ABSTRACT
The Arthus reaction is a rare adverse reaction that usually occurs after vaccination with large and more severe local reactions, belonging to type Ⅲ hypersensitivity reaction. This reaction is characterized by pain, swelling, induration (Tissue that becomes firm) and edema, even accompanied by severe necrosis or ulceration at the injection sites. However, most of mild cases generally can be cured without treatment, and only severe cases need to be treated with anti-allergy. Therefore, this adverse reaction is often ignored by people.
We searched PubMed, Web of Science and Chinese database (CNKI database and Wan Fang database) for published studies using the terms “Arthus reaction” or “Arthus phenomenon”, combined with “vaccine”, with no date or language restrictions for all publications before January 28, 2019. Only 30 cases of Arthus reaction were found, of which only one case died.4 cases of Arthus reaction post-dose-1 were reported in the review. The proportion of Arthus reaction occurred after the first, second and third injections in those case reports was 13.3%, 50.0%, and 23.3%, respectively. Arthus reaction was determined according to the clinical symptoms (The symptoms which were observed by the researchers, such as red, swelling and painful with itching at or around the injection sites). The specific causes of Arthus reaction after one dose of vaccination are not described in detail in literatures. Therefore, it could be hypothesized that the case has a pre-existing specific IgG (Such as pre-existing antibody, etc.) to cause the Arthus reaction.
And 17 reported cases were observed in children younger than 6 y. In addition, we collected only 18 cases of bacterial vaccine-induced Arthus reaction and 12 cases of viral vaccines. However, there are no other data (Such as the total number and incidence rate of vaccination) in literatures, so we cannot compare statistically significant differences. At presents, no previous reviews of vaccine-induced Arthus reaction have been found. Thus, a systematic review about vaccine-associated Arthus reaction is urgently needed to deepen people‘s understanding and concern of this phenomenon. In this manuscript, we retrospectively reviewed the description of the discovery process and mechanisms of Arthus reaction, a description of the characteristics of Arthus reaction cases, reporting the Arthus reaction cases in China during 2010–2015, diagnostic criteria and general treatment, preventive measures of Arthus reaction, and challenges remaining to be investigated in the future.
KEYWORDS:
Introduction
In the past century, the vaccine-preventable infectious diseases have been completely eradicated (Smallpox) or hugely reduced (Such as, polio, measles, pertussis, and diphtheria) with the widespread use of vaccines. Since the threats of these infectious diseases are no longer feared, the public and mass media pay more attention to vaccine safety in current era. The Adverse Events Following Immunization (AEFI) surveillance system mainly reports adverse reactions or events, which include fever, local redness and swelling, induration, Arthus reaction, and other symptoms.Citation1 Yet the Arthus reactions have been infrequently and rare reported after vaccinations.Citation2,Citation3
The Arthus phenomenon was first noted in rabbits; subsequently, similar reactions were observed in a variety of animal species, such as, guinea pigs, rats, and dogs, as well as in humans.Citation4,Citation5 This phenomenon can be elicited by vaccines, drugs (Insulin injection), insect bites, and so on.Citation6 Repeated injections of certain vaccines is prone to occurred Arthus reaction, and this phenomenon is usually characterized by localized acute small-vessel inflammation at the injection site.Citation7 The most common clinical manifestations of Arthus reaction are local tissue hardening, accompanied by obvious redness, swelling, and pain, diameter less than 5.0 cm at or around the site following the injection in mild cases, but in some severe cases the diameter of the redness or swelling can spread to the entire upper arm or extend to the injected upper arm from shoulder to elbow.Citation8 Those clinical manifestations usually can persist for one week, or even persisting for few months, without scar left after healing. However, the mild necrosis at the injection site, sclerosis in the deep tissue, and even severe necrosis and ulceration in local tissues, skin, and muscles can be found in severe cases. Nevertheless, the relatively low incidence of the Arthus reaction is often leading to a neglection in the vaccine clinical studies and routine vaccination practice. Here, we briefly describe the discovery process and mechanisms of the Arthus reaction, and summarize the Arthus reaction reported to deepen people‘s understanding and concern of this phenomenon.
What are the discovery process and mechanisms of Arthus reaction?
