ABSTRACT
Immunization is one of the most important public health interventions to contrast infectious disease; however, many people nowadays refuse vaccination. Vaccine hesitancy (VH) is due to several factors that influence the complex decision-making process. Information technology tools might play an important role in vaccination programs. In particular, immunization information systems (IISs) have the potential to improve performance of vaccination programs and to increase vaccine uptake. This review aimed to present IIS functionalities in order to counter VH. In detail, we analyzed the automatic reminder/recall system, the interoperability of the system, the decision support system, the web page interface and the possibility to record adverse events following immunization. IIS could concretely represent a valid instrument to increase vaccine confidence, especially trust in both health-care workers and decision makers. There are not enough trials aimed to evaluate the efficacy of IIS to counter VH. Further researches might focalize on this aspect.
Introduction
Vaccine hesitancy
Although vaccines represent one of the safest and most effective public health tools available to prevent and control infectious diseases, they are victims of their own success. Vaccine concern is as old as the vaccine itself, and although vaccines are safer than before, a recent review highlights discrepancy between scientific evidence and general perception.1 There is no unique form of vaccine hesitancy (VH), nor a single reason behind this hesitancy: reasons are contextual (cultural, religious and geographical) and sometimes even vaccine specific.Citation2 According to the Strategic Advisory Group of Experts Working Group on Vaccine Hesitancy: “Vaccine hesitancy refers to delay in acceptance or refusal of vaccination despite availability of vaccination services”.Citation3 From the literature, it is known that vaccine hesitance is a continuum between full acceptors and total refusers.Citation4 Moreover, the 3 C model emphasizes complacency, convenience and confidence as factors that can influence the parents’ complex decision-making process on immunization. Complacency is determined by the reduction of perceived disease risk, due to the low incidence of the infection. Confidence is the trust in vaccine safety and effectiveness, and the trust in policy makers who decide for vaccinations. Convenience refers to the comfort, appeal and the quality of the service (real or perceived), including economic and geographic factors, but also the ability of people to understand priority placed on immunization.Citation3,Citation5,Citation6 As shown in the literature, the different proposed methods (i.e., reminder/recall and educational workshops) failed to motivate the total refusers compared to late/selective refusers or cautious acceptors; nevertheless, communication approaches should take into account the various degrees of hesitancy.Citation7 The main public health issue related to VH is that the higher the VH, the less the vaccination rate, with decrease of herd immunity and higher risk of “old disease” outbreaks.Citation8 In other words, the increasing VH could jeopardize the individual and societal ability to prevent the impact of vaccine preventable diseases (VPD). To bridge VH, World Health Organization (WHO) proposes the use of a proactive and methodological communication strategy to face the misinformation and to contrast the anti-immunization movement. Tailored programs could reduce the unvaccinated pocket population, interrupt the infective transmission chain and reach the aims of eradication (polio) and elimination (measles and rubella).Citation9 Recently a review, published by Mayo Clinic experts, described what approach should be taken toward addressing VH.Citation10 They suggest (1) improving reminder/recall communications; (2) spreading vaccine schedules among physicians; (3) reducing as much as possible missed opportunities to delivery vaccines; (4) addressing hesitancy; (5) using a standard protocol; (6) having clear recommendations and (7) increasing pediatrician or general practitioner (GP) involvement in an immunization information system (IIS). Due to the cultural heterogeneity in VH, the proposed multiple approach should be tailored for each country, since it covers many of the key factors like offering an easy access to vaccination, communication mediated through health-care workers (HCWs), availability of information for action and understanding the reasons of VH.
IISs
IISs are confidential, electronic population-based systems storing individual-level data on vaccines received within a given geopolitical area.Citation11 These electronic registries store and provide access to consolidate personal immunization information. IIS has the potential to improve the performance of vaccination programs and to increase vaccine uptake. The importance of IIS and information technology tools for the vaccination programs was recognized by the Council of the European Union, in the Council Conclusions on 6 June 2011 on childhood immunization, followed by the Council Conclusions 1 December 2014 on vaccination: “…consider introducing or further developing immunisation information systems, including improved registration, where applicable, and pharmacovigilance systems”.
Indeed IIS is also mentioned in the European Vaccine Action plan 2015–2020, the most recently launched global effort by the WHO.Citation12 Furthermore, European Centre for Disease Prevention and Control (ECDC) recently published a technical guidance aimed to provide support in plan, operation, management or continuous enhancement of IIS.Citation13
IISs are also known as immunization registries, and in the majority of cases, data are entered by HCWs, whilst sometimes, the general population may also enter data, followed by a GP’s approval. IISs are integrated systems in which all the entire process of vaccination is managed and recorded including the logistical aspect of the management of the vaccination services. So often, IIS is able to generate reminder and recall notifications, and it is largely used to assess vaccination coverage within a defined geographic area. Moreover, because in some countries, the vaccination services are private, the functions dedicated to recording of the vaccinated population are well separated from the other dedicated to the management of vaccination process and call of the people. Most IISs have additional capabilities, such as monitoring vaccine stocks to facilitate timely procurement of vaccines in order to limit wastage and ensure adequate supplies, as well as monitoring of adverse events following immunization (AEFI) reporting, and communicating with other health information systems, in particular with civil and cancer registries. The interoperability with civil registries allows the maintenance of birth-to-death vaccination histories, while the interconnection with the cancer registry is useful for efficacy/effectiveness study, particularly for vaccine-preventable cancers such as Hepatitis B virus (HBV) or HPV. Moreover, IIS can provide accurate data on which to make informed vaccination decisions and better protect against vaccine-preventable diseases. IISs have the potential to improve the performance of vaccination programs and to increase vaccine uptake; the strength of IIS is to provide decision makers with support for a vaccine strategy aimed to evaluate the efficacy of such vaccine policy and to improve program management. In our historical context, where VH is one of the most important challenges in the VPD field and since VH is a personal/community behavior choice in a specific context and for specific vaccines, it is important to know who, why and where VH is distributed (in socio-cultural, political, religious and geographical context). Even if the hesitants do not strictly match with not vaccinated, because it is possible that hesitants delay the vaccination; IIS could help to fight VH through recording additional information regarding reasons for delay, interruption or refusal vaccinations. However, a review by Schuster et al. revealed gaps in knowledge especially due to the paucity of studies from middle- and low-income settings.Citation1 This is one of the possible applications of IIS for VH, also in the low- and middle-income countries, especially because several incoming countries are developing or piloting these instruments. The ECDC provided the last updating data on IIS implementation among European countries,Citation14 while WHO made available data for the other developing countries.Citation15
Study aim
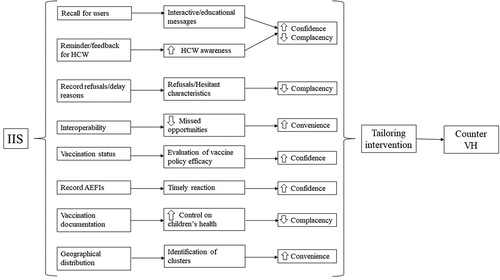
The aim of this review was to present the advantages coming from the use of IIS as a tool able to counter VH. It is extremely important to take into account that VH is only one determinant of vaccine uptake and several other factors impact on that, such as vaccines supply, availability and accessibility to immunization services. In this paper, we only presented the IIS applicability on countering VH. In particular, we focalized our attention on: (1) automatic reminder/recall; (2) assessment of vaccines refusers and vaccines recipients characteristics; (3) interoperability with other electronic registries and decision support system; (4) evaluation of vaccine program performance; (5) possibility to record AEFI; (6) social mobilization to promote vaccine programs and (7) geographical distribution and clusters of vaccine hesitants. Each of the following aspects of IIS is discussed in reference to the 3C model, as depicted in .
Methods
We conducted a narrative review, as a comprehensive qualitative synthesis of previously published information.Citation16 The original research articles were retrieved from PubMed and Embase on June 2016, using a combination of MeSH term and free text words. The search terms were electronic, computerized, registr*, register*, immunization, immunisation, vaccin*, “immunization registr*”, “immunisation registr*”, “vaccination registr*”, “vaccine hesitancy”, barrier*. Nevertheless, a manual check of the reference lists of the retrieved studies was carried out, in order to further identify proper articles. The articles were included in this narrative review whether they met the following inclusion criteria: (1) full text available; (2) articles using IISs as primary data source and (3) articles focusing on IIS’s functionalities useful to counter VH (as previously detailed).
Exclusion criteria were: (1) studies without original data (abstract, letters to editor, editorials, comments and commentaries) and (2) studies published in Congress proceedings and gray literature. No time filter was applied. Only articles in English were evaluated. Synthetic description of included articles is reported in , whilst papers considered more relevant were deeply discussed in the text.
Table 1. Characteristics extracted from the included studies.
Results
Automatic reminder/recall
One of the benefits of IIS is the potential for generating automatic reminders or recalls. This review identified 21 articles focused on reminder/recall and the IIS. Usually, the reminder/recall is developed to provide information about vaccination delivery, such as vaccines recommended or mandated for a child at a specific age and, in some cases, educational information is also provided.Citation17–Citation34 Nowadays, many IISs are developed with a built-in reminder system that automatically emits reminders. These automated systems can send reminders directly to people who are in due for a vaccination, or otherwise, other automatic feedback/reminders are sent to health professionals, in order to be updated on the patients who have to be called for the next vaccination.Citation13 In 2012, IIS was used, for the first time, as a tool able to evaluate the efficacy of two different methods of reminders (paper mail plus text messages versus paper mail).Citation32 Indeed, researchers developed a text-messaging platform integrated with the IIS aimed to estimate the list of persons to be contacted. At the end of the study (January–June 2009), 21.8% of the patients who received text messages compared to 9.2% (p = 0.02) who received only a letter were vaccinated. The same results were also obtained by Morris et al., who compared four different types of recall: postcard, text message, e-mail, phone call and nonintervention control group.Citation29 In this trial, the group who received a text message was the group with the highest rate of vaccination attendance (32.1% compared to 9.7% in the nonintervention group). Moreover, text message appeared to be the most effective system: fewer days passed between the reminder and when people were vaccinated (110 d compared to 234 d for nonintervention control).
Given the utility of a text-message reminder, the impact of (1) an educational and interactive text message, (2) educational only text-message reminder and (3) usual protocol (telephone appointment reminder with general information on vaccination) on flu vaccine coverage in children (aged 6 months–17 y) who were not vaccinated until mid-November 2011Citation26 was also compared. The results proved the beneficial effect of text messages, especially the educational plus interactive text message (p < 0.02 compared to usual care and p = 0.04 compared to only educative text message). The possible explanation, as the authors suggested is that the interactivity increased the sense of responsibility due to the parents’ active engagement that, in the 3C model, is represented by complacency. The text messages were sent by a platform, named EzVac, connected to the IIS. The IIS was also used to check the vaccination status and the timeliness of vaccinations. Recently, Suh and colleagues showed the greater adherence and cost-effectiveness of the recalls generated automatically from the centralized IIS compared to population-based recalls.Citation34
These results highlight how new technology such as text messages and IIS can improve vaccine coverage, especially if mobile phone numbers are recorded as a part of the registration process and if the IIS is connected to the civil registry.Citation132 This last option could serve as a way to geo-localize patients and to send them a more tailored messaging reminder, for example, messages with information on where and when they can be vaccinated or how to receive more information. These functionalities can contrast VH by increasing convenience. Indeed, according to the 3C model, providing information on the vaccination delivery might influence convenience due to an increased perceived quality of services. Clark et al. conducted a cross-sectional web-based national survey of parents of children 0–17 y old, evaluating the experiences and preferences about reminder/recall immunization messages.Citation18 They found that 76% of parents received vaccination notices by mail, by e-mail and call to mobile phone and that none received a text message. However, only 33% of the parents preferred to still receive notices by mail, while 3% preferred to receive text messages, and 56% of parents indicated that they would be willing to register their cell phone number to receive future call or text messages with information regarding immunization. The IIS could be a useful tool to record parental preferences about reminder/recall message modes. In general, the new technologies could help to increase parental empowerment regarding child vaccination, thanks to educational and tailored text messages, based on the characteristics of child, vaccinations and doses. However, to better understand text messaging, the methodologies and the applicability of this innovative system, other studies are ongoing.Citation133 In particular, we expect future studies on the application of social software (e.g., WhatsApp) that could represent the next and cheaper strategy compared to the text messages.
