ABSTRACT
Rotavirus gastroenteritis imposes a heavy burden on low- and middle-income countries. The World Health Organization defines the Eastern Mediterranean region (WHO-EMRO) as a diverse area in terms of socioeconomic status and health indicators. Rotavirus vaccination has been introduced, at least partially, in 19 out of the 22 EM countries; however, vaccine coverage remains low, and data on rotavirus disease burden is scarce.
Available data on rotavirus prevalence, seasonality, vaccination status, and genotype evolution was systematically compiled following a literature review that identified 165 relevant WHO-EMRO epidemiology studies published between 1990 and 2017.
Although the infectious agents responsible for acute gastroenteritis vary over time, rotavirus remained the leading cause of acute gastroenteritis in children, as seen in 76.3% of reviewed publications. Younger children (<2 years old) were at higher risk and thus increased vaccination coverage and surveillance systems are required to reduce the rotavirus gastroenteritis burden in WHO-EMRO countries.
Introduction
Rotavirus (RV) is the leading cause of diarrheal morbidity and mortality in young children worldwide and causes severe acute gastroenteritis (AGE) requiring hospitalization, and if dehydration is not treated in time leads to mortality especially in the developing world.Citation1 By the age of five years, nearly every child will have had an episode of RV gastroenteritis (RVGE), one in five of them will visit a clinic, one in 65 will be hospitalized, and approximately 1 out of 293 will eventually have a fatal outcome.Citation2,Citation3 According to a report, more than 90% of RVGE deaths in 2013 occurred in 72 low-income and low-middle-income countries.Citation4 Implementation of an RV vaccination in national immunization programs (NIPs) reduces the RV disease burden substantially.Citation5 Countries need to have adequate knowledge and information on local burden, trends, and age distribution of disease to help decision-makers consider the introduction of an RV vaccine as part of their immunization programs.Citation6
The Eastern Mediterranean region, as defined by the World Health Organization (WHO-EMRO region, ), is a geographically and socioeconomic diverse area with varying health indicators. At present, the region has a population of over 656 million (81.4 million (12.4% of the total population) – is under 5 years of age).Citation7 RV-associated mortality and morbidity also vary considerably in the region: the annual morbidity rates among children under five years of age ranged from 0 to 112/100,000 with an average mortality rate of 39/10,000 per year.Citation5,Citation8 Low-income countries (e.g. Afghanistan, Pakistan, Sudan, Yemen, and Somalia) had a higher mortality rate due to RVGE compared with countries where the per capita income was high (e.g. Saudi Arabia and Kuwait). However, the overall hospital and health center visits due to RVGE among children under five years of age was similar in both high- and low-income WHO-EMRO countries.Citation5,Citation8,Citation9
Figure 1. WHO-EMRO region countries (i.e. Afghanistan, Bahrain, Djibouti, Egypt, Iran (Islamic Republic of), Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Occupied Palestine Territory, Oman, Pakistan, Qatar, Somalia, Sudan, Syrian Arab Republic, Tunisia, United Arab Emirates (UAE), and Yemen) included in the study.
UAE: United Arab Emirates.
An RV vaccine has been introduced as part of the NIPs in 15 out of the 22 countries (albeit partially in Pakistan and Palestine) in the WHO-EMRO region. Vaccine coverage is predominantly suboptimal, with the exception of Saudi Arabia, with 97% vaccine coverage, and burden of disease data are still scarce.1,Citation6,Citation10 Furthermore, a lack of surveillance systems prevents decision makers from understanding the magnitude of the problem and the need for RVGE prevention through vaccination.Citation11
The purpose of this literature review is to assess the burden of RVGE in the pediatric population and to summarise the current status of recommendations for RV vaccination across the WHO-EMRO region. Moreover, this review will also be useful as a baseline for post-vaccination surveillance for both AGE and RVGE.
Results
Four hundred and twenty-six articles were identified through the search process as of 5th December 2017. Two hundred and thirteen publications were excluded and 213 were included for full-text review. One hundred and sixty-five publications were included in the qualitative assessment (). The countries and number of associated references from which published data were obtained are: Afghanistan (1),Citation12 Bahrain (3), Citation13–Citation15 Egypt (17),Citation16–Citation32 Iran (37),Citation33–Citation68 Iraq (3),Citation41,Citation69,Citation70 Jordan (9),Citation71–Citation79 Kuwait (2),Citation80,Citation81 Lebanon (3),Citation82–Citation84 Libya (5),Citation85–Citation89 Morocco (9),Citation90–Citation98 Oman (4),Citation99–Citation102 Palestine (2),Citation103,Citation104 Pakistan (15),Citation105–Citation119 Qatar (1),Citation120 Saudi Arabia (21),Citation121–Citation141 Somalia (2),Citation142,Citation143 Sudan (4),Citation144–Citation147 Tunisia (20),Citation148–Citation167 United Arab Emirates (UAE) (3),Citation168–Citation170 and Yemen (4).Citation171–Citation174 No study related to Djibouti could be retrieved.