The Arthus reaction is a localized inflammatory response, belonging to a typical local subacute type III hypersensitivity reaction. The Arthus reaction was discovered by Nicolas Maurice Arthus in 1903Citation9–Citation11”: Arthus subcutaneously injected 5 cc. of horse serum into rabbits per 6 d. After the third injection, the slight infiltration was observed at the injection site, persisting 2 or 3 d. After the fourth injection, he noted that a local oedematous reaction occurred and the absorption of serum became slow. After the fifth injection, the infiltration became more severe, and edematous did not disappear until after 5 or 6 d. After the sixth injection a white (not containing pus and absolutely sterile), solid, compact and thick mass was produced in the subcutaneous cellular tissue, lasting for several weeks. After the seventh injection the skin became rapidly red, then blanched and dried, and even leading to a gangrenous plaque. The general condition of the rest of the animal (Without the injection of 5 cc. horse serum) is good.” This phenomenon was soon repeated in many laboratories, and was entitled the Arthus phenomenon (Or Arthus reaction) by Nicolle in 1907.Citation12
The mechanism of this phenomenon has been investigated in many studies.Citation13–Citation18 This reaction is mediated by intradermal injections of antigen into a local skin site of an animal subject preexisting large amounts of immunoglobulin G (IgG) antibodies specific to the antigen. The antigen will diffuse into the walls of local blood vessels and the immune complexes which are comprised of antigen, antibody, and complement in vessels precipitating close to the injection site. Subsequently, the C3a, C4a, and C5a complements are activated by the immune complexes, and the activation of FcγRIII (Binding immune complexes) on the localized mast-cells induces their degranulation with an increase in local vascular permeability, resulting in an inflammatory response. Besides the chemotactic factors C3a, C5a, and C5b67 attract large numbers of neutrophils to immune-complexes followed by lytic enzymes release, which can lead to the injury of vessel walls with the development of hemorrhage, edema, thrombi, local ischemia, and necrosis. Eventually, the series actions can lead to localized tissue and vascular damage, which are characterized by redness, swelling, pain, erythema, central blanching, induration, petechiae, and occasionally by tissue necrosis.” These signs and symptoms usually begin 2–12 h after exposure to the antigen, develop gradually in the next few hours and most of them resolve within one week without sequelae.Citation19
Case of vaccine-related arthus reaction
Literature search
We searched PubMed and Web of Science databases for published studies using the terms (MeSH terms, title terms, and abstract terms) “Arthus reaction” or “Arthus phenomenon”, combined with “vaccine” in English language, with no date or language restrictions for all publications before January 28, 2019. Published Chinese studies or reports on Arthus reactions post-vaccination were also identified from the CNKI database and Wan Fang database using the same search terms and strategy.
Inclusion and exclusion criteria
We included the published literatures on Arthus reactions in this reviews by following inclusion criteria: (1) studies provided sufficient data to describe the Arthus reaction/phenomenon; (2) Arthus reaction following the vaccination or immunization; (3) Original case reports or clinical studies. The exclusion criteria were as follows: (1) for duplicate publications; (2) irrelevant papers; (3) Arthus reaction in animal experiments; (4) Arthus reaction/phenomenon not caused by vaccination/immunization.
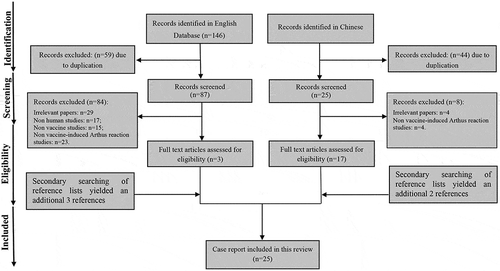
A total of 146 relevant literatures were found in English language database (Pubmed and Web of Science), and 87 of them were included after excluding duplicate documents (). Then, a total of 84 literatures were excluded due to the reports of irrelevant papers (n = 29), Arthus reaction in animals (n = 17), non-vaccine studies (n = 15) and Arthus reaction/phenomenon not caused by vaccination/immunization (n = 23). 3 studies were included after reviewing the full texts of the remaining literatures. Secondary searching of reference lists yielded an additional three references. On the other hand, 69 relevant Chinese literatures were found in CNKI database and Wan Fang database. Then, 44 duplicate literatures and 8 literatures were excluded for some reasons. The main reasons for exclusion of studies are provided in . Secondary searching of reference lists yielded an additional two references. There were 6 studies in English database and 19 studies in Chinese database. Finally, 25 literatures met the inclusion criteria, and only 30 cases were found in the 25 literatures to the case reports of vaccine-related Arthus reaction in human beings (1 of the 25 literatures reported six cases of Arthus reactionCitation20) ().