Usually, this reminder/recall approach is designed for the user but several studies evaluate the possibility to also send a reminder to the HCW.Citation35–Citation37 This particular type of feedback/reminder is based on the vaccine coverage data and on the best evidence-based practice. Different organizational models are in place in different countries; however, the main advantage is that HCW can personalize immunization care, tailor counseling and lastly move from administrative to involvement tasks. Brousseau and colleagues demonstrated how providing feedback to vaccinators is an effective strategy to improve vaccine coverage and reduce vaccine delay.Citation36 In this study, IIS was used to identify the clinics that had administered the highest number of doses of DTaP–polio–Hib, pneumococcal, meningococcal and mumps, measles and rubella (MMR) in 2007 in Quebec City, to calculate the vaccine coverage rate before and after the intervention and to establish the number of vaccination delays. During the two feedback sessions (before and after the intervention), authors presented the coverage data, the vaccine delays data obtained for the previous years and the best evidence practice; moreover, they also surveyed the organizational characteristic of the clinics. After a 12-month period, they found an increased number of administered doses and an increase in proportion of vaccines administered in time. A statistically significant increase was observed for DTaP–polio–Hib and pneumococcal (both +9% p < 0.001) using the 1-week delay definition. No significant statistics were observed for the 1-month definition. Moreover, after the intervention (provide feedback to vaccinators), four of the ten respondent clinics changed their habits, encouraging multiple injections and two of them improved nurse contribution. Among these four clinics, the vaccine delay was significantly decreased. The increasing proportion of infants immunized within a 1-month delay ranged from 32% to 44.6% (p < 0.001) for pneumococcal vaccine. The proportion of infants immunized without delay for MMR increased from 27.4% to 67.6% (p < 0.001) and from 56.5% to 80.9% (p < 0.001) for meningococcal.
Finally, these data highlight the important role played by IIS in vaccination reminders both to parent/patients and to HCW. Indeed, with educational text messages, parents can improve their empowerment on vaccination and are also facilitated with respect to the immunization schedule, while HCW can update, in real time, the immunization status of their patients. This shows that IIS could counteract VH by increasing the number of opportunities during which hesitant parents could discuss immunization with professionals. According to the 3C model, this could be beneficial to increase confidence and to contrast complacency.
Characteristics of vaccines refusers and vaccines recipients
Several recent reports confirm that the “new” outbreaks of measles and pertussis, for example, start in unvaccinated individuals and then spread to children whose vaccination may have failed. Due to these fundamental public health issues and the decreased immunization coverage, it is extremely important to know refusal and recipients characteristics. We identified 25 papers aimed to explore the characteristics of refusal or recipients.Citation7,Citation38–Citation61 Wei and colleagues used the information from the IIS to assess refusal status and then retrieved the racial, education and income characteristics from the census tract.Citation58 In this study, refusers had higher education levels and incomes p < 0.03 compared to non-refusers. Moreover, the refusers had no well-child visit and, compared to non-refusers, a higher percentage of refusers took antibiotics or seizure medications (p = 0.0003).
Van Lier and colleagues, using IIS, were able to outline the incomplete vaccination status of children in the Netherlands. The partially immunized children had at least one parent born out of the Netherlands or in no Western country; even a low socioeconomic status was associated with low vaccination coverage.Citation56 Another example comes from the study by MacDonald et al. where they assess, through a postal questionnaire, the reasons for no vaccination, or partial immunization, in children 2 y old during the period May 2008–April 2009 in Edmonton, Canada.Citation48 The obtained results show that concerns about vaccine safety, lack of awareness about disease severity and susceptibility and lack of trust in the health institutions and government were the most frequent reasons for partial immunizations, whereas children attending day care who had regular contact with a pediatrician and had at least one parent working outside the home were the most likely to complete the vaccinations. From these studies, it is noticeable how IIS might resolve the issue related to identification of unvaccinated people, especially if vaccine refusals are recorded as a part of the registration process and if the IIS is connected to pediatricians or family doctors’ software (electronic medical record systems). This function of IIS, if concretely used, could be important to contrast “complacency”, because it could allow analysis of parents’ reluctance.
Moreover, IISs may allow to record parents’ reasons of vaccinations refusal. Beard et al. published in 2016 a study evaluating the trend of vaccination objections.Citation38 People more affected by vaccine objections were the groups aged 12 months to 7 y old, in the lowest 10% of postcodes regarding socioeconomic status, while children born overseas had less registered objections.
Health programs rarely have the ability to track and follow-up vaccination refusers. It may seem to be expensive and time consuming but previous epidemiological studies have shown that refusers are able to transmit diseases to vaccinated individuals (taking into account vaccine efficacy and full immunization of people) when the two groups are mixed in a crowded area. Also Italy, with a subnational IIS – a national IIS, in Italy, is currently establishingCitation134 – was able to analyze reason of vaccinations refusal.Citation135 The IIS can provide the needed fundamentals to record the refusal status and the reasons for refusal.
Through IIS, it could also be possible to study the profile of people vaccinated and the characteristics of those who complete vaccination on time. Martinez-Baz et al., using a population-based vaccination registry (IIS), evaluated the proportion of persons vaccinated against influenza in Navarre, Spain, in the 2010–2011 season.Citation49 The aim was to analyze the factors influencing continued adherence to influenza vaccination in people older than 65 y and in those with major chronic conditions, who are considered at high risk of influenza complications.
Tan et al. extrapolated immunization records from the North Caroline Immunization Registry (their IIS) to evaluate the characteristics of girls who completed the HPV vaccination and completed it on time.Citation54 They stratified for several sociodemographic characteristics and they found that ethnicity and race was one of the most important factors influencing the completeness and on time delivery of the vaccine doses (59% vs. 43% p < 0.001 white vs. black, 51% vs. 47% p < 0.001 non-Hispanic vs. Hispanic for both completeness and on time). Comparing the funding type, those whose vaccine was privately funded were more likely than those that were publicly funded to complete the vaccine schedule and do it on time (both p < 0.001). Approximately 50% of those who completed the vaccination on time were immunized at pediatrician, GP clinics or local health units (both p < 0.001).
Leveraging IIS is possible to define the characteristics of vaccines refusers, which is extremely important in order to tailor the vaccination campaign. It is particularly true if we look at the parents’ fear on vaccine safety. Clearly, the opportunity to understand better the characteristics of target population depends on the type and quality of data recorded. Geographic or demographic data, such as reasons for not vaccinated, could not be available in all IISs.
Interoperability, missed opportunities and decision support system
One of the possible reasons for a low specific vaccine rate is missed opportunities; through this review, six manuscripts were retrieved and analyzed.Citation62–Citation67 Verani and colleagues performed a retrospective evaluation of 2001–2005 influenza seasons (using data from New York Citywide Immunization Registry) aimed to assess the prevalence of missed opportunities in children aged 6–23 months, among a practice network in New York.Citation66 Missed opportunities were defined as clinic visits during which the patients eligible for vaccination did not received vaccine. Missed opportunities had occurred in 82.2% of all vaccine-eligible visits, but with a remarkable decrease during the 5 y of study that was followed by an increasing coverage rate. Daley et al. conducted a prospective cohort study evaluating the frequency, reasons and the characteristics of missed opportunities for flu vaccinations in children aged 6–72 months with high-risk conditions, among four pediatrics clinics in Denver, during the 2002–2003 influenza season.Citation63 They extracted the vaccination status from the IIS, and the information about number, reasons and characteristics of clinical visits from billing databases. Also in this study, the missed opportunities were around 80%. Daley et al. surveyed parents of unvaccinated children to understand the reasons for no immunization. In the majority of the cases (29%), the reason was lack of physician recommendation, followed by low perception of flu risk (23%) and lack of particular reason (24%), while 13% were worried about potential vaccination risk. Only in 6% of cases was there a real opposition to the vaccinations, while in 5% of the cases, the reason was parental barriers. We can see that, probably, around 89% of unvaccinated children could have been immunized if they had received recommendations, information and education on vaccines. The missed opportunities for flu vaccine are particularly high, and interoperability between IIS and GP software, for instance, might reduce these occurrences. In fact, it is plausible that physicians can fail to recommend immunizations if they are not aware of both vaccination status or vaccine indications for their patients. Having this information available might increase the number of occasions in which HCW and parents can deal with the issue of vaccinations, addressing parents’ doubts and insecurities and thus reducing VH. Indeed, offering tailored counseling is extremely important in countering VH. Interoperability and DSS are extremely important for this aspect, as reported in the four articles found in the literature.Citation68–Citation71 Steven and colleagues present a brilliant example of bidirectional interface between electronic medical records and IIS. They developed a visual integrated interface by which physicians could easily and quickly acquire patients’ immunization information directly from IIS.Citation70 Of surveyed physicians, 68% feel more comfortable with the new interface; furthermore, they consider it much more efficient. IIS might provide decision support to the physician or to those who perform vaccinations, and assessment or feedback automatically generated can reduce missed opportunities. Hosseini and colleagues developed a method able to address interoperability within and between IISs; moreover, their main aim was to simplify and encourage the use of decision system support within the IIS.Citation68 Recently, Martinelli and colleagues combined three different data sources (hospital discharge registry, drug prescription registry and user fee exempt registry) with the IIS to identify patients with chronic diseases eligible for vaccination.Citation136 All the IISs are more operational when they are comprehensive and largely used by HCW. The decision support system is crucial to help physicians during their work, improving adherence to clinical guidelines and to provide alerts or recommendations in case of needed precautions. IIS equipped with decision system support could reduce the missed opportunities and improve the quality of the service. This is important if we take into account the impact of IIS on “convenience”. At the same time, IIS with decision support might help physicians to increase their trust in their own institution, which can act on “confidence”. The lack of trust may be a contributing factor to the increase in VH also in HCW.