Figure 2. Literature search strategy (limited to articles published between 1990 and 2017 with abstracts in English or French) using the keywords “rotavirus” and “[country name]”.
![Figure 2. Literature search strategy (limited to articles published between 1990 and 2017 with abstracts in English or French) using the keywords “rotavirus” and “[country name]”.](/cms/asset/68971396-e1d5-461f-9347-c19ec5119a90/khvi_a_1603984_f0002_b.gif)
Details on study subjects, dates, settings and calculated RV prevalence from the studies across the region is shown in Tables S1-S3. Table S1 presents data from the North African countries (Egypt, Morocco, Tunisia, Somalia, Sudan, and Libya), Table S2 includes the Middle Eastern countries (Saudi Arabia, Lebanon, Bahrain, Oman, Palestine, Qatar, UAE, Yemen, Jordan, Iraq, and Kuwait) and Table S3 shows data from the Asian countries (Iran, Pakistan, and Afghanistan). A total of 113,959 cases of confirmed RVGE are reported in this review, with the highest number from Pakistan (n = 13,546) and the lowest from Somalia (n = 213).
Prevalence of RVGE
The prevalence of RV infection from different studies across the three regions (Nord Africa, Middle East, and Asia) is shown in .
Table 1. Prevalence of RVGE among AGE cases in studies from 1990 to 2017, per country.
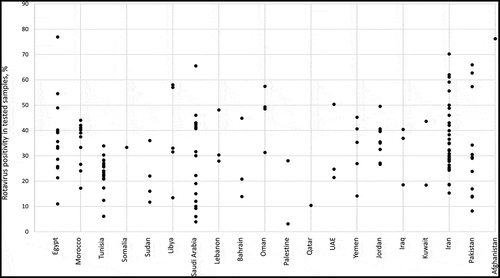
Among the 165 articles included in this review, 152 (92.1%) reported RV prevalence data. The mean RV prevalence among AGE cases in the EMRO countries with more than two studies was between 21.4% (Sudan) and 46.6% (Oman) and the median was between 19.0% and 48.9%. The prevalence of RV in AGE cases was over 20% in 121 (79.6%) of them, with the highest reported rates observed in Egypt (76.9%), Afghanistan (76.2%) and Iran (70.2%). (, )Citation66 There were differences in RV isolation rates among multiple studies conducted within the same country, e.g. Pakistan (8.2% to 65.9%), Saudi Arabia (3.9% to 65.5%) and Egypt (range: 11% to 76.9%). However, in other countries, prevalence appeared to vary less, as seen by a narrower distribution in for certain countries including Morocco (17.2% to 44%), Tunisia (6.1% to 33.9%), or Jordan (26.6% to 49.5%)
Figure 3. Prevalence of RVGE among cases of AGE in individual studies per country.
The overall incidence was reported for studies detailing prevalence over several years, except when the study was comparing pre- and post-vaccination periods. In this case, the latest value was retained, as more actual.
There were also important differences in inpatient and outpatient RV prevalence. For example, in Egypt, in the 2011–2012 period, and using the same technique, the RV prevalence was 29.9% for outpatients and 43.9% for inpatientsCitation;29 in Tunisia, this prevalence was 18.5% and 24.9%,Citation166 and in Bahrain 6% and 27.4%, respectively.Citation13
Changes in the detection of RVGE over time
In Jordan, the RVGE detection rates among AGE cases were fairly constant over a ten-year period (25% in 1995, 35.2% in 2002, 24.7% in 2003, and 25.8% in 2004).Citation76 On the other hand, the proportion of RVGE cases appears to have increased over time in Pakistan; as the RVGE rates were ranging from 8.2% to 29% in the earlier years (1985–1996),Citation105,Citation107,Citation116 while in the more recent years (2007–2014) the reported rates were ranging from 23.8 to 65.9.Citation110–Citation115,Citation118,Citation119 This can be due to the difference of sensibility of EIA used during these two different periods, or to the choice and characteristics of patients investigated.