Figure 1. Flow diagram of the process of literature selection on vaccine-induced Arthus reaction.
English database only includes Pubmed and Web of Science, and Chinese database includes Wanfang database and CNKI database.
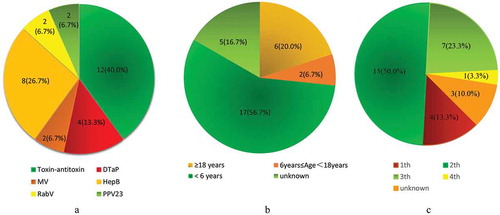
shows the details of the cases of vaccine-related Arthus reaction are recorded, including type of vaccines, author/reference/year of reporting, country, age, gender, schedule (injection times), dosage, inoculation sites or (and) routes, main manifestation of local skin and outcome. The Arthus reactions reported in English literatures were mainly before 1980, while in Chinese literatures were after 1980. One possible reason for this is that the China Ministry of Health (MOH) issued guidance for handling vaccine adverse reactions in 1980, while Arthus reaction has been discovered and studied in Western a long time ago (before 1980).Citation21 The most common clinical symptoms were redness, swelling, and induration around the injection site. Among these reported Arthus reaction cases, 29 cases recovered, and only 1 death case was reported ().Citation3,Citation10,Citation22–Citation26 In 1934, Ross FE reported that a 4.5-y-old boy suffered from local necrosis after multiple subcutaneous injections of tetanus antitoxin. He described the occurrence of a characteristic set of typical symptoms. These symptoms appear to be due to an allergic reaction between antibodies and specific antigens, resulting in the formation of toxic substances that cause local necrosis in the tissues. A few days later, the boy died of sepsis that night.Citation22 Most case reports of vaccine-related Arthus reactions mainly caused by Toxin-antitoxin (12, 40.0%), HepB (8, 26.7%), and DTaP (4, 13.3%). While the proportion of the case reports which caused by other vaccines is much lower, such as Rabies Vaccine (RabV, 2[6.7%]), 23-valent Pneumococcal Polysaccharide Vaccine (PPV23, 2[6.7%]), attenuated measles vaccine (MV, 2[6.7%]; ). The majority of reported cases were observed in children younger than 6 y (17, 56.7%), which may be associated with the situation that most recommended immunizations are administrated in children under 6 y of age.Citation27 In addition, 2 (6.7%) Arthus reaction cases were reported in children 6–18 y of age, and 6(20.0%) were found in adults age over 18 y (). Besides, we found that most of the Arthus phenomenon cases followed the booster injections (Which add up to 76.6%).
Table 1. Case reports of vaccine-related Arthus reaction.
The Arthus reaction in China‘s AEFI system during 2010–2015
The AEFI is a surveillance system to record medical incidents that take place after the immunizations, causes concern, and is believed to be caused by immunizations.Citation28 In China, the National surveillance program of AEFI was issued in 2010. During 2010–2015, a total of 103 cases of Arthus reactions were reported to the AEFICitation29–Citation33(). The highest annual reporting rate was 0.02 per million doses in 2010, while the lowest one was 0.0002 per million doses in 2013Citation29,Citation32 (). Moreover, cases of Arthus phenomenon were more prone to occur in DTaP, DTP, DT, HepB, and Rab-V (). Besides, the other vaccines were rarely reported, such as killed-virus & attenuated measles vaccine, Rabies Vaccine (Rab-V), 23-valent pneumococcal polysaccharide vaccine (PPV23), and influenza A (H1N1) vaccine, and so on.Citation24,Citation25,Citation29–Citation39 The overall incidence of Arthus reactions was very low compared with other common vaccine-related injection-site reactions. However, we should be aware that the AEFI is a passive surveillance system, and it is possible that some Arthus reaction cases may not be captured, and the incidence of Arthus reaction may be biased by underreporting.
Table 2. Number and Estimated Incidence Rate (Per million Doses) of Arthus reaction by vaccine and diagnosis in China, 2010–2015.
What are the diagnostic criteria, general treatment, and preventive measures of arthus reaction?
