ABSTRACT
The incidence of oropharyngeal cancer (OPC) related to infection with human papillomavirus (HPV) is rising, making it now the most common HPV-related malignancy in the United States. These tumors present differently than traditional mucosal head and neck cancers, and those affected often lack classic risk factors such as tobacco and alcohol use. Currently, there are no approved approaches for prevention and early detection of disease, thus leading many patients to present with advanced cancers requiring intense surgical or nonsurgical therapies resulting in significant side effects and cost to the health-care system. In this review, we outline the evolving epidemiology of HPV-related OPC. We also summarize the available evidence corresponding to HPV-related OPC prevention, including efficacy and safety of the HPV vaccine in preventing oral HPV infections. Finally, we describe emerging techniques for identifying and screening those who may be at high risk for developing these tumors.
Introduction
Head and neck cancer accounts for 3% of malignancies in the United States, with more than 63,000 Americans diagnosed with this disease and 13,000 dying from it annually.Citation1 Oropharyngeal cancer (OPC) represents a significant portion of head and neck cancers.Citation2 OPCs include those found in the soft palate, base of tongue, lingual and palatine tonsils, and surrounding tissues. While tobacco and alcohol exposure have long been established as risk factors for OPC, in recent decades there has been an increase in a subset of OPC linked to human papillomavirus (HPV).Citation3 HPV is a double-stranded DNA virus with predilection for squamous epithelium.Citation4 Cryptic epithelium overlying the tonsils and tongue base acts as a reservoir for the virus, providing access to its basal layer for viral replication.Citation2 Over time, malignant transformation can occur when viral oncoproteins disrupt tumor suppression genes in native tissue. Reticulated crypt epithelium in the oropharynx is unique to this anatomical location in the head and neck, and may explain why HPV is estimated to be five times higher in the oropharynx when compared to the oral cavity, larynx, or hypopharynx.Citation5 Although there are many types of HPV, the overwhelming majority of HPV-related OPC cases are caused by HPV16.
While data suggest an overall stable incidence of HPV-negative OPC, the incidence of HPV-related OPC is rising,Citation6 and it will continue to be a major factor in national health care related to cancer treatment. The rise in incidence of HPV-related OPC makes it now the most common HPV-related malignancy in the United States.Citation7,Citation8 According to the Centers for Disease Control and Prevention (CDC), there were 11,788 reported cases of cervical carcinoma and 18,917 cases of OPC, including 15,479 (82%) among men and 3438 (18%) among women in 2015.Citation7 In the United States, HPV DNA can now be identified in more than 70% of all new cases of OPC.Citation9 Similar trends are seen in Northern Europe.Citation10 Patients affected are typically in their fifth or sixth decade of life, have an earlier sexual debut, and a higher number of lifetime oral and vaginal sex partners than those affected by HPV-negative OPC.Citation8,Citation11,Citation12 In addition, these individuals are more often male, of higher socioeconomic status, and less likely to have a history of tobacco or alcohol abuse.Citation13
Oral HPV infection is the primary risk factor for HPV-related OPC, and over 90% of oral HPV infections are sexually acquired.Citation14 Therefore, it is no surprise that the number of oral sexual partners is the behavioral factor most strongly and specifically associated with OPC. Differences in sexual behavior between countries may contribute to the differences in global trends of HPV-related OPC.Citation10
In addition to viral exposure, concomitant tobacco use may also play a part in the development of HPV-related OPC. Gillison and colleagues performed a multivariable analysis inclusive of individuals aged 14–69 y, looking at factors independently associated with prevalent oral HPV including age, sex, lifetime number of sexual partners, and current smoking intensity.Citation15 Although adjustment for other factors dampened the first age-related peak in oral HPV prevalence, the bimodal age pattern remained statistically significant. Prevalence increased with number of lifetime sexual partners and number of cigarettes smoked per day. As a result, disease prevention efforts should also include attention to tobacco cessation.
Clinical behavior, treatment morbidity, and cost
The clinical behavior and presentation of HPV-related OPC are different from its HPV-negative counterpart. In patients with HPV-related OPC, the most common presenting symptom is a neck mass, while those with HPV-negative OPC are more likely to complain of a sore throat and dysphagia.Citation16 On presentation, they are more likely to have early T-stage (T1/T2) and advanced cervical nodal disease (N2/N3), when compared to HPV-negative tumors.Citation10,Citation17 However, while involvement of cervical lymph nodes reflects more advanced disease, such patients with HPV-related OPC have better survival outcomes and response to treatment than those with HPV-negative OPC.Citation18,Citation19
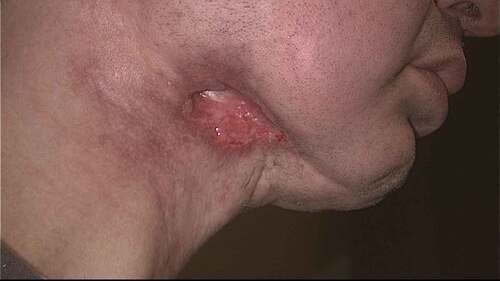
The standard treatment for OPC has been concurrent radiation and chemotherapy, with an increasing role for upfront surgical approach with advances in transoral surgery.Citation20 Although survival rates are high, long-term toxicity and poor functional outcomes are still a concern for patients who have survived their cancer ().Citation14,Citation21 Since the oropharynx is crucial to important everyday functions such as speech, swallow, and airway patency, both surgical and medical treatment of tumors in this area can result in significant morbidity. Patients can have long-lasting dysphagia and problems with speech such as velopharyngeal insufficiency from post-surgical changes, post-radiation effects, and chemotherapy. Additional side effects include nausea and vomiting, dry mouth, progression of dental disease, loss of taste, difficulty with mouth opening, and even osteoradionecrosis of the mandible ().Citation22-Citation26 While most of these issues improve over time with treatment and physical and/or speech therapy, some patients have long-standing dysfunction and require gastrostomy tubes for nutritional health, or tracheostomies for airway maintenance or pulmonary hygiene.Citation21 In fact, a retrospective review by Vatca et al. suggests that high-grade mucositis with concomitant weight loss from radiation therapy is worse in HPV-related OPC compared with HPV-negative OPC.Citation27 Thus, efforts are underway to investigate the feasibility of de-intensified chemoradiation therapy and to expand the indications for surgical therapy in hopes of decreasing acute and long-term morbidity and improving functional outcomes.Citation28,Citation29
Table 1. Adverse effects of nonsurgical therapy.