IIS as an instrument to measure vaccine program performance
The concerns of health policy makers about the growing phenomenon of VH force them to promote public health strategies within civil society and among HCW. IIS is a tested tool to evaluate the efficacy of vaccine policy through the assessment of changing vaccine coverage rates before and after policy intervention. Related to this topic, 22 manuscripts were included in this study.Citation72–Citation93 Cates and colleagues assessed vaccine coverage after 3 months of social marketing experiments aimed to facilitate conversation among adolescents/parents and physicians about HPV vaccination.Citation74 They compared the data from IIS of two different counties (one where they performed the intervention and the other as a control) and the probability to get vaccinated was 34% higher in the intervention county. Isaac et al. consulted the Manitoba IIS to assess the efficacy of home visiting programs on vaccine coverage.Citation80 They found higher complete vaccinations in children aged 1–2 y in the families enrolled in the home visiting program compared to control. Grant and colleagues have shown that to address vaccine concerns during antenatal periods is the best method to improve the rate of parents agreeing with full immunization of their child.Citation44 They surveyed both pregnant women and their partners about the intentions of future infant immunization; then the child’s immunization status was assessed by IIS. The results show the highest proportion of timely vaccinations in children whose mother and partner were involved in the decision process; moreover, timeliness was also associated with a mother’s decision to fully immunize, independently of the mother’s demographics and partner’s intention. However, the timeliness was much higher if both mother and partner agreed with complete immunization. Nevertheless, 22% of partners versus 14% of pregnant women hadn’t decided about vaccinations, which might reflect the lesser partner engagement with physicians. Addressing parental concern through educational interventions is associated with increasing coverage rates.Citation44 Suryadevara et al. demonstrated the effectiveness of multicomponent community-based interventions in increasing vaccine coverage among poor families.Citation91 They performed a face-to-face interview, investigating parental concern and at the same time providing practical information about where, why and how to perform vaccinations; additionally, they offered vaccines on site. Nine months after the intervention, the children with “vaccine-complete” status increased from 28% to 45%; in adolescents, the HPV vaccination had increased 16%, 8% for meningococcal vaccine. Finally, the flu vaccine had a 17% increase compared to 8% in one county (without intervention), during the same period.
Kharbanda et al. evaluated changes on coverage rates, before and after the introduction of school mandated immunization.Citation82 Data on immunization coverage were extracted from IIS (EzVAC: web-based immunization registry of New York). They evaluated the coverage rate of diphtheria and pertussis (Tdap) and meningitis (MCV4) (vaccines required by the mandate) in three overlapping cohorts of adolescents aged 11–14 y, in three consecutive years: pre mandate, the first year of mandate and the following year. Data show a remarkable increase of coverage rates for both vaccines, which was stunning throughout the study period (Tdap coverage moved from 29% in pre mandate era to 58% during the first year, to 83% during the following year. Data are also similar for MCV4). Also, Simpson and colleagues, through IIS, found an increasing coverage rate after changes in the school entry mandate, requiring meningococcal vaccinations for all 11–18 y old adolescents as CDC Advisory Committee on Immunization Practices recommended in 2007.Citation89
Possibility to record AEFI
As a consequence of the high safety and efficacy vaccines, the perception of infectious disease severity is decreasing, conversely increasing vaccine concern. This scenario imposes an increase and renewal of surveillance strategies of adverse events after immunizations, especially in light of the introduction of newly licensed vaccines. The integration between IIS and the AEFI registry can be important to identify new and rare adverse reactions, to recognize new potential risk factors, to verify the safety of new licensed vaccine through post-marketing studies and to be reactive in case of suspected adverse events reported by the media, as discussed in the 24 articles presented in this review,Citation94–Citation116,Citation137 because the trust building process is very complex and long, and it could be undermined in an instant.Citation138 In fact, in the case of a health crisis (i.e., new outbreak, alleged AEFIs), public opinion is formed within the first 24 h; that is why health institutions need to provide timely, transparent, true, coherent and credible information,Citation138,Citation139 particularly for vaccinations that are administered routinely in healthy people to prevent disease.
In Valencia, Spain, the IIS was set up in 2002, and in 2005, recording of AEFIs was allowed through the IIS. From the analysis of the period 2005–2011, including information about vaccine safety and reported AEFIs according to patient characteristics (age and sex) and type of vaccine administered, it was possible to identify an increase in local reported reactions due to the switch from DTaP to Tdap.Citation94 Another example of IIS connected to AEFI surveillance system is the Alberta IIS, where AEFIs related to HPV vaccination were explored.Citation108 Among 195,270 women who received the vaccine, only 192 reported AEFIs. They were also able to know the type of AEFI reported, how many days after the immunization, associated dose, if hospitalization was required and the outcome. Among the AEFIs found, in the majority of cases, they happened after the first dose (n = 117). The most common AEFIs reported were 90 allergic reactions, 32 rush, 34 unusual reactions and 23 swelling or pain. Out of these, only five were hospitalized, four within 42 d after vaccination and one after 110 d. All the hospitalized women were alive, the ICD-10 diagnostic codes were available for three of them: one was “other physical therapy”, one was “chest pain unspecified” and the last one was “phlebitis and thrombophlebitis of other deep vessels of lower extremities”. Dey et al. used the Australian IIS – where AEFIs are routinely reported – to detect an early-onset signal of adverse reactions.Citation101 After a punctual evaluation of the AEFIs recorded, the increased rate was clearly imputable to one specific vaccine manufacturer when compared to the others. It is an important example of timely and sensitive methods to assess adverse events associated with immunization. It was also possible because of traceability of vaccine lots, highlighting the relevance of accuracy in reported batches.
Social mobilization to promote vaccine programs
Previous studies evaluated the association between maintaining immunization records and the increase of vaccine coverage rates.Citation140 McElligott and Darden conducted a national validated survey aimed to assess availability of vaccination records among households with children between 19 and 35 months of age and assess if updating the vaccination database may increase the vaccination rate.Citation141 After stratification for numerous variables (ethnicity, parent education level, number of children at home, poverty status), they found a statistically significant relationship between having a vaccination record and immunization rate, for all variables. Moreover, having vaccination records increased the odds of being updated compared to not having vaccine records by 62%. These increasing vaccination rates among the group with vaccination records highlight the importance for parents to have a record, in order to have more control of children’s health, and for HCW to double check the vaccination status at every visit. One of the new challenges for IIS is migration, which can reduce a person’s own data availability; however, two papers have shown potential solution to this aspect.Citation117,Citation118 Wilson et al. proposed mobile phone software as a possible solution.Citation118 In fact, apps connected to IIS might consolidate data from multiple sources and, after an internal validation, it can provide a platform where people are engaged with their own vaccine information. Moreover, it can be consulted in all possible settings, increasing people’s awareness and accuracy in vaccine rate estimation. Control vaccination rates are essential to modulate public health efforts and to increase people’s awareness on vaccines that may dominate VH. An example of advocacy is the pro-vaccination campaign launched on Instagram by an Italian mother who was worried about the decreasing vaccination rate.Citation142 This “case-report” approach, also used by anti-vaccine movements, was aimed to motivate reluctant parents to vaccinate their child. This proactive movement overflow in a very short time into all other social networks is the needed evidence of a bottom-up approach.Citation142 Actually, Brunson in her anthropological study evaluated the role played by social networks (in person and sources of information) on parents’ vaccination decisions. This study has shown that both people and social media are essential to formulate vaccination decisions. In particular, among those who decide to get their child vaccinated, the people network was supportive of a conformal recommendation, instead of un-(under)immunized parents. Conversely, the highest percentage of network people recommended nonconformity was found in un-(under)immunized parents. HCWs were considered for both groups, the second important network member after their own partner; other network members included were friends, family members, coworkers, midwives and university professors. This study suggests that social networks largely influence vaccination decisions in both groups. Furthermore, it is essential to develop vaccine promotion programs engaging the whole community, instead of just parents, because of the high importance of parents’ network members.Citation143 In general terms, positive social mobilization in vaccine programs (even through implementation of IIS) might be crucial to reduce VH, increase “confidence” (3Cs model) and consequently increase vaccination rates.
Geographical distribution, challenges and barriers and clusters of vaccine hesitant
Increasing evidence shows a relationship between geographical clustering of unvaccinated and localization of VPD outbreaks. In this review, we synthetized results from 13 studies.Citation119–Citation131 Eccles et al., using a geographical visualization method and IIS, assessed how geographical distribution of those who refuse vaccine had changed during a certain period and over time, identifying specific areas of non-vaccinated.Citation125 This geographical distribution has high public health impact, both to identify areas with health systems or ethnic–religious barriers and to identify areas with sanitary issues. In fact, known reasons for un-immunization are health-care access barriers, such as the time needed to reach the health care unit or the presence of public transportation or accessible parking; ethnic–religious barriers and socioeconomic barriers such us poverty, immigrant status, high residential density, material deprivation and high violent crime rates, as Charland and colleagues have shown.Citation119 These factors are part of “convenience” of the 3C model. IIS is a good instrument to assess vaccine coverage and vulnerability of unvaccinated people; moreover, it is a powerful instrument for public health investigations. Thompson et al. employed this instrument to assess vaccination rates and geographical distribution after the outbreak of measles in Disneyland, Florida;Citation128 while Teng and colleagues, after the worst cholera outbreak in Haiti, were able to monitor the massive campaign and through the global positioning system mapped the vaccination post locations and the geographical distribution of community coverage.Citation127 IIS and geographical data are also decisive to assess the equity access of vulnerable populations such as the Australian Aboriginal. A study evaluating the Indigenous vaccination rate in relation to accessibility or remoteness, (graded in five categories, according to Accessibility/Remoteness Index of Australia) found that pneumococcal conjugate vaccine immunization coverage ranged between 0.06% in very remote areas and 28.8% in accessible districts.Citation121 However, the coverage was suboptimal even in highly accessible areas with a range between 2.7% and 92.2% among Indigenous children aged 3 months. Using the same index, it was also possible to assess the timeliness of the first three doses of diphtheria, tetanus, pertussis (DTP), Haemophilus influenza type b and MMR vaccines among Aboriginal children (aged at least 36 months in accordance with Australian vaccine schedules) even in relation to remoteness.Citation122 Timeliness and completeness of vaccination data and Indigenous status were assessed by Australian IIS. Delayed vaccine delivery was 3–5 times higher among Indigenous children compared to non-Indigenous children. In particular, for the last DTP dose, the delay was higher among Indigenous children living in remote areas compared to Indigenous children residing in accessible areas.
Trogdon and Ahn, using data from IIS, found a geographical cluster of vaccination coverage in North Carolina. The geographical areas, based on ZIP code, tended to have vaccination coverage similar to their neighbors.Citation130,Citation144 Geolocalization could also be useful to drive allocation of scarce governmental resources in initiatives where it is needed most.Citation120 Indeed, during an epidemic outbreak, vaccine campaign not only needs to first target people with a higher risk (for complications or for epidemiological reasons) but it also needs to take into account the geographical distribution of the outbreak. Keeling and White, with their mathematical model demonstrated the importance to first vaccinate the geographical areas with the higher transmission rates of the previous years,Citation145 because the spatial heterogeneity could reflect the potential sociodemographic heterogeneity.
Discussion
Because of the complexity and the dynamism of vaccine skepticism, it is important for public health institutions to invest as much as possible in studies evaluating vaccine safety and communication strategies. In fact, it is essential to advocate to people about relevance, safety and vaccine effectiveness,Citation146 to offer them a dedicated website to easily find precise scientific information in plain language and, finally, to teach them how to elaborate search strategies and how to flush out fake websites.
Development of IIS could generate beneficial effects for several aspects of immunization policy, such as estimation of vaccination coverage, vaccine efficacy and safety. Particularly, in this review, we presented the principal potential functions of IIS useful to reduce VH in an empowered way for both health-care workers and general population. Through the 3C model of VH, we discussed the beneficial aspects of IISs. We focalized our attention on (a) automatic reminder/recall, (b) characteristics of vaccines refusers and vaccines recipients, (c) interoperability, missed opportunities and decision support system, (d) IIS as an instrument to vaccine program performance, (e) possibility to record AEFI, (f) social mobilization to promote vaccine programs, (g) geographical distribution and cluster of vaccine hesitants.