Nowadays, the diagnostic criteria and principles of management of the Arthus reaction caused by vaccines have not been clearly defined. In general, the cases of Arthus reaction were diagnosed by physicians or expert panel according to the subject‘s vaccination status (e.g., immunization program, dose, injection site), main clinical manifestations (e.g., limb local swelling, induration, and other symptoms), and other relevant information. Besides, they also conducted an individual causality assessment-to comprehensive determine whether it belongs to the Arthus reaction.
Most Arthus reactions reported were mild and usually self-limited and generally did not need treatment. However, for some moderate cases, they could be treated with anti-allergy drugs as required, such as diphenhydramine, 25 to 50 mg per adult, 0.5 to 1.0 mg/kg per child, 3 times per day, or promethazine, 25 to 50 mg per adult, 1.0 mg/kg per child, 3 times per day. For severe cases with tissue necrosis could be taken cortisone 2 times a day, each 50 mg, intramuscular injection, once a week.Citation9,Citation10 Meanwhile, keeping the injected site clean to prevent secondary infection and promote the regeneration of necrotic tissue. Some treatments also could relieve symptoms (swelling or pain), such as cold compresses cold the affected limb around the injection site, acetaminophen and limb elevation.Citation19
Some studies found that the pre-antibody level of individual, dosage of vaccine, and booster immunization may be related to the occurrence of the vaccine-induced Arthus reaction.Citation3 Theoretically, we could prevent this phenomenon by controlling these risk factors for individuals. Although we invested a lot of manpower, material resources and money to implement these preventive measures, the benefits may be extremely low due to the Arthus reaction is a rare adverse reaction. Thus, it is an economical and practical way to take precautions for vaccinator who developed Arthus reaction. In order to prevent this phenomenon, it may need to be spaced at longer intervals and antibody levels should be monitored to determine when boosting is needed for those individuals. For example, Advisory Committee on Immunization Practices (ACIP) has recommended that persons who experienced an Arthus reaction after a dose of tetanus toxoid–containing vaccine should not receive Td more frequently than every 10 y, even for tetanus prophylaxis as part of wound management.Citation2 Besides, if persons experienced an Arthus reaction after diphtheria toxoid-containing vaccine, they should use tetanus toxoid for booster to reduce the occurrence of Arthus reaction rather than Tdap.Citation40 Moreover, some experts recommend that if Arthus reaction occurs at the initial dose in the primary infant series in a child under 6 months, the next doses need to be postponed for several months until the reduction of sero-antibodies. If the child is younger than 6 months when the second dose expires, this should be deferred until the child is 6 months of age; the third dose should be given 2 months apart. If the following dose is at the age of 6 months, there is no need to delay because circulating maternal antibodies will be significantly reduced.Citation41
Challenges and future work
The vaccine-induced Arthus reactions as a relatively rare adverse reaction are frequently overlooked. In this review, we have documented the discovery process, mechanism, diagnostic criteria of Arthus phenomenon, summarized the previous reported Arthus reaction cases after the vaccination, and discussed the general treatment and preventive measures. We hope this review could refresh the awareness and recognition of people about the complexity of the Arthus phenomenon in order to better prevent the Arthus reaction.
Indeed, we found in the Chinese database the number and incidence rate of Arthus reaction induced by various vaccines in China, but during the sample period of the English database, we did not find the relevant surveillance data. Thus, we cannot compare whether there is a difference in the incidence rate of Arthus reaction between the China and the rest of the world. On the other hand, the cases in English literature mainly occurred before 1980. The Arthus case has indeed declined since 1980. We have not yet found out why the Arthus reaction has decreased. It can be assumed that the vaccine is improved (updated) or the Arthus reaction is underreported.
So far, there is still no unified definition and diagnostic standard for Arthus reaction; thus, we recommend the public health sector experts to accurate and unify the definition and the diagnostic criteria for Arthus reaction. Notably, the incidence of reported Arthus reaction within the context of the total immunized population is often extremely low.Citation2,Citation3 Then in order to overcome challenges of the Arthus reaction reporting rate following vaccination, some physicians of expert panels maybe have advised the inclusion of rare Arthus reaction into national and provincial-level hospitals owned a local Hospital Information System (HIS).Citation42 Meanwhile, the improvements of reporting rate related to Arthus reaction will ensure a better understanding and awareness of this phenomenon. Mechanistic studies have been valuable in better understanding Arthus reaction action but currently, mechanisms of Arthus reaction are less well understood.Citation43 Hence, researcher in the scientific research institutions is still needed into the mechanisms underlying potential vaccine-associated Arthus reaction in an immunized population. The general public is difficult to accurately and scientifically understand the Arthus reaction if not carefully researched. It is important to note that Arthus reactions usually occur at and around the injection site,Citation3,Citation24 and in order to better prevent this phenomenon, vaccinators are advised to regularly care and clean the same injection site.