Although HPV appears to be a distinct risk factor from smoking in the development of OPC, tobacco exposure can also play a role.Citation15 Although patients with HPV-positive OPC are less likely to be smokers, those who do have a significant current or past smoking history have been found to have significantly worse disease control with treatment.Citation13,Citation30,Citation31 Maxwell and colleagues found that current tobacco users with advanced, HPV-positive OPC are at higher risk of disease recurrence compared with never-tobacco users after chemoradiation therapy. In their study cohort, 35% of HPV-positive ever-tobacco users recurred compared with only 6% of HPV-positive never-users and 50% of HPV-negative patients.Citation30
In addition to the morbidity from treatment and risk for cancer-related mortality, there are significant costs associated with the management of OPC, with one study showing an estimated cost of $140,000 per new patient in the first 2 y of treatment and surveillance, not accounting for additional costs due to loss of productivity.Citation32 Based on numbers from 2004 to 2007, it was estimated that the mean lifetime cost per new case of HPV-related head and neck cancer was $43,200 with a total annual cost in the United States of $306 million.Citation33 Another study by Moore et al. compared cost between different treatment modalities and found that the mean cost of therapy (private payers/government payers) ranged from $37,435/$15,664 in those treated with surgery alone to $198,285/$57,429 when chemoradiation was employed.Citation34
Rationale for prevention and screening
In response to the emerging epidemic of HPV-related OPC, and the morbidity and costs associated with treating these cancers, much attention has turned to the prevention and early detection of disease. A United States national initiative called Healthy People 2020 aims to decrease the number of deaths due to OPC by 10%, mainly through improving immunization rates to reduce preventable infections.Citation35 Current vaccination rates are low, especially in males who are most often affected by these cancers.Citation36 Recent statistics estimate that about 65% of girls and 56% of boys between ages 13 and 17 y have received the first dose of the HPV vaccine.Citation37 Moreover, due to the lack of level one evidence showing a reduction of premalignant lesions and OPC with vaccination, prevention of OPC is not an approved vaccine indication by the Food and Drug Administration (FDA). This creates a barrier to increasing public education and awareness on a large scale.
In addition to the challenges posed in implementing successful prevention campaigns, there is an increasing need to develop effective screening techniques to allow for early detection of disease, especially for those who have not received the vaccine. Challenges to screening include lack of a precursor lesion and a long latency period between exposure to virus and onset of disease. Multiple studies suggest that the timing between exposure to HPV virus and development of cancer exceeds a decade, and can be as long as 30 y.Citation10,Citation38 In this review, we provide an update on the current status of HPV-related OPC prevention in the United States as well as evolving approaches to screening. In addition, we outline some of the challenges posed to these efforts as well as potential areas for future study.
Prevention of HPV-related OPC
Lifestyle modifications
The behavioral risk factor most associated with HPV-related OPC is oro-genital sex. As a result, primary prevention through safe sexual practices such as condoms and barrier contraceptives is a critical prevention strategy. Gupta et al. performed a cross-sectional analysis of National Health and Nutrition Assessment Survey from 2009 to 2014 to see if there is a correlation between barrier contraceptive and prevalence of oral HPV 16/18 infection. They found that after adjusting for all variables associated with HPV positivity, individuals reporting barrier use were significantly less likely to be HPV 16/18 positive when compared to those not using barrier during oro‐genital sex. It is important to note that this population had not received the HPV vaccine.Citation39 Therefore, barrier contraceptive is an important prevention strategy for those who are beyond the eligible age for vaccination. While the CDC recommends use of barrier contraceptives such as condoms and dental dams during oro-genital sex to reduce transmission of sexually transmitted infections, there needs to be stronger emphasis on the correlation between this and decreasing risk of developing OPCs.
As mentioned above, Gillison et al. demonstrated that tobacco smoking also significantly correlates with oral HPV infection,Citation15 and such use can negatively impact survival if OPC develops.Citation30 As a result, efforts of smoking cessation are critical in an effort to reduce oral HPV infection as well as the development of OPC and other cancers.
