi:https://doi.org/10.1586/14760584.2013.845529.
- Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, Ella KM. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: a multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis. 2015;61:393–402. doi:https://doi.org/10.1093/cid/civ295.
- Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, Rappuoli R, Szu S, Saul A, Martin LB. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011;29:712–20. doi:https://doi.org/10.1016/j.vaccine.2010.11.022.
- Jaurigue JA, Seeberger PH. Parasite carbohydrate vaccines. Front Cell Infect Microbiol. 2017;7:248.
- Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc. 2005;77:293–324. doi:https://doi.org/10.1590/S0001-37652005000200009.
- Link-Gelles R, Taylor T, Moore MR. Forecasting invasive pneumococcal disease trends after the introduction of 13-valent pneumococcal conjugate vaccine in the United States, 2010-2020. Vaccine. 2013;31:2572–77. doi:https://doi.org/10.1016/j.vaccine.2013.03.049.
- Thanawastien A, Cartee RT, Griffin TJ, Killeen KP, Mekalanos JJ. Conjugate-like immunogens produced as protein capsular matrix vaccines. Proc Natl Acad Sci U S A. 2015;112:E1143–51. doi:https://doi.org/10.1073/pnas.1425005112.
- Giannini G, Rappuoli R, Ratti G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 1984;12:4063–69. doi:https://doi.org/10.1093/nar/12.10.4063.
- Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother. 2013;9:2505–23. doi:https://doi.org/10.4161/hv.26109.
- Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun. 1991;59:4555–61.
- Martin DG, Jarvis FG, Milner KC. Physicochemical and biological properties of sonically treated Vi antigen. J Bacteriol. 1967;94:1411–16.
- Rondini S, Micoli F, Lanzilao L, Pisoni I, Di Cioccio V, Saul AJ, Martin LB. Characterization of Citrobacter sp. line 328 as a source of Vi for a Vi-CRM(197) glycoconjugate vaccine against Salmonella Typhi. J Infect Dev Ctries. 2012;6:763–73. doi:https://doi.org/10.3855/jidc.2495.
- Jang H, Yoon YK, Kim JA, Kim HS, An SJ, Seo JH, Cui C, Carbis R. Optimization of Vi capsular polysaccharide production during growth of Salmonella enterica serotype Typhi Ty2 in a bioreactor. J Biotechnol. 2008;135:71–77. doi:https://doi.org/10.1016/j.jbiotec.2008.02.018.
- Green MR, Pastewka JV, Peacock AC. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973;56:43–51. doi:https://doi.org/10.1016/0003-2697(73)90167-X.
- Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem. 1949;180:249–61.
- Axelsen NH, Brock E. Electroimmunoassay (Rocket Immunoelectrophoresis). Scan J Immunol. 1983;17:103–06. doi:https://doi.org/10.1111/j.1365-3083.1983.tb04005.x.