In general, potential factors (such as type of vaccine, the dosage and doses of vaccine, time interval, and pre-antibody level) were carefully considered and well controlled in order to prevent of Arthus reaction. We collected only 18 cases of bacterial vaccine-induced Arthus reaction and 12 cases of viral vaccines. However, there are no other data (such as total number and incidence of vaccinations) in literatures, so we cannot compare statistically significant differences. Studies have shown that the higher dosages of vaccine can cause Arthus reaction.Citation44 Retrospective studies, especially case reports, may not be able to describe the relationship between the case with Arthus reaction and the dosage of vaccine in a comprehensive and detailed. This indicates an urgent need for more research between the dosage of vaccine and Arthus reaction. On the other hand, of the 30 reported cases of Arthus reaction, 22 occurred after the second and third doses of the vaccine. Therefore, it could be hypothesized that cases who receive multiple revaccinations do have a sufficient concentration of type-specific antibody to cause an Arthus reaction in the published literature.Citation45 Arthus reactions are more likely to occur after frequently repeated injections of the same vaccine.Citation46 Thus, appropriate spacing at longer intervals between injections may be an effective measure to avoid or reduce this phenomenon. Other possibilities are primary prevention of Arthus reaction in sensitized or at-risk children (the majority of reported cases were observed in children younger than 6 y). This may be due to poor immunization in children compared to adults, and multiple or intensive revaccination campaigns under 6 y of age, which are consistent with the above the doses of vaccine.
This study has three limitations worthy of mention. First, we obtained vaccine-related Arthus reaction cases mainly from the CNKI database, Wan Fang database, PubMed and Web of Science databases. However, we exclude the other non-English published literature. Therefore, there may be selective bias in our review. Second, we collected only 18 cases of bacterial vaccine-induced Arthus reaction and 12 cases of viral vaccines. The cases of Arthus reaction are descriptive studies or case reports. These cases may not be Arthus reaction. Thus, there is ascertainment bias because observers might expect more bacterial cases, and viral cases are under-reported. Finally, the number and incidence rate of Arthus reaction in are derived from the China‘s AEFI System. There may be ascertainment bias and reporting bias. In summary, the public health scholars and related personnel are encouraged to standardize the definition of Arthus reaction and to establish diagnostic criteria for Arthus reaction in the future. And then it is recommended that the health sector experts and their staff will closely observe and monitor the early symptoms of Arthus reaction following vaccination and reduce its severity and harmfulness. The national health sector has the right and responsibility to observe and improve the AEFI surveillance system so as to early detection. Importantly, the National Institutes of Health further improve the prevention and treatment of Arthus reaction in the future. And researchers in the scientific research institutions are needed into the mechanisms underlying potential vaccine-associated Arthus reaction in an immunized population. On the positive side, the above recommendations and measures may contribute to early detection, accurate diagnosis, and effective treatment of Arthus reaction.
Abbreviations
| ACIP | = | Advisory Committee on Immunization; |
| AEFI | = | adverse events following immunization; |
| BCG | = | Bacilli Calmette-Guérin Vaccine; |
| DTaP | = | Diphtheria Tetanus and Acellular Pertussis Combined Vaccine; |
| DT | = | Diphtheria and Tetanus Combined Vaccine; |
| DTP | = | Diphtheria, Tetanus, Pertussis Combined Vaccine; |
| HepA-L | = | Hepatitis A Attenuated Live Vaccine; |
| HepB | = | Hepatitis B Vaccine; |
| Hib | = | Haemophilus Influenzae Type b Polysaccharide Conjugate Vaccine; |
| InfV | = | Influenza Vaccine; |
| JEV-L | = | Japanese Encephalitis Attenuated Live Vaccine; |
| MPV-A | = | Group A meningococcal polysaccharide Vaccine; |
| MPV-AC | = | Group A and C meningococcal polysaccharide Vaccine; |
| MR | = | Measles and Rubella Combined Attenuated Live Vaccine; |
| MMR | = | Measles, Mumps and Rubella Combined Attenuated Live Vaccine; |
| OPV | = | Oral Poliomyelitis Attenuated Live Vaccine; |
| PPV23 | = | 23-valent Pneumococcal Polysaccharide Vaccine; |
| PPCV7 | = | 7-valent Pneumococcal Polysaccharide Conjugate Vaccine; |
| Rab-V | = | Rabies Vaccine; |
| VarV | = | Varicella attenuated live Vaccine |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Liu Dawei GB, Cao lingsheng, Zang Ling and Liang Xiaofeng. Study on the surveillance of adverse events following immunization in China, 2005–2006. Chin J Vaccines Immunization. 2007;13(6):505–13.