Vaccination
In 2006, the pharmaceutical company, Merck and Co., introduced a quadrivalent HPV vaccine called Gardasil 4. The vaccine protected against HPV 6, 11, 16, 18 with the initial goal of preventing HPV-related cervical cancer in women.Citation40 Since the introduction of Gardasil 4, a bivalent (Cervarix) and a nine-valent vaccine (Gardasil 9) were also created and approved for use. Gardasil 9 is the most recently approved vaccine, and its safety was ascertained in clinical trials with over 15,000 participants prior to its FDA approval.Citation41 In addition to the original four HPV strains covered by the quadrivalent version, Gardasil 9 also protects against HPV types 31, 33, 45, 52, 58 and thus covers strains that cause over 90% of HPV-related cancers including HPV-related OPC.
By 2011, the CDC recommended routine HPV vaccination for both girls and boys between 9 and 26 y old for the prevention of cervical and anogenital HPV cancers. Ideally, the vaccine would be administered at 11 or 12 y of age with the goal of preceding sexual debut and capturing the robust immune response that is mounted at that age.Citation40,Citation42 In theory, later vaccination would be less effective as many individuals would have already contracted a persistent infection by a high-risk HPV virus prior to that time, and may therefore not be protected. However, recent studies have shown that the immune response to HPV vaccination in men aged 27–45 y was comparable to those observed in younger men but with unknown efficacy rates of preventing persistent HPV infections in these older patients.Citation43 On October 5, 2018, the FDA approved a supplemental application for the Gardasil 9 Vaccine, expanding its approved use to include women and men aged 27–45 y.
Though the CDC recognizes that persistent infection with oncogenic HPV types can cause cancers at non-cervical sites, the FDA has not officially approved the vaccine for prevention of OPCs. This contributes to a lack of awareness regarding the correlation between HPV vaccination and prevention of OPC, even among medical professionals. This was highlighted by a study in Louisiana by Mehta and colleagues surveying members of the Louisiana Chapter of the American Academy of Pediatrics.Citation44 The study demonstrated that 15.5% of the pediatricians polled were not aware of the link between OPC and HPV, and less than half knew that HPV-related OPC incidence was increasing. Despite CDC recommendations, the study found that only 89.7% of pediatricians routinely recommended the HPV vaccine, while 5.2% occasionally offered or only at caregiver request and the remaining 5.2% did not offer the vaccine at all. This, combined with the fact that less than 1% of the US population in 2014 recognized HPV as a risk factor for development of head and neck cancer,Citation45 may in part be responsible for lower vaccine uptake compared to the others in the adolescent series.
Lack of FDA approval for use of the vaccine to prevent HPV-mediated OPC stems from the inability of clinical trials to directly demonstrate vaccine efficacy against oropharyngeal HPV-related disease. Until relatively recently, regulatory agencies required a clinical disease end point for trials regarding HPV vaccine efficacy. Precancerous lesions, which would serve as a disease end point, are not established in OPCs. Furthermore, since cancer may not develop for many years after initial infection with the virus, a trial showing a correlation between increasing vaccine use and decreasing OPC rates may not be available for many decades.
In 2014, the World Health Organization recommended that efficacy against incidence and persistent HPV infection can be a surrogate for disease risk.Citation46 A number of studies have provided evidence that HPV vaccination decreases the rate of HPV-related infections and are likely to reduce the incidence of cancers of the oropharynx. A double-blind randomized controlled trial conducted in Costa Rica by Herrero et al. evaluated the efficacy of the bivalent vaccine in reducing oral HPV infection 4 y after vaccination. They observed a 93.3% reduction of prevalent oral HPV 16 and 18 infections in the vaccine arm compared to the control arm.Citation47 Another recent study by Hirth et al. concluded that vaccine-type oral HPV prevalence was lower in individuals who received the HPV vaccine compared to unvaccinated individuals.Citation48 Similar results were shown by Chaturvedi et al.Citation36 Their study demonstrated that HPV vaccination was associated with an estimated 88% reduction in prevalence of vaccine type oral HPV 6, 11, 16, 18 infections among vaccinated young adults in the US. As noted in the prior studies, findings consistently show a significant decrease in these HPV infections in the vaccinated population compared to the unvaccinated men and women in the United States. However, because of a vaccination rate of only 18.3% between 2011 and 2014 among individuals 18–33 y of age, the population-level effect of HPV vaccination on oral HPV 6,11,16,18 was a modest 17%.Citation36 Other recent studies have addressed concerns that vaccination against some but not all HPV types may introduce a competitive advantage for non-vaccine types. This would lead to an eventual decrease effectiveness of the vaccine. A study by Tota et al. obtained data from the Costa Rica Vaccine trial and PATRICIA trial to compare incidence of non-protected HPV infections across the trial arms after 4 y. Their results revealed similar or higher incidence of non-protected HPV types in the control arm compared with the HPV arm across all their analyses, which supports that type replacement is unlikely to occur among vaccinated individuals.Citation49 This further supports vaccination as a primary prevention strategy.
Currently available HPV vaccines are 90–100% effective in preventing genital HPV infections, and the global cervical cancer burden is projected to be dramatically reduced by 2050 due to both the vaccine and the improved cervical screening.Citation10 However, unlike the Papanicolaou test for cervical cancer, there is no current reliable screening method to detect precancerous OPCs. As a result, expanding the use of the HPV vaccine is that much more critical when reducing the number of patients affected by HPV-related OPC. It has been estimated that, by vaccinating boys and men, 5416 and 51,168 additional cases of HPV-related OPC would be prevented at 50 and 100 y, respectively.Citation50 Additionally, the societal cost of HPV vaccination has been predicted to be well below the $50,000/Quality-Adjusted Life Year threshold used to determine cost-effectiveness of public health initiatives.Citation51 Such evidence strongly supports continued efforts to improve vaccination rates in both girls and boys.