In relation to automatic reminder/recall, IIS feedback to vaccine providers might reduce vaccine delay and missed vaccination opportunities. IIS might also be an instrument to assess vaccine providers’ performance and to assign incentives. Actually, with a reminder/recall automatic system, IIS can increase people compliance to vaccination and vaccine knowledges whether the reminder is also associated with educational information. Whilst, if the feedback/reminder, based on vaccine coverage, is send to the health care workers, it could increase the communication opportunities on vaccines and reduce the missed opportunities. Furthermore, IIS could be useful to combine several types of activities (financial incentives, share strategy and policy)Citation83 and provide basic information on vaccine counseling during the feedback, in order to be able to experience both health prevention and promotion. IIS could be a very useful instrument for HCW and for public health program managers to identify characteristics of vaccines refusers and vaccines recipients. Indeed, IIS is strategic to assess, monitor and address the determinants of hesitancy and to sustain efforts to enhance vaccination confidence and uptake. Further, the reduction of missed opportunities, thanks to IIS, may, in addition, increase the frequency of recommendations from HCW to patients. This can reinforce the perception of the relevance of vaccinations among patients, resolve possible patient doubts or hesitancy about vaccines and can also transmit health information. In fact, despite 59–81% of surveyed US adults having used the internet to get health information,Citation147,Citation148 physicians remain the highest trusted information source among patients.Citation149
Regarding the evaluation of vaccine campaign performance, the IIS only represents an instrument to evaluate or compare different vaccine policies: how they impact vaccine coverage and cost effectiveness, and they give scientific support to policy makers, independently of potential coercion.Citation146 IIS could concretely represent a valid instrument to increase “confidence”, and especially trust, in both HCW and decision makers.
The possibility to record AEFIs in IIS might help to generate spot signals in the safety surveillance system. It would also help to identify a specific questionable lot and consequently to retrospectively identify who received the vaccine and from which specific lot. Additionally, it allows activation of the specific action required. In other words, IIS represents an excellent instrument to record, and to make available, more information on the event compared to the standard form for AEFI system.
Post-licensure surveillance of AEFIs is an integral part of immunization programs. IIS provides useful information, such as trends and signals that can be detected. In particular, IIS with AEFI records allowed easy and quick evaluation of potential adverse events and, subsequently, planning of a timely, credible and complete communication campaign, avoiding the spread of misleading information.Citation150 An example of this could be the so-called Fluad case during the 2014/2015 influenza vaccination campaign in Italy.Citation151 Indeed, after an erroneous report of four suspected deaths caused by administration of influenza vaccine, the influenza vaccine uptake dramatically dropped.Citation150 Certainly, the concept of “balanced information” in this case is essential. However, the availability of timely and accurate data may contribute in preventing misinformation.Citation152 It is particularly true considering that low trust in institution and fear of vaccine safety are the most frequent reasons of VH. In this sense, the possibility to record AEFIs could help in countering VH. In other words, IISs may easily evaluate vaccines safety also through individuals data linkage with other electronic systems that are part of the e-Health initiatives which are developing quickly and they will be very useful to general population, vaccine providers and health authorities. Clearly, IIS with AEFIs, and consequently its rapid consultation, may increase people’s “confidence”, improving trust particularly in vaccine safety. Moreover, it is noteworthy that continuous recommendations from physicians, updated education on vaccines for HCWs, traceability of immunizations records and dissemination of scientific evidence in plain language are milestones in facing vaccine delay or hesitancy. All of these are potential IIS functions that can improve the quality of the service, increasing “convenience” and “confidence”. The use of IIS at full operating speeds might represent an efficient tool to bridge the gap in vaccine coverage rates. Lastly, the possibility to geolocalize in detail the districts with low vaccination rates might underline the presence of potential issues, can allow to know more in depth the characteristics of the people in these areas and may support more tailored interventions to face specific needs.
Lastly, IIS can also reduce entry errors. Indeed, because IIS is an electronic system, the data entry could be carried out electronically, for instance vaccine bench codes, bar codes or drop-down menus could be used instead of manual data entry. Such innovative immunization surveillance system may be extremely useful also in rural area and in developing countries where computing infrastructures are very limited.Citation153 Inversely, mobile phones are very promising, because they are cheaper, easily used by HCW, with low power consumption and ubiquitous. Furthermore, mobile app can also be useful for cross-border travelers who have to show the International Certificate of Vaccination, for instance yellow fever, when arriving in countries where this is mandatory. Digital immunization passports could be beneficial for both public health purpose and users. In fact, if information could be stored centrally, more data could be recorded and the digital identification could be less prone to forgery.Citation154 Blockchain is a real-time digital technology that allows any user to figure out who owns what, where and when within a hypothetical supply chain.Citation155 This technology would be very helpful in those situations where vaccine supply chain should be warranted and a supply chain disruption may affect seriously vaccine uptake, which is the case of vaccine delivery in developing countries. In addition, the blockchain technology may ensure secure data access and patient privacy when it comes to distribute information coming from IIS.
Nevertheless, IIS is not without potential limitations. Researchers during IIS studies often face other obstacles, such as data sharing and confidentiality, or the overestimation of the denominator used to calculate the coverage rate.Citation156 The number of people who moved to another state or region but remain active in the IIS could explain this. Indeed, the completeness and accuracy of the denominator is one of the limits of IIS. It could be due principally to the absence of a unique identifier number assigned to the citizens or the absence of multiple sources for denominator data, which are characteristics considered important for IIS in order to fully support the immunization programs. Several possibilities can be offered to address this problem. For instance, reminder/recall systems can help to identify the cross-border child, the system could allow their citizens to update their own informationCitation108 or an IIS interconnected with civil registries could reduce this bias.Citation157 Timeliness is another aspect that should be taken into account. Indeed, in order to reduce missing data or data entry error, the time between vaccination administration and data record should be reduced. Finally, the adoption of electronic devices requires a huge investment both in terms of time and financial resources.Citation158 Another potential limit of the IIS is the upgrade of the functionalities such as the cross-talk between different registries. Registries may differ in terms of aims (e.g., cancer registry, civil registry) and, in the case of subnational IIS, different counties or districts could have different software.Citation159 Nevertheless, the potential benefits in terms of vaccine program quality, high vaccine rates, decrease of social disparities and VH, are invaluable. Indeed, IIS also represents an instrument to evaluate or compare different vaccine policies, how they can affect vaccine coverage and cost effectiveness and give scientific support to policy makers. IIS could concretely represent a valid instrument to increase “confidence” and “convenience”, especially trust in both health-care workers and decision makers and to reduce “complacency”. Nowadays, VH is one of the most important issues in public health; therefor, it is mandatory for public health workers to find new strategies able to address this problem. Currently, the international public health institutions are focusing on communication, but this could not be enough. Developing and improving IISs could represent one useful tool to improve communication, confidence and convenience on immunization programs. VH is a complex phenomenon where complacency, confidence and convenience are the three main decision factors. The results of this review show that IISs are important instruments to counter VH; nevertheless, there are not enough trials aimed to evaluate the efficacy of IIS to contrast VH. Further researches should focalize on this aspect.
Lastly, the present review had some limitations. Indeed, due to the newness of this topic, there is a wide variation in the indexed terms used in PubMed and Embase to describe IIS. However, to the best of our knowledge, this is the first review analyzing the application of IIS in order to counter VH.
Conclusion
In conclusion, some of the potential applications of IISs are get data on refusers’ characteristics, such as geographic distribution; be aware of the personal immunization status, which appear much more important in the current context of migration/globalization and social mobilization; get data on potential adverse effects following immunization, increasing the vaccine’s confidence and people trust.Citation160 The use of IIS is a promising tool useful for both vaccine providers and vaccine recipients as well as public health policy makers and epidemiologists.Citation13,Citation161 It allows access to flexible analyses that cannot be done using other vaccination data sources. It is able to reduce the burden of manual paper reporting systems, to facilitate quarterly vaccine coverage reports instead of aggregate data, to increase the accuracy of the data and to track the administered doses.Citation162 Recent systematic reviews gave an overview of the possible IIS uses in public health.Citation163,Citation164 However, Curran and colleagues have stated that the IIS is probably used below its real potential, as shown by an exponential increase of published articles only in the last few years.Citation156
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors would like to thank Ms. Christina Drace, a native speaker, for English revision.
Additional information
Funding
References
- Schuster M, Eskola J, Duclos P; Hesitancy SWGoV. Review of vaccine hesitancy: rationale, remit and methods. Vaccine. 2015 Aug 14;33(34):4157–60. doi:10.1016/j.vaccine.2015.04.035. PubMed PMID: 25896380.
- Hickler B, Guirguis S, Obregon R. Vaccine special issue on vaccine hesitancy. Vaccine. 2015 Aug 14;33(34):4155–56. doi:10.1016/j.vaccine.2015.04.034. PubMed PMID: 25896381.
- MacDonald NE; Hesitancy SWGoV. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015 Aug 14;33(34):4161–64. doi:10.1016/j.vaccine.2015.04.036. PubMed PMID: 25896383.
- Leask J, Kinnersley P, Jackson C, Cheater F, Bedford H, Rowles G. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi:10.1186/1471-2431-12-154. PubMed PMID: 22998654; PubMed Central PMCID: PMCPMC3480952.
- World Health Organization Regional Office for Europe, editor. Working group on vaccine communications. 2011 Oct 13–14. Istanbul (Turkey): WHO.
- The SAGE Vaccine Hesitancy Working Group. What influences vaccine acceptance: a model of determinants of vaccine hesitancy; 2013 [accessed 2019 Jan 20]. https://www.who.int/immunization/sage/meetings/2013/april/1_Model_analyze_driversofvaccineConfidence_22_March.pdf?ua=1
- Forbes TA, McMinn A, Crawford N, Leask J, Danchin M. Vaccination uptake by vaccine-hesitant parents attending a specialist immunization clinic in Australia. Hum Vaccin Immunother. 2015;11(12):2895–903. doi:10.1080/21645515.2015.1070997.
- Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014 Apr 17;32(19):2150–59. doi:10.1016/j.vaccine.2014.01.081. PubMed PMID: 24598724.
- World Health Organization. Addressing vaccine hesitancy; 2016. http://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/.
- Jacobson RM, St Sauver JL, Finney Rutten LJ. Vaccine Hesitancy. Mayo Clin Proc. 2015 Nov;90(11):1562–68. PubMed PMID: 26541249. doi:10.1016/j.mayocp.2015.09.006.
- Immunization information systems progress–United States, 2006. MMWR Morb Mortal Wkly Rep. Centers for Disease Control and Prevention, 2008 Mar 21;57(11):289–91. PubMed PMID: 18354373; eng.
- World Health Organization. European vaccine action plan 2015–2020. editor. Copenhagen (Denmark): Regional Office for Europe; 2014.
- European Centre for Disease Prevention and Control. Designing and implementing an immunisation information system. Stockholm, Sweden: ECDC; 2018.
- Derrough T, Olsson K, Gianfredi V, Simondon F, Heijbel H, Danielsson N, Kramarz P, Pastore-Celentano L. Immunisation information systems - useful tools for monitoring vaccination programmes in EU/EEA countries, 2016. Euro Surveill. 2017 Apr 27;22(17). doi:10.2807/1560-7917.ES.2017.22.17.30519. PubMed PMID: 28488999; PubMed Central PMCID: PMC5434883.
- World Health Organization. A case for better immunization information systems; 2013. http://www.who.int/immunization/programmes_systems/supply_chain/optimize/better_immunization_information_systems.pdf.
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006 Autumn;5(3):101–17. doi:10.1016/S0899-3467(07)60142-6. PubMed PMID: 19674681; PubMed Central PMCID: PMC2647067.