- JiaKai Y. Analysis on surveillance data of adverse events followings immunization in China, 2013. Chin J Vaccines Immunization. 2015;21(2):121–3.
- Froehlich H, Verma R. Arthus reaction to recombinant hepatitis B virus vaccine. Clin Infect Dis. 2001;33(6):906–08. PMID:11512098. doi:10.1086/322585.
- Liu D, Wu W, Li K, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33(32):4041. doi:10.1016/j.vaccine.2015.04.060.
- Phillip Waalkes T, Coburn H. Serotonin, histamine, and the Arthus phenomenon. J Allergy. 1960;31(2):181–84. doi:10.1016/0021-8707(60)90041-1.
- Wan-Yao LI, Cao XJ, Wan-Shan LI. The investigation of Arthus reaction caused by apitherapy. J Clin Acupuncture Moxibustion. 2010;26(01):57-58.
- Sutter RW. Adverse reactions to tetanus toxoid. JAMA. 1994;271(20):1629. PMID:8182821. doi:10.1001/jama.1994.03510440091052.
- Stetson CA Jr. Similarities in the mechanisms determining the Arthus and Shwartzman phenomena. J Exp Med. 1951;94(4):347–58. PMID:14888816. doi:10.1084/jem.94.4.347.
- Arthus NM. Injections repetees de serum de cheval cuez le lapin[J]. C R Soc Biol. 1903;(55):817–25.
- Tumpeer IH. The Arthus phenomenon: a serologic study in a syphilitic child with a fatal reaction to transfusion. Am J Dis Children. 1933;45(2):343–54. doi:10.1001/archpedi.1933.01950150116008.
- Maroney JA. Arthus phenomenon Report of a Clinical Case. N Engl J Med. 1934;211(3):106–07. doi:10.1056/NEJM193407192110304.
- Benjamin W, Zweifach L. ed. The inflammatory process: CHAPTER 19–the Arthus reaction; 1965. doi:10.1001/jama.1965.03090150103043.
- Stratton KR, Howe CJ, Johnston RB; Vaccine Safety Committee IoM, National Academy of Sciences. Adverse events associated with childhood vaccines: evidence bearing on casuality. National Academies Press; 1993.
- Facktor MA, Bernstein RA, Fireman P. Hypersensitivity to tetanus toxoid. J Allergy Clin Immunol. 1973;52(1):1–12. doi:10.1016/0091-6749(74)90074-8.
- Cruse REL JM. Atlas of immunology, 3rd ed. CRC Press; 2010.
- Cruse REL JM. Illustrated dictionary of immunology, 3rd ed. CRC Press; 2009.
- Kindt RAG TJ, Osborne BA, Kuby J. Kuby Immunology, 4th ed. Freeman and Company; 2000.
- Parham P. The immune system. 3rd ed. New York (NY): Garland Science; 2009.
- WenDi W. Analysis on surveillance data of adverse events following immunizatin in China, 2012. Chin J Vaccines Immunization. 2014;20:1–12.
- Gatewood WE, Baldridge CW. Tissue hypersensitiveness following the administration of toxin-antitoxin. J Am Med Assoc. 1927;88(14):1068–71. doi:10.1001/jama.1927.02680400024009.
- Cao LS, Yuan P. Thinking of China national immunization program information management system construction. Chin J Vaccines Immunization. 2010;16(6):553-57.
- Ross FE. The Arthus phenomenon: report of a case. J Am Med Assoc. 1934;103(103):563–563. doi:10.1001/jama.1934.72750340002008b.