A reduction in RVGE occurrence is clearly evident after the introduction of RV vaccination in some countries. For instance, there was a 41.5% reduction in RV detection rate in the post-vaccination period compared to the pre-vaccination period in Morocco.Citation96 In Yemen there was a steady decline in RV detection following vaccine introduction; the detection rates during the pre-vaccination period was 43.8% and 37.4% in 2009 and 2010, respectively, but declined during the post-vaccination period to 17.9% in 2013 and to 10.5% in 2014.Citation174 In the same study, a 75.9% reduction in hospitalization due to RV diarrhea was reported over five years (2009: 43.8%; 2014: 10.5%). Banajeh et al.Citation173 also showed that in Yemen, after vaccination had started, the prevalence of RVGE decreased by 48% and the hospitalization rate due to RV diarrhea declined from 42.9% during the pre-vaccination period (2007/2011) to 18.5% in the post-vaccination period (2013/2014). A similar pattern was observed in Saudi Arabia, where the RVGE prevalence was reduced from 12% to 46% to 9.2% over a three-year period following the introduction of the RV vaccine in 2013.Citation141
Concomitant enteropathogens
About one fourth (38 of 165) of the reviewed publications concomitantly reported isolation of pathogens other than RV. However, RV was most frequently responsible for AGE cases in 29/38 (76.3%), second most frequent in 5/38 (13.2%) and third most frequent in 3/38 (7.9%) of the studies. Norovirus, Astrovirus, and Adenovirus were other common viral pathogens detected in AGE cases ().
Table 2. Distribution of enteropathogens isolated in AGE cases in the WHO-EMRO region.
RVGE seasonality patterns
A seasonality pattern of RVGE was reported in 95 of the 165 (57.6%) reviewed studies. Most studies (66/95, 69.5%) reported a peak of RVGE in the cold season from November to April. However, some studies also showed exceptional RVGE peaks occurring in late summer.Citation22,Citation111,Citation154,Citation172 Studies from Iran, Bahrain, and Saudi Arabia did not observe any seasonal variation of RVGE.Citation13,Citation39,Citation132
Detection techniques used
A total of 126 out of 147 studies that described RVGE prevalence also reported the isolation technique used for RV detection: 97 studies (77%) used enzyme immunoassay (EIA), 12 (9.5%) used latex agglutination (LA), 25 (19.8%) used polymerase chain reaction (PCR), 4 (3.2%) used polyacrylamide gel electrophoresis (PAGE) techniques, and a comparison with electron microscopy (EM) was made in 2 studies. Some of the studies also compared detection rates based on which techniques had been used. In Egypt, the positivity of RV detection varied between 34% and 39.5% according to LA and EIA techniques, respectively.Citation16 In Jordan, the sensitivity of EM and EIA RV detection techniques were 18.9% vs. 39.6%, respectively.Citation72 In Egypt, the sensitivity was higher when PCR (76.9%) was used as compared to EIA (67.7%).Citation30
Nosocomial RV infection
Nosocomial infection due to RV is also common. A study in Iraq showed that 32.4% of hospitalized children had nosocomial infections and 18.5% of them were due to RV.Citation41 The nosocomial infection rate was 33.3% in Morocco,Citation92 and in three different studies from Iran, the reported rates were 26.3%, 18.5% and 30%.Citation38,Citation41,Citation54
Disease severity and mortality rates
In Morocco, 1 child per 32 RV-infected children was hospitalized and 1 child per 389 RV-infected children died due to RVGE in a group of children under 5 years of age.Citation91 Another Moroccan study reported that 30% of hospitalizations for diarrhea were due to RVGE, with a mortality rate of 40–50%.Citation90 In Pakistan, 1 child per 40 infected children under 5 years of age had an episode of severe RVGE per year.Citation117 A meta-analysis spanning from 1989 to 2004 estimated 19,933 deaths due to RVGE in Pakistan, constituting 3.8% of the global RVGE mortality.Citation109 In Iran, 2,700 deaths due to RVGE occurred per year; it was estimated that 13–40% of all cases of diarrhea were due to RV and that 30% of them required hospitalization.Citation37,Citation40,Citation47
RV genotype and evolution of genotypes post-vaccine introduction
The genotype distribution of RV isolates in different studies was also reviewed. We evaluated the genotype combinations, where both G and P capsid protein combinations are reported. Studies conducted in 13 WHO-EMRO region countries (Bahrain, Egypt, Iran, Iraq, Jordan, Libya, Lebanon, Morocco, Oman, Pakistan, Saudi Arabia, Tunisia, and Yemen) reported RV genotype distribution (). Studies from Afghanistan, Palestine, Qatar, Somalia, and Sudan did not report on genotype distribution; Kuwait and UAE reported only G- or P-type, and were therefore not included in this evaluation. Overall, the genotype distribution was diverse across the region.