- Chung RJ, Walter EB, Kemper AR, Dayton A. Keen on teen vaccines: improvement of adolescent vaccine coverage in rural North Carolina. J Adolesc Health. 2015 May;56(5 Suppl):S14–6. PubMed PMID: 25863548; eng. doi:10.1016/j.jadohealth.2014.10.272.
- Clark SJ, Butchart A, Kennedy A, Dombkowski KJ. Parents‘ experiences with and preferences for immunization reminder/recall technologies. Pediatrics. 2011;128(5):e1100–e5. doi:10.1542/peds.2010-3664.
- Crawford N, Royle J, Sonja Elia RN, South M, Buttery J. Effect of a postcard immunisation reminder in hospital outpatients: A randomised controlled trial. J Paediatr Child Health. 2011;47:20. doi:10.1111/j.1440-1754.2010.01995.x.
- Custis CL, Helgerson SD, Murphy JS, Parry CA, Nett RJ. Evaluation of vaccination recall letter system for medicaid-enrolled children aged 19-23 months - Montana, 2011. Morb Mortal Wkly Rep. 2012;61:811–15.
- Dombkowski KJ, Cowan AE, Harrington LB, Allred NJ, Hudson E, Clark SJ. Feasibility of initiating and sustaining registry-based immunization recall in private practices. Acad Pediatr. 2012 Mar-Apr;12(2):104–09. PubMed PMID: 22321815; eng. doi:10.1016/j.acap.2012.01.002.
- Dombkowski KJ, Harrington LB, Dong S, Clark SJ. Seasonal influenza vaccination reminders for children with high-risk conditions: a registry-based randomized trial. Am J Prev Med. 2012 Jan;42(1):71–75. PubMed PMID: 22176850; eng. doi:10.1016/j.amepre.2011.09.028.
- Dombkowski KJ, Reeves SL, Dong S, Stevenson J, Clark SJ. Assessing the burden of undeliverable immunization reminder and recall notifications. Prev Med. 2011 Dec;53(6):424–26. PubMed PMID: 22001688; eng. doi:10.1016/j.ypmed.2011.09.014.
- Dombkowski KJ, Costello LE, Harrington LB, Dong S, Kolasa M, Clark SJ. Age-specific strategies for immunization reminders and recalls: A registry-based randomized trial. Am J Prev Med. 2014;47(1):1–8. doi:10.1016/j.amepre.2014.02.009.
- Dombkowski KJ, Cowan AE, Potter RC, Dong S, Kolasa M, Clark SJ. Statewide pandemic influenza vaccination reminders for children with chronic conditions. Am J Public Health. 2014;104(1):e39–44. doi:10.2105/AJPH.2014.302167.
- Hofstetter AM, Vargas CY, Camargo S, Holleran S, Vawdrey DK, Kharbanda EO, Stockwell MS. Impacting delayed pediatric influenza vaccination: A randomized controlled trial of text message reminders. Am J Prev Med. 2015;48(4):392–401. doi:10.1016/j.amepre.2014.10.023.
- Kempe A, Saville A, Dickinson LM, Eisert S, Reynolds J, Herrero D, Beaty B, Albright K, Dibert E, Koehler V, et al. Population-based versus practice-based recall for childhood immunizations: a randomized controlled comparative effectiveness trial. Am J Public Health. 2013 Jun;103(6):1116–23. doi:10.2105/ajph.2012.301035. PubMed PMID: 23237154; PubMed Central PMCID: PMCPMC3619016. eng.
- Kempe A, Saville AW, Dickinson LM, Beaty B, Eisert S, Gurfinkel D, Brewer S, Shull H, Herrero D, Herlihy R. Collaborative centralized reminder/Recall notification to increase immunization rates among young children a comparative effectiveness trial. JAMA Pediatr. 2015;169(4):365–73. doi:10.1001/jamapediatrics.2014.3670.
- Morris J, Wang W, Wang L, Peddecord KM, Sawyer MH. Comparison of reminder methods in selected adolescents with records in an immunization registry. J Adolesc Health. 2015;56(5):S27–S32. doi:10.1016/j.jadohealth.2014.10.274.
- Saville AW, Beaty B, Dickinson LM, Lockhart S, Kempe A. Novel immunization reminder/recall approaches: rural and urban differences in parent perceptions. Acad Pediatr. 2014;14(3):249–55. doi:10.1016/j.acap.2014.02.003.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. Jama. 2012 Apr 25;307(16):1702–08. doi:10.1001/jama.2012.502. PubMed PMID: 22535855; eng.
- Stockwell MS, Kharbanda EO, Martinez RA, Lara M, Vawdrey D, Natarajan K, Rickert VI. Text4Health: impact of text message reminder-recalls for pediatric and adolescent immunizations. Am J Public Health. 2012 Feb;102(2):e15–21. PubMed PMID: 22390457; PubMed Central PMCID: PMCPMC3483980. doi:10.2105/AJPH.2011.300331.
- Stockwell MS, Catallozzi M, Camargo S, Ramakrishnan R, Holleran S, Findley SE, Kukafka R, Hofstetter AM, Fernandez N, Vawdrey DK. Registry-linked electronic influenza vaccine provider reminders: A cluster-crossover trial. Pediatrics. 2015;135(1):e75–e82. doi:10.1542/peds.2014-1115.
- Suh CA, Saville A, Daley MF, Glazner JE, Barrow J, Stokley S, Dong F, Beaty B, Dickinson LM, Kempe A. Effectiveness and net cost of reminder/recall for adolescent immunizations. Pediatrics. 2012 Jun;129(6):e1437–45. PubMed PMID: 22566415. doi:10.1542/peds.2011-1714.
- Allison MA, Daley MF, Barrow J, Crane LA, Beaty BL, Allred N, Kempe A. High influenza vaccination coverage in children with high-risk conditions during a vaccine shortage. Arch Pediatr Adolesc Med. 2009 May;163(5):426–31. PubMed PMID: 19414688; eng. doi:10.1001/archpediatrics.2009.30.
- Brousseau N, Sauvageau C, Ouakki M, Audet D, Kiely M, Couture C, Pare A, Deceuninck G. Feasibility and impact of providing feedback to vaccinating medical clinics: evaluating a public health intervention. BMC Public Health. 2010;10:750. doi:10.1186/1471-2458-10-750. PubMed PMID: 21129216; PubMed Central PMCID: PMCPMC3017028. eng.
- Crawford NW, Barfield C, Hunt RW, Pitcher H, Buttery JP. Improving preterm infants‘ immunisation status: a follow-up audit. J Paediatr Child Health. 2014 Apr;50(4):314–18. PubMed PMID: 24372963; eng. doi:10.1111/jpc.12481.
- Beard FH, Hull BP, Leask J, Dey A, McIntyre PB. Trends and patterns in vaccination objection, Australia, 2002-2013. Med J Aust. 2016 Apr 18;204(7):275. doi:10.5694/mja15.01226. PubMed PMID: 27078604; eng.
- Bernhardt H, Barker D, Reith DM, Broadbent RS, Jackson PM, Wheeler BJ. Declining newborn intramuscular vitamin K prophylaxis predicts subsequent immunisation refusal: A retrospective cohort study. J Paediatr Child Health. 2015 Sep;51(9):889–94. PubMed PMID: 25873083; eng. doi:10.1111/jpc.12887.
- Feemster KA, Spain CV, Eberhart M, Pati S, Watson B. Identifying infants at increased risk for late initiation of immunizations: maternal and provider characteristics. Public Health Rep (Washington, DC: 1974). 2009 Jan-Feb;124(1):42–53. doi:10.1177/003335490912400108. PubMed PMID: 19413027; PubMed Central PMCID: PMCPMC2602930. eng.
- Feiring B, Laake I, Molden T, Cappelen I, Haberg SE, Magnus P, Steingrimsdottir OA, Strand BH, Stalcrantz J, Trogstad L. Do parental education and income matter? A nationwide register-based study on HPV vaccine uptake in the school-based immunisation programme in Norway. BMJ Open. 2015;5(5):e006422. doi:10.1136/bmjopen-2014-006422. PubMed PMID: 25991445; PubMed Central PMCID: PMCPMC4442157. eng.
- Gold R, Naleway AL, Jenkins LL, Riedlinger KK, Kurosky SK, Nystrom RJ, Kurilo MB. Completion and timing of the three-dose human papillomavirus vaccine series among adolescents attending school-based health centers in Oregon. Prev Med. 2011 Jun;52(6):456–58. PubMed PMID: 21539853; eng. doi:10.1016/j.ypmed.2011.04.010.
- Gowda C, Dong S, Potter RC, Dombkowski KJ, Dempsey AF. A population-level assessment of factors associated with uptake of adolescent-targeted vaccines in Michigan. J Adolesc Health. 2013 Oct;53(4):498–505. PubMed PMID: 24054080; eng. doi:10.1016/j.jadohealth.2013.07.022.
- Grant CC, Chen MH, Bandara DK, Marks EJ, Gilchrist CA, Lewycka S, Carr PE, Robinson EM, Pryor JE, Camargo CA, et al. Antenatal immunisation intentions of expectant parents: relationship to immunisation timeliness during infancy. Vaccine. 2016 Mar 8;34(11):1379–88. doi:10.1016/j.vaccine.2016.01.048. PubMed PMID: 26850758; eng.
- Gupta R, Alkhateeb FM, Latif DA, Farley KN. Parental attitudes affecting compliance with the recommendation for two doses of 2009 pandemic influenza A (H1N1) vaccine in children less than 10 years of age in West Virginia. W V Med J. 2013 Mar-Apr;109(2):10–14. PubMed PMID: 23600099; eng.
- Hofstetter AM, Natarajan K, Martinez RA, Rabinowitz D, Vawdrey DK, Stockwell MS. Influenza vaccination coverage and timeliness among children requiring two doses, 2004-2009. Prev Med. 2013 Mar;56(3–4):165–70. PubMed PMID: 23219757; eng. doi:10.1016/j.ypmed.2012.11.018.
- Lin W, Xiong Y, Tang H, Chen B, Ni J. Factors associated with delayed measles vaccination among children in Shenzhen, China: a case-control study. Hum Vaccin Immunother. 2014;10(12):3601–06. doi:10.4161/21645515.2014.979687. PubMed PMID: 25668667; PubMed Central PMCID: PMCPMC4514074. eng.
- MacDonald SE, Schopflocher DP, Vaudry W. Parental concern about vaccine safety in Canadian children partially immunized at age 2: A multivariable model including system level factors. Hum Vaccin Immunother. 2014;10(9):2603–11. doi:10.4161/21645515.2014.970075.
- Martinez-Baz I, Aguilar I, Moran J, Albeniz E, Aldaz P, Castilla J. Factors associated with continued adherence to influenza vaccination in the elderly. Prev Med. 2012 Sep;55(3):246–50. PubMed PMID: 22759626; eng. doi:10.1016/j.ypmed.2012.06.020.
- Nadeau JA, Bednarczyk RA, Masawi MR, Meldrum MD, Santilli L, Zansky SM, Blog DS, Birkhead GS, McNutt LA. Vaccinating my way - Use of alternative vaccination schedules in New York State. J Pediatr. 2015;166(1):151–6.e1. doi:10.1016/j.jpeds.2014.09.052.
- Riise OR, Laake I, Bergsaker MA, Nokleby H, Haugen IL, Storsaeter J. Monitoring of timely and delayed vaccinations: a nation-wide registry-based study of Norwegian children aged < 2 years. BMC Pediatr. 2015;15:180. doi:10.1186/s12887-015-0487-4. PubMed PMID: 26563381; PubMed Central PMCID: PMCPMC4643514. eng.
- Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics. 2012 Jul;130(1):32–38. PubMed PMID: 22711719; eng. doi:10.1542/peds.2011-3154.
- Schmitt K, Thompson DR. HPV vaccine timeliness and completion rates, Florida 2007-2011. J Womens Health. 2013;22:40.
- Tan W, Viera AJ, Rowe-West B, Grimshaw A, Quinn B, Walter EB. The HPV vaccine: are dosing recommendations being followed? Vaccine. 2011 Mar 21;29(14):2548–54. doi:10.1016/j.vaccine.2011.01.066. PubMed PMID: 21300098; eng.
- van Keulen HM, Otten W, Ruiter RA, Fekkes M, van Steenbergen J, Dusseldorp E, Paulussen TW. Determinants of HPV vaccination intentions among Dutch girls and their mothers: a cross-sectional study. BMC Public Health. 2013;13:111. doi:10.1186/1471-2458-13-111. PubMed PMID: 23388344; PubMed Central PMCID: PMCPMC3570492. eng.
- van Lier A, van de Kassteele J, de Hoogh P, Drijfhout I, de Melker H. Vaccine uptake determinants in The Netherlands. Eur J Public Health. 2014;24(2):304–09. doi:10.1093/eurpub/ckt042.
- Wagner KS, van Wijgerden JC, Andrews N, Goulden K, White JM. Childhood vaccination coverage by ethnicity within London between 2006/2007 and 2010/2011. Arch Dis Child. 2014 Apr;99(4):348–53. PubMed PMID: 24347574; eng. doi:10.1136/archdischild-2013-304388.
- Wei F, Mullooly JP, Goodman M, McCarty MC, Hanson AM, Crane B, Nordin JD. Identification and characteristics of vaccine refusers. BMC Pediatr. 2009;9:18. doi:10.1186/1471-2431-9-18. PubMed PMID: 19261196; PubMed Central PMCID: PMCPMC2667392. eng.
- Wilson S, Lim G, Seo C, McIntyre M, Fediurek J, Deeks S. School-based vaccination programs in Ontario: vaccine coverage and non-medical exemptions. Paediatr Child Health (Canada). 2014;19(6):e45. doi:10.1093/pch/19.6.e35-27.
- Wilson SE, Seo CY, Lim GH, Fediurek J, Crowcroft NS, Deeks SL. Trends in medical and nonmedical immunization exemptions to measles-containing vaccine in Ontario: an annual cross-sectional assessment of students from school years 2002/03 to 2012/13. CMAJ Open. 2015 Jul-Sep;3(3):E317–23. PubMed PMID: 26457292; PubMed Central PMCID: PMCPMC4596119. eng. doi:10.9778/cmajo.20140088.
- Woestenberg PJ, van Lier A, van der Maas NA, Drijfhout IH, Oomen PJ, de Melker HE. Delayed start of diphtheria, tetanus, acellular pertussis and inactivated polio vaccination in preterm and low birth weight infants in the Netherlands. Pediatr Infect Dis J. 2014 Feb;33(2):190–98. PubMed PMID: 24168985; eng. doi:10.1097/inf.0000000000000106.
- Berling I, Stephenson J, Cashman PM, Loten C, Butler M, Durrheim D. Are emergency departments missing an opportunity to increase childhood immunisation rates? EMA - Emerg Med Australas. 2011;23:20.
- Daley MF, Beaty BL, Barrow J, Pearson K, Crane LA, Berman S, Kempe A. Missed opportunities for influenza vaccination in children with chronic medical conditions. Arch Pediatr Adolesc Med. 2005 Oct;159(10):986–91. PubMed PMID: 16203946; eng. doi:10.1001/archpedi.159.10.986.
- Oltean HN, Lofy KH, Goldoft MJ, DeBolt CA. Human papillomavirus vaccination in washington state: estimated coverage and missed opportunities, 2006-2013. Public Health Rep. 2016;131(3):474–82. doi:10.1177/003335491613100313.
- Shingler S, Hunter K, Graham D. Opportunities taken: the need for, and effectiveness of secondary care opportunistic immunisation. J Paediatr Child Health. 2011;47:22–23. doi:10.1111/j.1440-1754.2010.01885.x.
- Verani JR, Irigoyen M, Chen S, Chimkin F. Influenza vaccine coverage and missed opportunities among inner-city children aged 6 to 23 months: 2000-2005. Pediatrics. 2007 Mar;119(3):e580–6. PubMed PMID: 17332178; eng. doi:10.1542/peds.2006-1580.
- Way AS, Durrheim DN, Vally H, Massey PD. Missed immunisation opportunities in emergency departments in northern New South Wales, Australia. J Paediatr Child Health. 2012 Jan;48(1):66–70. PubMed PMID: 21988697; eng. doi:10.1111/j.1440-1754.2011.02188.x.
- Hosseini M, Ahmadi M, Dixon BE. A service oriented architecture approach to achieve interoperability between immunization information systems in Iran. AMIA Annu Symp proc/AMIA Symp AMIA Symp. 2014;2014:1797–805.
- Rajamani S, Bieringer A, Muscoplat M. Characterizing the access of clinical decision support offered by immunization information system in minnesota. Online J Public Health Inform. 2015;7(3):e227. doi:10.5210/ojphi.v7i3.6282. PubMed PMID: 27252795; eng.
- Stevens LA, Palma JP, Pandher KK, Longhurst CA. Immunization registries in the EMR Era. Online J Public Health Inform. 2013;5(2):211. doi:10.5210/ojphi.v5i2.4696. PubMed PMID: 23923096; PubMed Central PMCID: PMCPMC3733755. eng.
- Swenson CJ, Appel A, Sheehan M, Hammer A, Fenner Z, Phibbs S, Harbrecht M, Main DS. Using information technology to improve adult immunization delivery in an integrated urban health system. Jt Comm J Qual Patient Saf. 2012 Jan;38(1):15–23. PubMed PMID: 22324187; eng.
- Ali H, Zwar N, Wild J. Improving childhood immunisation coverage rates–evaluation of a divisional program. Aust Fam Physician. 2009 Oct;38(10):833–35. PubMed PMID: 19893825; eng.
- Brotherton JM, Murray SL, Hall MA, Andrewartha LK, Banks CA, Meijer D, Pitcher HC, Scully MM, Molchanoff L. Human papillomavirus vaccine coverage among female Australian adolescents: success of the school-based approach. Med J Aust. 2013 Nov 4;199(9):614–17. PubMed PMID: 24182228; eng.
- Cates JR, Diehl SJ, Crandell JL, Coyne-Beasley T. Dissemination strategies to promote hpv vaccination among preteen boys: embracing the transition to adolescence through three-way conversations among provider, parent and preteen. J Adolesc Health. 2015;56(2):S14–S5. doi:10.1016/j.jadohealth.2014.10.274.
- Clayton JL, Potter RC, Wells EV, Carlton CA, Boulton ML. Influenza vaccination of Michigan children by provider type, 2010-2011. Am J Prev Med. 2014 Jul;47(1):46–52. PubMed PMID: 24854780; eng. doi:10.1016/j.amepre.2014.03.002.
- Ernst KC, Pogreba-Brown K, Rasmussen L, Erhart LM. The effect of policy changes on hepatitis A vaccine uptake in Arizona children, 1995-2008. Public Health Rep (Washington, DC: 1974). 2011 Jul-Aug;126(Suppl 2):87–96. doi:10.1177/00333549111260S211. PubMed PMID: 21812173; PubMed Central PMCID: PMCPMC3113434. eng.
- Feiring B, Laake I, Molden T, Haberg SE, Nokleby H, Seterelv SS, Magnus P, Trogstad L. Do selective immunisation against tuberculosis and hepatitis B reach the targeted populations? A nationwide register-based study evaluating the recommendations in the Norwegian Childhood Immunisation Programme. Vaccine. 2016 Apr 12;34(17):2015–20. doi:10.1016/j.vaccine.2016.02.060. PubMed PMID: 26947498; eng.
- Hull BP, Menzies R, Macartney K, McIntyre PB. Impact of the introduction of rotavirus vaccine on the timeliness of other scheduled vaccines: the Australian experience. Vaccine. 2013 Apr 8;31(15):1964–69. doi:10.1016/j.vaccine.2013.02.007. PubMed PMID: 23422140; eng.
- Humiston SG, Schaffer SJ, Szilagyi PG, Long CE, Chappel TR, Blumkin AK, Szydlowski J, Kolasa MS. Seasonal influenza vaccination at school: A randomized controlled trial. Am J Prev Med. 2014;46(1):1–9. doi:10.1016/j.amepre.2013.08.021.
- Isaac MR, Chartier M, Brownell M, Chateau D, Nickel NC, Martens P, Katz A, Sarkar J, Hu M, Burland E, et al. Can opportunities be enhanced for vaccinating children in home visiting programs? A population-based cohort study. BMC Public Health. 2015;15:620. doi:10.1186/s12889-015-1926-8. PubMed PMID: 26149681; PubMed Central PMCID: PMCPMC4494701. eng.
- Kansagra SM, Papadouka V, Geevarughese A, Hansen MA, Konty KJ, Zucker JR. Reaching children never previously vaccinated for influenza through a school-located vaccination program. Am J Public Health. 2014;104(1):e45–e9. doi:10.2105/AJPH.2014.302167.
- Kharbanda EO, Stockwell MS, Colgrove J, Natarajan K, Rickert VI. Changes in Tdap and MCV4 vaccine coverage following enactment of a statewide requirement of Tdap vaccination for entry into sixth grade. Am J Public Health. 2010 Sep;100(9):1635–40. PubMed PMID: 20634463; PubMed Central PMCID: PMCPMC2920954. eng. doi:10.2105/ajph.2009.179341.
- Melinkovich P, Hammer A, Staudenmaier A, Berg M. Improving pediatric immunization rates in a safety-net delivery system. Jt Comm J Qual Patient Saf. 2007 Apr;33(4):205–10. PubMed PMID: 17441558.
- Moore AM, Burgess S, Shaw H, Banks C, Passaris I, Guest C. Achieving high immunisation rates amongst children in the Australian Capital Territory: a collaborative effort. Aust Health Rev. 2011 Feb;35(1):104–10. PubMed PMID: 21367341; eng. doi:10.1071/ah10769.
- Moss JL, Gilkey MB, Griffith T, Bowling JM, Dayton AM, Grimshaw AH, Quinn B, Brewer NT. Organizational correlates of adolescent immunization: findings of a state-wide study of primary care clinics in North Carolina. Vaccine. 2013 Sep 13;31(40):4436–41. doi:10.1016/j.vaccine.2013.06.092. PubMed PMID: 23845803; PubMed Central PMCID: PMCPMC3798154. eng.
- Moss JL, Reiter PL, Dayton A, Brewer NT. Increasing adolescent immunization by webinar: a brief provider intervention at federally qualified health centers. Vaccine. 2012 Jul 13;30(33):4960–63. doi:10.1016/j.vaccine.2012.05.042. PubMed PMID: 22652406; eng.
- Potter RC, DeVita SF, Vranesich PA, Boulton ML. Adolescent immunization coverage and implementation of new school requirements in Michigan, 2010. Am J Public Health. 2014;104(8):1526–33. doi:10.2105/AJPH.2014.301910.