Screening for HPV-related OPC
As previously mentioned, the most common presenting symptom of HPV-related OPC is a lateral neck mass. Thus, many patients have developed regional metastasis at presentation, and by definition have more advanced disease. In fact, one study showed that of 1907 patients with HPV-related OPC, 73% were diagnosed with advanced locoregional disease.Citation17 As a result, there is a tremendous need to develop an approach to identify occult lesions at an earlier stage to allow for successful and less morbid treatment. For a screening technique to be beneficial, it is first necessary to determine a high-risk population. An ideal test would be cheap, minimally invasive, and appropriately sensitive to identify a high proportion of subclinical lesions (i.e. a high negative predictive value).
While there are currently no effective screening methods and no precancerous lesion correlates for HPV-related OPC, there are emerging techniques. These advances show promise through serologic testingCitation14 and imagingCitation52,Citation53 that may aid in identifying high-risk individuals as well as subclinical lesions, respectively. Here, we outline some of these approaches that may allow for a more focused assessment of those predisposed to developing HPV-related OPC.
Population screening
Population screening for OPC is difficult since at present there are no precursor lesions. Moreover, the mucosal surface of the oropharynx is much more challenging to examine than the cervix as many lesions start in the reticulated epithelium at the depth of tonsillar tissue crypts, thus concealing them from visual inspection. As a result, efforts have focused on first narrowing the population down into those at highest risk for development of disease. Men aged 50–65 y with multiple sexual partners would be an appealing target demographic for screening programs given the higher incidence of these tumors in this demographic group.Citation33 However, further definition of the true level of risk of such a group is currently under investigation.Citation54
Oral HPV screening
An initial approach considered for assessment was to use oral HPV screening. This technique evaluates for the presence of HPV DNA in saliva and can be even used to focus specifically on high-risk HPV such as HPV 16. However, while this technique can be effective in demonstrating active HPV infection, it is of little utility in screening for HPV-related OPC as the majority of individuals either go on to clear the infection or fail to progress to malignancy. Consequently, the use of oral HPV screening has been discouraged as a screening technique to identify OPC.Citation55
HPV serology
One area that has shown particular promise in assessing high-risk populations is to screen for serum antibodies to HPV 16 proteins. Such an approach may allow for at-risk individuals to be identified prior to progression of disease. In an early study by Mork et al., serum positivity to the L1 capsid protein of HPV 16 conferred a 14-times increased risk of developing OPC, when linking findings from a Nordic serum bank and tumor registries.Citation56 While these findings were encouraging, these antibodies represent the body’s cumulative exposure to HPV 16 and are not specific to anatomic site. Moreover, they do not reflect expression of HPV oncoproteins necessary for carcinogenesis.
In 2013, Kreimer and colleagues identified that serum antibodies to the E6 oncoprotein of HPV 16 were a better marker for predicting cancer.Citation38 In this important study, participants came from the European Prospective Investigation Into Cancer and Nutrition cohort and 638 patients who went on to develop head and neck cancer and 1599 controls with no evidence of cancer were evaluated. Pre-diagnostic serum samples were collected and analyzed for antibodies against multiple HPV 16 proteins as well as other subtypes of HPV. All told, HPV 16 E6 seropositivity conferred a 274 times increased risk of developing OPC, being present in 34.8% of OPC patients compared to 0.6% in other head and neck cancer patients and control patients. This positive finding was found on average 6 y before diagnosis and was observed in some instances more than 10 y before the cancer was found. Such seropositivity has been shown to result in a 10-y cumulative risk of developing HPV-related OPC of 6.2% in men and 1.3% in women, compared to 0.04% in seronegative controls.Citation57 Additional study has also shown that higher pretreatment HPV 16 E6 antibody titers can predict recurrence, while E6 and E7 titers decreased when reassessed in the early and late posttreatment setting.Citation58
Transcervical ultrasound
In individuals with HPV-related cancer in a neck mass, as well as those found to be high risk by either positive serology screening or other methods, transcervical ultrasound can be used as an additional means for assessment. The use of ultrasound has long been standard in the evaluation of thyroid lesions and cervical adenopathy, and it can aid in guidance of fine needle aspirations, especially in cystic nodal disease like what is often observed in HPV-related cancers.
In addition to assessment of nodal disease, the use of transcervical ultrasound has now been applied to evaluate the oropharynx in an effort to identify occult tumors, especially those developing in crypts of the palatine and lingual tonsils, not visible on surface examination.Citation59,Citation60 While such an approach to screening is not widely utilized, at present, it is particularly appealing in that it is relatively cheap, minimally invasive, and results in no exposure to ionizing radiation or intravenous contrast.
Mucosal imaging
The lack of an identifiable early lesion presents a significant challenge when evaluating for premalignant and malignant lesions of the oropharynx. The conventional approach following a head and neck physical examination is to offer fiber-optic nasopharyngoscopy using standard white light imaging (WLI). While the optics of contemporary endoscopes allows for improved definition and magnification, small and superficial lesions may be missed due to the subtle difference in appearance of normal and abnormal mucosa. Two techniques that have been applied to augment this aspect of mucosal screening are narrow band imaging (NBI) and endoscopic lifetime imaging.