- Duan XuYun CS, RongGuang Z, ChengYan Z. One case of Arthus reaction caused by inoculation of Diphtheria, Tetanus and Acellular Pertussis Vaccine. China Rural Health. 2013;(6):61–61. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_zgncws201306033.
- Buser F. Side reaction to measles vaccination suggesting the Arthus phenomenon. N Engl J Med. 1967;277(5):250–51. PMID: 4226465. doi:10.1056/NEJM196708032770507.
- ChengGang D. A case report of measles Vaccine induced Arthus reaction. J Prev Med Inf. 2008;24(5):400.
- Na Meng WC, QingKun Z, Xia L, YunDong Y, Xian Z. One case of Arthus reaction [J]. J Dermatol Venereol. 2016;38(5):383–84.
- China NHaFPCotPsRo. Immunization schedules and instructions for vaccines of the National Immunization Program, the Chinese CDC, 2017, http://www.scwst.gov.cn/fw/cxfw/jbyffw/201705/t20170517_13548.html.
- Organization WH; Western Pacific Regional Office WHO. editor. Immunization safety surveillance: guidelines for managers of immunization programmes on reporting and investigating adverse events following immunization. Manila: World Health Organization; 1999. https://trove.nla.gov.au/version/44427879 World Health Organization, Regional Office for the Western Pacific, 1999. Libraries Australia.
- Wu Wendi LD, Keli L, Disha X, Jingshan Z, Lingsheng C, Lei C, Ping Y, Huaqing W. Analysis on adverse events following immunization surveillance in China, 2010. Chin J Vaccines Immunization. 2012;18(5):385–97.
- Wu WLK, Jingshan Z, Dawei L, Disha X, Hong Y, Lei C, Lingsheng C, Ping Y, Li L, Wang H. Analysis on surveillance data of adverse events following immunization in China, 2011. Chin J Vaccines Immunization. 2013;19(2):97–109.
- Wu Wendi LD, Keli L, Zheng J, Disha X, Yamin W, Lei C, Lingsheng C, Ping Y, Huaqing W, Li L. Analysis on surveillance data of adverse events following immunization in China, 2012. Chin J Vaccines Immunization. 2014;20:1–12, 66.
- Ye Jiakai LK, Disha X, Wendi W, Dawei L, Jingshan Z, Lei C, Lingsheng C, Ping Y, Huaqing W, Li L. Evaluation of the adverse events following immunization information management system in China, 2013. Chin J Vaccines Immunization. 2015;21:121–31.
- Ye Jiakai LK, Disha X, Wendi W, Dawei L, Jingshan Z, Lei C, Lingsheng C, Ping Y, Jian C, Huaqing W. Analysis of surveillance for adverse events following immunization in China, 2014. Chin J Vaccines Immunization. 2016;22:125–37.
- Zhang Yan LS. A case report of Arthus reaction caused by Rabies Vaccine. Tianjin J Nurs. 1996;4(1):37–38.
- Min W. One case of Arthus reaction caused by intramuscular injection of rabies vaccine. J Hubei Inst Nationalities (Med Ed). 1997;14:37–37.
- HongXing S. Short-term Inoculation of 23-valent pneumococcal polysaccharide vaccine causes Arthus reaction: a case report. Modern Prev Med. 2006;33:1731–1731.
- Long Yinghong WY. Inoculation of 23-valent pneumococcal polysaccharide vaccine causes Arthus response: one case report. World Latest Med Inf. 2014;14:207–207.
- Xiao-Feng L, Li L, Da-Wei L, Ke-Li L, Wen-Di W, Bao-Ping Z, et al. Safety of influenza a (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med. 2011;364(7):638–74
- Ye Jiakai LK, Disha X, Wu W, Jingshan Z, Lei C, Lingsheng C, Jian C, Dawei L, Huaqing W. Surveillance of adverse events following immunization in China, 2015. Chin J Vaccines Immunization. 2017;23:481–492, 511.
- Kretsinger K, Broder KR, Cortese MM, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by. Morb Mortal Wkly Rep Recommendations Rep. 2006;55(RR–3):1–42.
- Hakim MS, Wang WS, Bramer WM, Geng J, Huang F, de Man RA, Peppelenbosch MP, Pan Q. The global burden of hepatitis E outbreaks: a systematic review. Liver Int. 2017;37(1):19–31. doi:10.1111/liv.13237.