- Rehn M, Uhnoo I, Kuhlmann-Berenzon S, Wallensten A, Sparen P, Netterlid E. Highest vaccine uptake after school-based delivery - a county-level evaluation of the implementation strategies for HPV catch-up vaccination in Sweden. PLoS One. 2016;11(3):e0149857. doi:10.1371/journal.pone.0149857. PubMed PMID: 26974977; PubMed Central PMCID: PMCPMC4790890. eng.
- Simpson JE, Hills RA, Allwes D, Rasmussen L. Uptake of meningococcal vaccine in Arizona schoolchildren after implementation of school-entry immunization requirements. Public Health Rep (Washington, DC: 1974). 2013 Jan-Feb;128(1):37–45. doi:10.1177/003335491312800106. PubMed PMID: 23277658; PubMed Central PMCID: PMCPMC3514719. eng.
- Sull M, Eavey J, Papadouka V, Mandell R, Hansen MA, Zucker JR. Adolescent vaccine co-administration and coverage in New York City: 2007-2013. Pediatrics. 2014;134(6):e1576–e83. doi:10.1542/peds.2013-3604.
- Suryadevara M, Bonville CA, Ferraioli F, Domachowske JB. Community-centered education improves vaccination rates in children from low-income households. Pediatrics. 2013 Aug;132(2):319–25. PubMed PMID: 23837177; eng. doi:10.1542/peds.2012-3927.
- Teplow-Phipps R, Papadouka V, Benkel DH, Rosenthal S, Soren K, Stockwell M. Factors associated with early uptake and series completion of HPV vaccination in male and female adolescents. J Adolesc Health. 2014;54(2):S12. doi:10.1016/j.jadohealth.2013.10.040.
- Ward K, Dey A, Hull B, Quinn HE, Macartney K, Menzies R. Evaluation of Australia‘s varicella vaccination program for children and adolescents. Vaccine. 2013 Feb 27;31(10):1413–19. doi:10.1016/j.vaccine.2012.12.052. PubMed PMID: 23290837; eng.
- Alguacil-Ramos AM, Muelas-Tirado J, Garrigues-Pelufo TM, Portero-Alonso A, Diez-Domingo J, Pastor-Villalba E, Lluch-Rodrigo JA. Surveillance for adverse events following immunization (AEFI) for 7 years using a computerised vaccination system. Public Health. 2016 Jun;135:66–74. doi:10.1016/j.puhe.2015.11.010. PubMed PMID: 26976484; eng.
- Baker MA, Nguyen M, Cole DV, Lee GM, Lieu TA. Post-licensure rapid immunization safety monitoring program (PRISM) data characterization. Vaccine. 2013 Dec 30;31(Suppl 10):K98–112. doi:10.1016/j.vaccine.2013.04.088. PubMed PMID: 24331080; eng.
- Bakken IJ, Aaberg KM, Ghaderi S, Gunnes N, Trogstad L, Magnus P, Håberg SE. Febrile seizures after 2009 influenza A (H1N1) vaccination and infection: A nationwide registry-based study. BMC Infect Dis. 2015;15(1). doi:10.1186/s12879-015-1263-7.
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, Booy R, Bines JE. Intussusception following rotavirus vaccine administration: post-marketing surveillance in the National Immunization Program in Australia. Vaccine. 2011 Apr 5;29(16):3061–66. doi:10.1016/j.vaccine.2011.01.088. PubMed PMID: 21316503; eng.
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, Bines J, McIntyre PB. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia‘s National Immunization Program. Clin Infect Dis. 2013 Nov;57(10):1427–34. PubMed PMID: 23964090; eng. doi:10.1093/cid/cit520.
- Conlin AM, Bukowinski AT, Gumbs GR. Analysis of pregnancy and infant health outcomes among women in the National Smallpox Vaccine in Pregnancy Registry who received Anthrax Vaccine Adsorbed. Vaccine. 2015 Aug 26;33(36):4387–90. doi:10.1016/j.vaccine.2015.05.054. PubMed PMID: 26049005; eng.
- De Wals P, Deceuninck G, Ouakki M, Boulianne N, De Serres G, Danzig L. Analysis of mortality following a mass immunization campaign with serogroup C meningococcal conjugate vaccine: methodological difficulties and imperfect solutions. Vaccine. 2009 May 21;27(24):3223–27. doi:10.1016/j.vaccine.2009.02.055. PubMed PMID: 19446195; eng.
- Dey A, Gidding HF, Menzies R, McIntyre P. General practice encounters following seasonal influenza vaccination as a proxy measure of early-onset adverse events. Vaccine. 2014 Apr 17;32(19):2204–08. doi:10.1016/j.vaccine.2014.02.044. PubMed PMID: 24613527; eng.
- Gold M, Dugdale S, Woodman RJ, McCaul KA. Use of the Australian Childhood Immunisation Register for vaccine safety data linkage. Vaccine. 2010 Jun 11;28(26):4308–11. doi:10.1016/j.vaccine.2010.04.021. PubMed PMID: 20430123; eng.
- Håberg SE, Trogstad L, Gunnes N, Gjessing HK, Samuelsen SO, Cappelen I, Engeland A, Aavitsland P, Madsen S, Buajordet I, et al. The 2009 influenza pandemic, vaccination during pregnancy and fetal death: A national registry-based study in Norway. Pharmacoepidemiol Drug Saf. 2012;21:382.
- Heier MS, Gautvik K, Wannag E, Bronder KH, Midtlyng E, Kamaleri Y, Storsæther J. Narcolepsy in Norwegian children and adolescents after Pandemrix‘ vaccination. J Sleep Res. 2012;21:30.
- Hu Y, Li Q, Lin L, Chen E, Chen Y, Qi X. Surveillance for adverse events following immunization from 2008 to 2011 in Zhejiang Province, China. Clin Vaccine Immunol. 2013 Feb;20(2):211–17. PubMed PMID: 23239804; PubMed Central PMCID: PMCPMC3571262. eng. doi:10.1128/cvi.00541-12.
- Kiraly N, Koplin JJ, Crawford NW, Bannister S, Flanagan KL, Holt PG, Gurrin LC, Lowe AJ, Tang ML, Wake M, et al. Timing of routine infant vaccinations and risk of food allergy and eczema at one year of age. Allergy. 2016 Apr;71(4):541–49. doi:10.1111/all.12830. PubMed PMID: 26707796; eng.
- Liang XF, Li L, Liu DW, Li KL, Wu WD, Zhu BP, Wang HQ, Luo HM, Cao LS, Zheng JS, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med. 2011;364(7):638–47. doi:10.1056/NEJMoa1008553.
- Liu XC, Bell CA, Simmonds KA, Svenson LW, Russell ML. Adverse events following HPV vaccination, Alberta 2006-2014. Vaccine. 2016 Apr 4;34(15):1800–05. doi:10.1016/j.vaccine.2016.02.040. PubMed PMID: 26921782; eng.
- Lloyd-Johnsen C, Justice F, Donath S, Bines JE. Retrospective hospital based surveillance of intussusception in children in a sentinel paediatric hospital: benefits and pitfalls for use in post-marketing surveillance of rotavirus vaccines. Vaccine. 2012 Apr 27;30(Suppl 1):A190–5. doi:10.1016/j.vaccine.2011.11.015. PubMed PMID: 22520131; eng.
- Macartney KK, Gidding HF, Trinh L, Wang H, McRae J, Crawford N, Gold M, Kynaston A, Blyth C, Yvonne Z, et al. Febrile seizures following measles and varicella vaccines in young children in Australia. Vaccine. 2015;33(11):1412–17. doi:10.1016/j.vaccine.2014.10.071.
- Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: A population- and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275(2):172–90. doi:10.1111/joim.12150.
- Rouleau I, De Serres G, Skowronski DM, Drolet JP, Lemire C, Toth E, Landry M. Risk factors associated with anaphylaxis and other allergic-like events following receipt of 2009 monovalent AS03-adjuvanted pandemic influenza vaccine in Quebec, Canada. Vaccine. 2014;32(28):3480–87. doi:10.1016/j.vaccine.2014.04.059.
- Rousseau MC, El-Zein M, Conus F, Legault L, Parent ME. Bacillus Calmette-Guérin (BCG) vaccination in infancy and risk of childhood diabetes. Paediatr Perinat Epidemiol. 2016;30(2):141–48. doi:10.1111/ppe.12263.
- Schurink-van ‘T Klooster TM, De Ridder MAJ, Kemmeren JM, Van Der Lei J, Dekker F, Sturkenboom M, De Melker HE. Determination of a possible association between human papillomavirus (HPV) vaccination and migraine. Pharmacoepidemiol Drug Saf. 2014;23:151.
- Stehr-Green P, Galloway Y, Kieft C, McNicholas A. The risk of bronchiolitis hospitalisation following administration of a group B meningococcal vaccine in New Zealand. N Z Med J. 2007;120(1263):U2746. PubMed PMID: 17972966; eng.
- Van der Maas N, Wesselo C, Phaff T, Vermeer-de Bondt P, Oostvogels B. Adverse events following bivalent human papillomavirus vaccination reported to the enhanced passive surveillance system of the Netherlands. Pharmacoepidemiol Drug Saf. 2010;19:S324.
- Robison SG. Addressing immunization registry population inflation in adolescent immunization rates. Public Health Rep. 2015;130(2):161–66. doi:10.1177/003335491513000209.
- Wilson K, Atkinson KM, Deeks SL, Crowcroft NS. Improving vaccine registries through mobile technologies: A vision for mobile enhanced Immunization information systems. J Am Med Inform Assoc. 2016;23(1):207–11. doi:10.1093/jamia/ocv055.
- Charland KM, de Montigny L, Brownstein JS, Buckeridge DL. Clinic accessibility and clinic-level predictors of the geographic variation in 2009 pandemic influenza vaccine coverage in Montreal, Canada. Influenza Other Respi Viruses. 2014;8(3):317–28. doi:10.1111/irv.12227.
- Fu LY, Cowan N, McLaren R, Engstrom R, Teach SJ. Spatial accessibility to providers and vaccination compliance among children with medicaid. Pediatrics. 2009 Dec;124(6):1579–86. PubMed PMID: 19933734; eng. doi:10.1542/peds.2009-0233.
- Hull BP, Deeks S, Menzies R, McIntyre PB. What do we know about 7vPCV coverage in Aboriginal and Torres Strait Islander children? A 2007 update. Commun Dis Intell Q Rep. 2008 Jun;32(2):257–60. PubMed PMID: 18767426; eng.
- Hull BP, McIntyre PB. Timeliness of childhood immunisation in Australia. Vaccine. 2006 May 15;24(20):4403–08. doi:10.1016/j.vaccine.2006.02.049. PubMed PMID: 16569467; eng.
- Barbaro B, Brotherton JM. Assessing HPV vaccine coverage in Australia by geography and socioeconomic status: are we protecting those most at risk? Aust N Z J Public Health. 2014;38(5):419–23. doi:10.1111/1753-6405.12218.
- Brien S, Kwong JC, Charland KM, Verma AD, Brownstein JS, Buckeridge DL. Neighborhood determinants of 2009 pandemic A/H1N1 influenza vaccination in Montreal, Quebec, Canada. Am J Epidemiol. 2012 Nov 15;176(10):897–908. doi:10.1093/aje/kws154. PubMed PMID: 23077284; PubMed Central PMCID: PMCPMC3662406. eng.
- Eccles KM, Bertazzon S. Applications of geographic information systems in public health: A geospatial approach to analyzing MMR immunization uptake in Alberta. Can J Public Health = Revue Canadienne De Sante Publique. 2015 Sep-Oct;106(6):e355–61. PubMed PMID: 26680425; eng. doi:10.17269/cjph.106.4981.