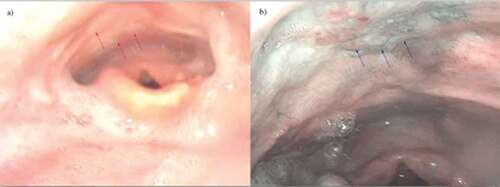
NBI was first described for use in the gastrointestinal tract by Sano in 2001.Citation61 Using this technology, filters of spectral regions centered at 415 nm (blue light) and 540 nm (green light) are used, excluding other signals from visible spectrum, thus highlighting the vascularity near the tissue surface. Images are obtained through the nasopharyngoscope that can allow for identification of subclinical lesions (). Such an approach was used in a multicentered randomized controlled trial and found NBI detected superficial mucosal lesions of the head and neck more often than WLI (100% vs. 8%) and had a sensitivity and accuracy of finding lesions of 100% and 86.7%, respectively.Citation62 This technique has also been shown to be helpful in identifying the primary lesion in patients with neck cancer with an unknown head and neck primary site. One study by Ni and colleagues evaluated 53 patients with cervical lymph node metastases with no identifiable primary site seen on physical examination, computed tomography scan (CT), magnetic resonance imaging scan (MRI), and laryngoscopy. The use of NBI allowed for identification of lesions in 47% patients.Citation63 Similarly, Hayashi et al. used NBI to screen 46 of such patients and a primary was identified in 35% of individuals. The study did not report specifically on percentage of lesions positive for HPV.Citation64
Figure 2. Patient with T1N2A oropharyngeal cancer status post definitive chemoradiation. (a) Distal chip video laryngoscopy revealed post-treatment radiation edema with no evidence of mucosal disease delineated by red arrows. (b) NBI revealed a mucosal abnormality delineated by blue arrows. Biopsy was consistent with high-grade dysplasia. Image is borrowed with permission from Dr Peter Belafsky.
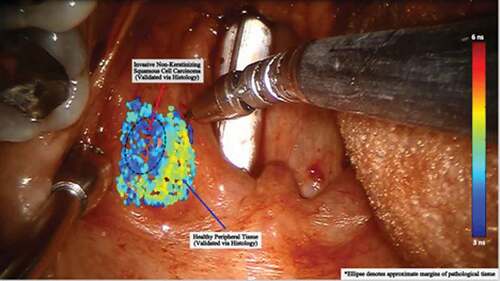
Lifetime-resolved laser-induced imaging is another approach that has shown recent promise in mucosal imaging. Using this technique, laser energy is applied to a surface and the reflected signal representing the induced autofluorescence of the tissue is captured. Data have shown that the autofluorescence characteristics of normal, dysplastic, and cancerous head and neck mucosa are different, suggesting that this technique may have a role in cancer screening ().Citation65,Citation66 Despite their promise, all mucosal imaging techniques have limitations in OPC screening as many tumors originate in the depths of tonsillar crypts and thus are not well seen on surface evaluation.
Figure 3. This intraoperative image shows the use of lifetime tissue autofluorescence for identification of pathologic mucosa. A laser is aimed at the surface and the signal returning from the tissue is interpreted. The signal for pathologic mucosa has been shown to be distinctly different than normal and is demonstrated by a different shade on the color scale. Such an approach could be very useful in cancer screening. However, small submucosal lesions may avoid detection.
Future directions
There are currently no reliable biomarkers that can be used for tumor screening or to evaluate for cancer recurrence for head and neck cancer. With increased availability of robust sequencing technology, however, methods have been developed looking at saliva and serum tumor DNA as a potential approach. This concept of measuring serum DNA is particularly exciting as it would represent a minimally invasive way to assess for subclinical lesions and monitor for disease response and recurrence. Circulating cell-free DNA (cfDNA) has been proposed as one such method. This approach centers around the fact that when tissue dies, its DNA is released into serum and circulates extracellularly prior to being metabolized over the next 15–20 min. Consequently, any analysis provides a reflection of tissue undergoing rapid turnover as can be seen in active malignancy. Virally mediated cancers are particularly well suited for such “liquid biopsies” as they have known viral DNA incorporated into the host DNA that can be queried. In fact, this approach has demonstrated tremendous promise in screening for Epstein-Barr virus (EBV)-related nasopharyngeal cancer.Citation67 In a trial where 20,174 asymptomatic participants were screened, 309 individuals were found to have persistently elevated levels and 300 ultimately went on to undergo nasal endoscopy with or without MRI. Thirty-four of those subjected to further screening were found to have nasopharyngeal cancer, with the majority being either stage I or II, while only one patient with a negative initial test is found to have a nasopharyngeal cancer in the year following screening, thus yielding a sensitivity and specificity of the technique of 97.1% and 98.6%, respectively.
HPV-related OPC also appears appropriate for assessment with cfDNA. Such tumors often have nodal metastases with significant necrosis, thus likely shedding large amounts of tumor DNA. In addition, with the known component of the HPV DNA, as well as other differentiating patterns such as DNA methylation and fragment size, techniques are under development that may soon allow for early identification of these lesions through blood analysis. Wang et al. studied the potential for looking at tumor-specific DNA in saliva and serum in head and neck cancer patients.Citation68 They found oral cancer was more likely to show elevation in saliva tumor DNA while tumors in the oropharynx were more likely to have elevations in their serum levels. Additionally, the combined use of posttreatment saliva and plasma HPV 16 DNA positivity was found to be 69.5% sensitive and 90.7% specific in predicting recurrence within 3 y of therapy completion, suggesting a potential role in surveillance.Citation69 Similar findings were observed in a recent publication by Hanna and colleagues where HPV cfDNA levels correlated with total tumor burden, and higher levels as well as higher total tumor burden resulted in worse overall survival.Citation70 Moreover, using this method, cfDNA levels were also found to have a corresponding change at a median of 16 d prior to restaging scans reflecting either disease response or progression. With the promise seen from these early results, such methods are being used to prospectively evaluate high-risk individuals to determine if early lesions can be identified prior to symptom development. While these approaches alone would not be sufficient for surveillance, it could help augment current surveillance techniques such as history, physical examination, transcervical ultrasound, and positron emission tomography imaging.