- Liu D, Wu W, Li K, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33(32):4041–46. doi:10.1016/j.vaccine.2015.04.060.
- Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007;137(1):S46–S50. doi:10.1016/j.jcpa.2007.04.012.
- Cochrane CG. The cutaneous reaction to soluble antigen-antibody complexes: a comparison with the Arthus phenomenon. J Exp Med. 1958;108(5):591–604. PMID:13587844. doi:10.1084/jem.108.5.591.
- Walker FJ, Singleton RJ, Bulkow LR, Strikas RA, Butler JC. Reactions after 3 or more doses of pneumococcal polysaccharide vaccine in adults in Alaska. Clin Infect Dis Offl Publ Infect Dis Soc Am. 2005;40(12):1730–35. PMID: 15909258. doi:10.1086/430305.
- Wu Wenjun ea. China. Surveillance data of suspected abnormal vaccination surveillance. Chin J Vaccines Immunization. 2010;2012(05):385–97.
- Tumpeer IH, Matheson A, Straus DC. Arthus phenomenon in a syphilitic child. J Am Med Assoc. 1931;96(17):1373–76. doi:10.1001/jama.1931.02720430023007.
- Jianli L. One case report of Arthus reaction Induced by tetanus antitoxin injection. Chin Nurs Res. 2004;18(4):298–298.
- Chunfang L. Two cases of Arthus reaction. Chin Community Doctors. 2006;22(5):27–27.
- Zhitong L. A case report of pertussis diphtheria tetanus mixed vaccine(concentrated) Induced Arthus reaction. South China J Prev Med. 2009;11(5):560.
- Meiying H. One case of hypersensitivity reaction induced by diphtheria, tetanus and acellular pertussis vaccine injection. Chin J Vaccines Immunization. 2005;11:342–342.
- Yan Z. A case report of rabies vaccine induced local Arthus reaction. Tianjin J Nurs. 1996;(1):37–38.
- Min W. One case of Arthus reaction caused by intramuscular rabies vaccine. J Hubei Inst Nationalities (Med Ed). 1997;14(1):37–37.
- How to deal with Arthur reaction after vaccination. http://blog.sina.com.cn/s/blog_4869316f0100034j.html.
- Zhu XiangJun CY, ZhiGang G, YiMing S, Yonggang. Z. A case report of hepatitis B vaccine made by recombined DNA techniques in yeast induced Arthus reaction. Chin J Vaccines Immunization. 2008;8(5):270.
- ZhiCheng X. A case report of hepatitis B Vaccine made by recombined DNA techniques in yeast induced Arthus reaction. Chin J Vaccines Immunization. 2007;13(3):215.
- Zheng ZhiGang HL. One case report of Arthus reaction induced by hepatitis B vaccine injection. Modern Prev Med. 2007;34:2753.
- Yan DongMei SS, Qin. J. One case of Arthus reaction caused by inoculation of Hepatitis B vaccine. People‘S Mil Surgeon. 2009;52(5):560.
- A Case Report of Hepatitis B vaccine induced Arthus reaction. http://www.xzbu.com/6/view-33755.htm.
- JunHua X. A case of infantile hepatitis B vaccine induced by Arthus reaction. Chin-Foreign Women‘S Health. 2012;20:120.
- Hongxing S. A case report of Arthus reaction caused by reinoculation of 23-valent pneumococcal polysaccharide vaccine in a short term. Modern Prev Med. 2006;33(9):1731–1731.
- Yinghong L. A case report of 23-valent pneumococcal polysaccharide vaccine induced Arthus reaction. World Latest Med Inf. 2014;14(10):207–207.
- WenDi W. Analysis on adverse events following immunization surveillance in China, 2010. Chin J Vaccines Immunization. 2012;18(05):385–97.
- WenDi W. Analysis on Surveillance Data of adverse events following in China, 2011. Chin J Vaccines Immunization. 2013;19(2):97–109.
- JiaKai Y. Analysis on surveillance data of adverse events following immunizatin in China, 2014. Chin J Vaccines Immunization. 2016;22(2):125–37.
- JiaKai Y. Analysis on surveillance data of adverse events following immunizatin in China, 2015. Chin J Vaccines Immunization. 2017;23(5):481–92.