- Mueller S, Exeter DJ, Petousis-Harris H, Turner N, O‘Sullivan D, Buck CD. Measuring disparities in immunisation coverage among children in New Zealand. Health Place. 2012 Nov;18(6):1217–23. PubMed PMID: 23000894; eng. doi:10.1016/j.healthplace.2012.08.003.
- Teng JE, Thomson DR, Lascher JS, Raymond M, Ivers LC. Using Mobile Health (mHealth) and geospatial mapping technology in a mass campaign for reactive oral cholera vaccination in rural Haiti. PLoS Negl Trop Dis. 2014;8(7):e3050. doi:10.1371/journal.pntd.0003050. PubMed PMID: 25078790; PubMed Central PMCID: PMC4117440.
- Thompson KM, Logan GE. Characterization of heterogeneity in childhood immunization coverage in central florida using immunization registry data. Risk Anal. 2015 May 29. doi:10.1111/risa.12424. PubMed PMID: 26033542; Eng.
- Trogdon JG, Ahn T. Geospatial patterns in human papillomavirus vaccination uptake: evidence from uninsured and publicly insured children in North Carolina. Cancer Epidemiol Biomarkers Prev. 2015;24(3):595–602. doi:10.1158/1055-9965.EPI-14-1231.
- Trogdon JG, Ahn T. Geospatial patterns in influenza vaccination: evidence from uninsured and publicly insured children in North Carolina. Am J Infect Control. 2015 Mar 1;43(3):234–40. doi:10.1016/j.ajic.2014.11.022. PubMed PMID: 25637432.
- Wagner AL, Sun X, Montgomery JP, Huang Z, Boulton ML. The impact of residency and urbanicity on Haemophilus influenzae Type b and pneumococcal immunization in Shanghai Children: a Retrospective Cohort Study. PLoS One. 2014;9(5):e97800. doi:10.1371/journal.pone.0097800. PubMed PMID: 24828814; PubMed Central PMCID: PMCPMC4020859. eng.
- Odone A, Ferrari A, Spagnoli F, Visciarelli S, Shefer A, Pasquarella C, Signorelli C. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. 2015;11(1):72–82. doi:10.4161/hv.34313. PubMed PMID: 25483518; PubMed Central PMCID: PMC4514191.
- Kalan R, Wiysonge CS, Ramafuthole T, Allie K, Ebrahim F, Engel ME. Mobile phone text messaging for improving the uptake of vaccinations: A systematic review protocol. BMJ Open. 2014;4(8). doi:10.1136/bmjopen-2014-005130.
- Establishment of the national vaccine registry. [Istituzione dell’Anagrafe nazionale vaccini.]. Gazzetta ufficiale della Repubblica Italiana: Ministry of Health, n. 257. 2018 Sep 17.
- Iannazzo S, Rizzuto E, Pompa MG. Polio vaccination failure in Italy, years 2006-2010. Epidemiol Prev. 2014 Nov-Dec;38(6 Suppl 2):98–102. PubMed PMID: 25759353.
- Martinelli D, Fortunato F, Iannazzo S, Cappelli M, Prato R. Using routine data sources to feed an immunization information system for high-risk patients—a pilot study. Front Public Health. 2018;6:1–6. doi:10.3389/fpubh.2018.00037.
- Arnheim-Dahlstrom L, Hallgren J, Weibull CE, Sparen P. Risk of presentation to hospital with epileptic seizures after vaccination with monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine (Pandemrix): self controlled case series study. Bmj. 2012;345:e7594. doi:10.1136/bmj.e7594. PubMed PMID: 23274350; PubMed Central PMCID: PMCPMC3532724. eng.
- European Centre for Disease Prevention and Control. A literature review of trust and reputation management in communicable disease public health. Stockholm, Sweden: ECDC; 2011.
- Higgins O, Sixsmith J, Barry MM, Domegan C. A literature review on health information seeking behaviour on the web: a health consumer and health professional perspective. Stockholm, Sweden: ECDC; 2011.
- Rosenthal J, Rodewald L, McCauley M, Berman S, Irigoyen M, Sawyer M, Yusuf H, Davis R, Kalton G. Immunization coverage levels among 19- to 35-month-old children in 4 diverse, medically underserved areas of the United States. Pediatrics. 2004 Apr;113(4):e296–302. doi:10.1542/peds.113.4.e296. PubMed PMID: 15060256.
- McElligott JT, Darden PM. Are patient-held vaccination records associated with improved vaccination coverage rates? Pediatrics. 2010;125(3):e467–e72. doi:10.1542/peds.2009-0835.
- Rosselli R, Martini M, Bragazzi NL. The old and the new: vaccine hesitancy in the era of the Web 2.0. Challenges and opportunities. J Prev Med Hyg. 2016;57(1):E47–50. PubMed PMID: 27346940; PubMed Central PMCID: PMCPMC4910443.
- Brunson EK. The impact of social networks on parents‘ vaccination decisions. Pediatrics. 2013 May;131(5):e1397–404. PubMed PMID: 23589813. doi:10.1542/peds.2012-2452.
- Trogdon JG, Ahn T. Geospatial patterns in human papillomavirus vaccination uptake: evidence from uninsured and publicly insured children in North Carolina. Cancer Epidemiol Biomarkers Prev. 2015 Mar;24(3):595–602. PubMed PMID: 25576528. doi:10.1158/1055-9965.EPI-14-1231.
- Keeling MJ, White PJ. Targeting vaccination against novel infections: risk, age and spatial structure for pandemic influenza in Great Britain. J Royal Soc Interface. 2011 May 6;8(58):661–70. doi:10.1098/rsif.2010.0474. PubMed PMID: 20943682; PubMed Central PMCID: PMC3061093.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics. 2008 Jul;122(1):149–53. PubMed PMID: 18595998. doi:10.1542/peds.2008-0987.
- HarrisInteractive. Number of ‘cyberchondriacs’ - adults going online for health information - has plateaued or declined; 2010 Mar 30. http://www.harrisinteractive.com/news/newsletters/healthnews/HI_HealthCareNews2008Vol8_Iss8.pdf.
- Susannah F. The social life of health information. Washington (DC): Project PRCsIAL, editor; 2011.
- Hesse BW, Nelson DE, Kreps GL, Croyle RT, Arora NK, Rimer BK, Viswanath K. Trust and sources of health information: the impact of the Internet and its implications for health care providers: findings from the first Health Information National Trends Survey. Arch Intern Med. 2005 Dec 12–26;165(22):2618–24. doi:10.1001/archinte.165.22.2618. PubMed PMID: 16344419.
- Rosselli R, Martini M, Bragazzi NL, Watad A. The public health impact of the so-called “Fluad Effect” on the 2014/2015 influenza vaccination campaign in Italy: ethical implications for health-care workers and health communication practitioners. Adv Exp Med Biol. 2017;973:125–34. doi:10.1007/5584_2017_39. PubMed PMID: 28452003.
- Signorelli C, Odone A, Conversano M, Bonanni P. Deaths after Fluad flu vaccine and the epidemic of panic in Italy. Bmj. 2015 Jan 14;350:h116. doi:10.1136/bmj.h116. PubMed PMID: 25589037.
- Bonanni P. Enlarged free childhood vaccination offer in Italy proposed to curb the rise in the growing anti-vaccine message. Expert Rev Vaccines. 2018 Feb;17(2):103–05. PubMed PMID: 29257703. doi:10.1080/14760584.2018.1419069.
- Katib A, Rao D, Rao P, Williams K, Grant J. A prototype of a novel cell phone application for tracking the vaccination coverage of children in rural communities. Comput Methods Programs Biomed. 2015 Nov;122(2):215–28. PubMed PMID: 26363678. doi:10.1016/j.cmpb.2015.08.008.
- Wilson K, Atkinson KM, Bell CP. Travel vaccines enter the digital age: creating a virtual immunization record. Am J Trop Med Hyg. 2016 Mar;94(3):485–88. PubMed PMID: 26711516; PubMed Central PMCID: PMC4775877. doi:10.4269/ajtmh.15-0510.
- Mamoshina P, Ojomoko L, Yanovich Y, Ostrovski A, Botezatu A, Prikhodko P, Izumchenko E, Aliper A, Romantsov K, Zhebrak A, et al. Converging blockchain and next-generation artificial intelligence technologies to decentralize and accelerate biomedical research and healthcare. Oncotarget. 2018 Jan 19;9(5):5665–90. doi:10.18632/oncotarget.22345. PubMed PMID: 29464026; PubMed Central PMCID: PMC5814166.
- Curran EA, Bednarczyk RA, Omer SB. Evaluation of the frequency of immunization information system use for public health research. Hum Vaccin Immunother. 2013 Jun;9(6):1346–50. PubMed PMID: 23422024; PubMed Central PMCID: PMCPMC3901828. eng. doi:10.4161/hv.24033.
- Olsson K, Gianfredi V, Derrough T. Immunisation information systems in the EU and EEA - Results of a survey on implementation and system characteristics. Stockholm, Sweden: ECDC: European Centre for Disease Prevention and Control; 2017.
- Tozzi AE, Gesualdo F, D‘Ambrosio A, Pandolfi E, Agricola E, Lopalco P. Can digital tools be used for improving immunization programs? Front Public Health. 2016;4:36. doi:10.3389/fpubh.2016.00036. PubMed PMID: 27014673; PubMed Central PMCID: PMCPMC4782280. eng.
- D‘Ancona F, Gianfredi V, Riccardo F, Iannazzo S. Immunisation Registries at regional level in Italy and the roadmap for a future Italian National Registry. Ann Ig. 2018 Mar-Apr;30(2):77–85. PubMed PMID: 29465145. doi:10.7416/ai.2018.2199.
- Goldstein ND, Maiese BA. A brief review of vaccination coverage in immunization registries. Online J Public Health Inform. 2011;3(1). doi:10.5210/ojphi.v3i1.3385. PubMed PMID: 23569601; PubMed Central PMCID: PMCPMC3615778. eng.
- Sartori AM, Nascimento AF, Yuba TY, Soarez PC, Novaes HM. Methods and challenges for the health impact assessment of vaccination programs in Latin America. Rev Saude Publica. 2015;49. doi:10.1590/s0034-8910.2015049006058. PubMed PMID: 26759964; PubMed Central PMCID: PMCPMC4687821. eng.
- Metroka AE, Hansen MA, Papadouka V, Zucker JR. Using an immunization information system to improve accountability for vaccines distributed through the Vaccines for Children program in New York City, 2005–2008. J Public Health Manag Pract. 2009 Sep-Oct;15(5):E13–21. PubMed PMID: 19704302; eng. doi:10.1097/PHH.0b013e3181a8c31f.
- Groom H, Hopkins DP, Pabst LJ, Murphy Morgan J, Patel M, Calonge N, Coyle R, Dombkowski K, Groom AV, Kurilo MB, et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J Public Health Manag Pract. 2015 May-Jun;21(3):227–48. doi:10.1097/PHH.0000000000000069. PubMed PMID: 24912082.
- Patel M, Pabst L, Chattopadhyay S, Hopkins D, Groom H, Myerburg S, Morgan JM; Community Preventive Services Task F. Economic review of immunization information systems to increase vaccination rates: a community guide systematic review. J Public Health Manag Pract. 2015 May-Jun;21(3):253–62. doi:10.1097/PHH.0000000000000100. PubMed PMID: 24912081.