Limitations
Since this paper is a narrative review, there is a lack of objective and systematic selection criteria for the papers included. Papers included in discussing the topic range from case reports to randomized controlled trials and therefore vary in level of evidence. While we chose to only use papers which we felt provided high quality of information, lack of methodological selection of these papers lead to bias of our interpretation and conclusions.
Conclusions
HPV-related OPC carries significant morbidity, mortality, and substantial cost to the health-care system. With its increasing incidence, importance should be placed on prevention of this disease and early diagnosis through effective screening techniques. While new screening methods are still underway, emphasis should be placed on prevention through greater public awareness of risk factors associated with HPV, behavioral changes to mitigate these risks, and widespread use of the vaccine. Furthermore, while HPV-related OPC has improved survival compared to HPV-negative OPC, it is important to remember that tobacco users have significantly worse disease control with treatment. Although HPV-vaccination efforts show promise in protecting against oral HPV infection, clinical trials supporting vaccine efficacy against oropharyngeal HPV-related disease are currently lacking. This has contributed to a delay in the FDA’s approval of the vaccine for prevention of HPV-related OPC. Therefore, further research in showing vaccine efficacy is critical. For those already at risk due to prior exposure, development of effective screening techniques will be crucial to allow for early detection of subclinical lesions. While some methods appear useful in screening those in high-risk cohorts, other techniques have application in individual patient assessment as well as in disease surveillance. Further research is needed to determine the optimal means to combine these and other methods to allow for optimal disease prevention and early detection on a larger scale.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387.
- McLaughlin-Drubin ME, Munger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143(2):195–208. doi:10.1016/j.virusres.2009.06.008.
- Ducatman BS. The role of human papillomavirus in oropharyngeal squamous cell carcinoma. Arch Pathol Lab Med. 2018;142(6):715–18. doi:10.5858/arpa.2018-0083-RA.
- Munoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24(Suppl 3):S3/1–10. doi:10.1016/j.vaccine.2006.05.115.
- Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50(5):370–79. doi:10.1016/j.oraloncology.2013.11.004.
- American cancer society: cancer facts & figures. Atlanta, GA; 2017.
- Van Dyne EA, Mona Saraiya SJH, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus–associated cancers — United States, 1999–2015. 2018 [accessed 2018]. https://www.cdc.gov/mmwr/volumes/67/wr/mm6733a2.htm?s_cid=mm6733a2_w.
- Jemal A, Simard EP, Dorell C, Noone A-M, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et al. Annual report to the nation on the status of cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi:10.1093/jnci/djs491.
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–19. doi:10.1200/JCO.2007.14.1713.
- Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235–42. doi:10.1200/JCO.2015.61.6995.
- Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital. 2014;34(5):299–309.
- Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012;6(Suppl 1):S16–24. doi:10.1007/s12105-012-0377-0.
- Gillison ML, D'Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–20. doi:10.1093/jnci/djn025.
- Gillison ML, Alemany L, Snijders PJ, Chaturvedi A, Steinberg BM, Schwartz S, Castellsagué X. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(Suppl 5):F34–54. doi:10.1016/j.vaccine.2012.05.070.
- Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, Graubard BI, Chaturvedi AK. Prevalence of oral HPV infection in the United States, 2009-2010. Jama. 2012;307(7):693–703. doi:10.1001/jama.2012.101.
- McIlwain WR, Sood AJ, Nguyen SA, Day TA. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(5):441–47. doi:10.1001/jamaoto.2014.141.
- O‘Sullivan B, Huang SH, Su J, Garden AS, Sturgis EM, Dahlstrom K, Lee N, Riaz N, Pei X, Koyfman SA, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440–51. doi:10.1016/S1470-2045(15)00560-4.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi:10.1056/NEJMoa0912217.
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–69. doi:10.1093/jnci/djn011.
- Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97(8):701–07. doi:10.1002/jso.21012.
- Ward G, Mehta V, Moore M. Morbidity, mortality and cost from HPV-related oropharyngeal cancer: impact of 2-, 4- and 9-valent vaccines. Hum Vaccin Immunother. 2016;12(6):1343–47. doi:10.1080/21645515.2015.1095415.
- Tolentino Ede S, Centurion BS, Ferreira LHC, Souza APD, Damante JH, Rubira-Bullen IRF. Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients. J Appl Oral Sci. 2011;19:448–54.
- Irune E, Dwivedi RC, Nutting CM, Harrington KJ. Treatment-related dysgeusia in head and neck cancer patients. Cancer Treat Rev. 2014;40(9):1106–17. doi:10.1016/j.ctrv.2014.06.011.
- Kocak-Uzel E, Gunn GB, Colen RR, Kantor ME, Mohamed ASR, Schoultz-Henley S, Mavroidis P, Frank SJ, Garden AS, Beadle BM, et al. Beam path toxicity in candidate organs-at-risk: assessment of radiation emetogenesis for patients receiving head and neck intensity modulated radiotherapy. Radiother Oncol. 2014;111(2):281–88. doi:10.1016/j.radonc.2014.02.019.
- Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay M, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. doi:10.1016/S1470-2045(10)70290-4.
- McBride SM, Parambi RJ, Jang JW, Goldsmith T, Busse PM, Chan AW. Intensity-modulated versus conventional radiation therapy for oropharyngeal carcinoma: long-term dysphagia and tumor control outcomes. Head Neck. 2014;36(4):492–98. doi:10.1002/hed.23319.
- Vatca M, Lucas JT, Laudadio J, D’Agostino RB, Waltonen JD, Sullivan CA, Rouchard-Plasser R, Matsangou M, Browne JD, Greven KM, et al. Retrospective analysis of the impact of HPV status and smoking on mucositis in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemotherapy and radiotherapy. Oral Oncol. 2014;50(9):869–76. doi:10.1016/j.oraloncology.2014.06.010.
- Chera BS, Amdur RJ, Tepper J, Qaqish B, Green R, Aumer SL, Hayes N, Weiss J, Grilley-Olson J, Zanation A, et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2015;93(5):976–85. doi:10.1016/j.ijrobp.2015.08.033.
- Lorch JH, Hanna GJ, Posner MR, O’Neill A, Thotakura VL, Limaye SA, Rabinowits G, Sher DJ, Tishler RB, Haddad RI. Human papillomavirus and induction chemotherapy versus concurrent chemoradiotherapy in locally advanced oropharyngeal cancer: the Dana Farber Experience. Head Neck. 2016;38(Suppl 1):E1618–24. doi:10.1002/hed.24289.
- Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, Wolf GT, Prince ME, Moyer JS, Teknos TN, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16(4):1226–35. doi:10.1158/1078-0432.CCR-09-2350.
- Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, Spencer S, Harris J, Chung CH, Ang KK. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102–11. doi:10.1200/JCO.2011.38.4099.
- Lairson DR, Wu C-F, Chan W, Dahlstrom KR, Tam S, Sturgis EM. Medical care cost of oropharyngeal cancer among texas patients. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1443–49. doi:10.1158/1055-9965.EPI-17-0220.
- Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the annual direct medical costs of the prevention and treatment of disease associated with human papillomavirus in the United States. Vaccine. 2012;30(42):6016–19. doi:10.1016/j.vaccine.2012.07.056.
- Moore EJ, Hinni ML, Olsen KD, Price DL, Laborde RR, Inman JC. Cost considerations in the treatment of oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2012;146(6):946–51. doi:10.1177/0194599812437534.
- Healthy people 2020 [accessed 2018 Sept]. https://www.healthypeople.gov/node/3513/data-details.
- Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong Z-Y, Xiao W, Kahle L, Gillison ML. Effect of prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262–67. doi:10.1200/JCO.2017.75.0141.
- Sexually transmitted disease surveillance 2017. [ accessed 2019 Jan 13]. https://www.cdc.gov/std/stats17/other.htm.
- Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, Tjønneland A, Overvad K, Quirós JR, González CA, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708–15. doi:10.1200/JCO.2012.47.2738.
- Gupta A, Perkins RB, Ortega G, Feldman S, Villa A. Barrier use during oro-genital sex and oral human papillomavirus prevalence: analysis of NHANES 2009–2014. Oral Dis. 2019;25(2):609–16.
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63:1–30.
- Vesikari T, Brodszki N, van Damme P, Diez-Domingo J, Icardi G, Petersen LK, Tran C, Thomas S, Luxembourg A, Baudin M. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus gardasil(R) in 9-15-Year-old girls. Pediatr Infect Dis J. 2015;34(9):992–98. doi:10.1097/INF.0000000000000773.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–11. doi:10.1056/NEJMoa0909537.
- Giuliano AR, Isaacs-Soriano K, Torres BN, Abrahamsen M, Ingles DJ, Sirak BA, Quiterio M, Lazcano-Ponce E. Immunogenicity and safety of Gardasil among mid-adult aged men (27-45 years)–the MAM Study. Vaccine. 2015;33(42):5640–46. doi:10.1016/j.vaccine.2015.08.072.
- Mehta V, Holmes S, Master A, Leblanc B, Caldito LG, Bocchini J. Knowledge of HPV-related oropharyngeal cancer and use of human papillomavirus vaccines by pediatricians in Louisiana. J La State Med Soc. 2017;169:37–42.
- Luryi AL, Yarbrough WG, Niccolai LM, Roser S, Reed SG, Nathan C-AO, Moore MG, Day T, Judson BL. Public awareness of head and neck cancers: a cross-sectional survey. JAMA Otolaryngol Head Neck Surg. 2014;140(7):639–46. doi:10.1001/jamaoto.2014.867.
- Lowy DR, Herrero R, Hildesheim A. Primary endpoints for future prophylactic human papillomavirus vaccine trials: towards infection and immunobridging. Lancet Oncol. 2015;16(5):e226–33. doi:10.1016/S1470-2045(15)70075-6.
- Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329. doi:10.1371/journal.pone.0068329.
- Hirth JM, Chang M, Resto VA. Prevalence of oral human papillomavirus by vaccination status among young adults (18-30years old). Vaccine. 2017;35(27):3446–51. doi:10.1016/j.vaccine.2017.05.025.
- Tota JE, Struyf F, Merikukka M, Gonzalez P, Kreimer AR, Bi D, Castellsagué X, de Carvalho NS, Garland SM, Harper DM, et al. Evaluation of type replacement following HPV16/18 vaccination: pooled analysis of two randomized trials. J Natl Cancer Inst. 2017;109(7). doi:10.1093/jnci/djx007.
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–67. doi:10.1016/j.vaccine.2010.08.030.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28. doi:10.1111/j.1524-4733.2004.75003.x.
- Blanco RG, Califano J, Messing B, Richmon J, Liu J, Quon H, Neuner G, Saunders J, Ha PK, Sheth S, et al. Transcervical ultrasonography is feasible to visualize and evaluate base of tongue cancers. PLoS One. 2014;9(1):e87565. doi:10.1371/journal.pone.0087565.
- Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila). 2011;4(9):1378–84. doi:10.1158/1940-6207.CAPR-11-0284.
- ClinicalTrials.gov. National Library of Medicine (U.S.). HPV related oropharyngeal and uncommon cancers screening. Identifier NCT0289742; 2017 Mar 28 [accessed 2019 Jan 11]. https://clinicaltrials.gov/ct2/show/NCT02897427?id=NCT02897427&rank=1&load=cart.
- AHNS. AHNS prevention & early detection committee position statement on early detection of pre-malignant oral cancer. Council AE, Editor. American Head and Neck Society; 2017.
- Mork J, Lie AK, Glattre E, Hallmans G, Jellum E, Koskela P, Møller B, Pukkala E, Schiller JT, Youngman L, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–31. doi:10.1056/NEJM200104123441503.
- Kreimer AR, Johansson M, Yanik EL, Katki HA, Check DP, Lang Kuhs KA, Willhauck-Fleckenstein M, Holzinger D, Hildesheim A, Pfeiffer R, et al. Kinetics of the human papillomavirus type 16 e6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst. 2017;109(8). doi:10.1093/jnci/djx007.
- Fakhry C, Qualliotine JR, Zhang Z, Agrawal N, Gaykalova DA, Bishop JA, Subramaniam RM, Koch WM, Chung CH, Eisele DW, et al. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratification and recurrence of HPV-associated oropharyngeal cancer. Cancer Prev Res (Phila). 2016;9(2):135–41. doi:10.1158/1940-6207.CAPR-15-0299.
- Coquia SF, Hamper UM, Holman ME, DeJong MR, Subramaniam RM, Aygun N, Fakhry C. Visualization of the oropharynx with transcervical ultrasound. AJR Am J Roentgenol. 2015;205(6):1288–94. doi:10.2214/AJR.15.14299.
- Mydlarz WK, Liu J, Blanco R, Fakhry C. Transcervical ultrasound identifies primary tumor site of unknown primary head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2014;151(6):1090–92. doi:10.1177/0194599814549181.
- Sano Y,KM, Hamamoto Y. New diagnostic method based on color imaging using narrowband imaging (NBI) system for gastrointestinal tract. Gastrointest Endosc J. 2001;53:AB125.
- Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28(9):1566–72. doi:10.1200/JCO.2009.25.4680.
- Ni XG, Cheng -R-R, Lai S-Q, Zhang L, He S, Zhang Y-M, Wang G-Q. [Value of narrow band imaging endoscopy in the detection of unknown primary site with cervical lymph node metastasis of squamous cell carcinoma]. Zhonghua Zhong Liu Za Zhi. 2013;35:698–702.
- Hayashi T, Muto M, Hayashi R, Minashi K, Yano T, Kishimoto S, Ebihara S. Usefulness of narrow-band imaging for detecting the primary tumor site in patients with primary unknown cervical lymph node metastasis. Jpn J Clin Oncol. 2010;40(6):537–41. doi:10.1093/jjco/hyp197.
- Meier JD, Xie H, Sun Y, Sun Y, Hatami N, Poirier B, Marcu L, Farwell DG. Time-resolved laser-induced fluorescence spectroscopy as a diagnostic instrument in head and neck carcinoma. Otolaryngol Head Neck Surg. 2010;142(6):838–44. doi:10.1016/j.otohns.2010.02.005.
- Sun Y, Phipps JE, Meier J, Hatami N, Poirier B, Elson DS, Farwell DG, Marcu L. Endoscopic fluorescence lifetime imaging for in vivo intraoperative diagnosis of oral carcinoma. Microsc Microanal. 2013;19(4):791–98. doi:10.1017/S1431927613001530.
- Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, Chu SWI, Mak C, Tse IOL, Leung SYM, et al. Analysis of plasma epstein-barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377(6):513–22. doi:10.1056/NEJMoa1701717.
- Wang Y, Wang Y, Springer S, Mulvey CL, Silliman N, Schaefer J, Sausen M, James N, Rettig EM, Guo T, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7(293):293ra104. doi:10.1126/scitranslmed.aad3106.
- Ahn SM, Chan JYK, Zhang Z, Wang H, Khan Z, Bishop JA, Westra W, Koch WM, Califano JA. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2014;140(9):846–54. doi:10.1001/jamaoto.2014.1338.
- Hanna GJ, Supplee JG, Kuang Y, Mahmood U, Lau CJ, Haddad RI, Jänne PA, Paweletz CP. Plasma HPV cell-free DNA monitoring in advanced HPV-associated oropharyngeal cancer. Ann Oncol. 2018;29:1980–86. doi:10.1093/annonc/mdy251.