Table 3. RV genotype distribution (%) in WHO-EMRO countries.
G1P[8] was the most prevalent genotype combination in 7 of the 13 countries (i.e. Iran, Saudi Arabia, Jordan, Bahrain, Morocco, Iraq) for which data from the past 15 years are available ().Citation15,Citation34,Citation37,Citation45,Citation48,Citation57,Citation69,Citation78,Citation79,Citation90,Citation93–Citation96,Citation98,Citation100–Citation102,Citation130–Citation132,Citation138,Citation139,Citation141
G1P[8] and G2P[4] were the most common genotypes in Yemen and Egypt.Citation19,Citation23,Citation24,Citation27,Citation29,Citation32,Citation83,Citation84,Citation171,Citation172 In Lebanon, the genotype distribution varied over time and geographical location: the G4P[8] type disappeared from the northern part of the country in two out of the three studied seasons, while the G2P[4] genotype did not.Citation83 In Oman, the distribution pattern of the G2P[4] and G1P[8] RV genotypes also varied with time.Citation100–Citation102
In Tunisia, both G1P[8] and G3P[8] were common, followed by G4P[8].Citation148,Citation158,Citation159,Citation162–Citation164,Citation166 RV genotype G9 was first detected in 2004 in Tunisia and from 2010, there was an increase in the prevalence of related genotypes (10.3–37.5%).Citation165,Citation166
In Pakistan, the distribution of genotype combinations was heterogeneousCitation110–Citation112,Citation117,Citation118 and G12P[6] was found to be one of the most prevalent RV genotype in 2014.Citation115
The introduction of RV vaccines had a substantial effect on G1P[8] detection. In Saudi Arabia, a decrease was noted in G1P[8] detection from 51% to 37.1% after the vaccine was introduced, but G2P[4] increased from 21.6% to 33.3%.Citation141
In Morocco, a periodic decrease in the detection of G1P[8] genotype was reported: 57% one-year post-vaccination and 54.7% two years post-vaccination.Citation96 However, G2P4 was detected in 15% of samples in the pre-vaccination period and in 54% and 33% of samples, respectively, in the second and third years following the vaccine introduction. An increase was also seen for G9P[8] (7.8% in the pre-vaccination period, 16.6% and 67% in the second and third year of the post-vaccination period, respectively).
In Yemen, the G1P[8] detection rate increased from 45.5% to 87.5% and the G2P[4] detection rate decreased from 76.5% to 0.2% after vaccine implementation.Citation173 In another study in Yemen, the G1P[8] detection rate increased from 15% in the pre-vaccination period to 31% in the post-vaccination period and became the predominant genotype, followed by G9P[8] (27.5%).Citation174
In Iran, there was also an emergence of new RV genotypes over time, with G12P[8] being detected for the first time in 2016.Citation57 In Egypt, genotype G6P[14] was first reported in 2011.Citation26
Like in other parts of the world, mixed or non-typeable RV strains have also been detected in the WHO-EMRO region. In Iran, mixed genotype combinations, including G1P[4] and G1P[8], were detected in two studies, and the rate of mixed genotypes varied from 2.7% to 33.3%.Citation47,Citation49 Non-typed (Nt) strain detection in Iran ranged from 5% to 40.9%.Citation34,Citation68 In Pakistan, Egypt and Tunisia, the detection rates for mixed RV genotypes were 6.7%, 7% and 5.9%, respectively.Citation19,Citation113,Citation148
Vaccination status
The demographic characteristics and the type of RV vaccine used in the WHO-EMRO countries are shown in . An RV vaccine was available in 19 (86.4%) of the 22 WHO-EMRO countries; either publicly (10/22), both publicly and privately (5/22), or only privately (4/22). No RV vaccine was available in three (13.6%) WHO-EMRO countries (Iran, Somalia, and Syria). Fifteen (72.7%) WHO-EMRO countries had introduced an RV vaccine in their NIP (Afghanistan, Bahrain, Djibouti, Iraq, Jordan, Kuwait, Libya, Morocco, Pakistan, Palestine, Qatar, Saudi Arabia, Sudan, UAE, and Yemen). Six (27.3%) of the 22 EMRO countries were eligible for GAVI support. ().
Table 4. Demographics and RV vaccination status of the 22 WHO-EMRO countries.
Discussion
This review evaluated the RV infection status in the WHO-EMRO region, identified reductions in RV prevalence and mortality following implementation of RV vaccination and described existing RV surveillance systems in the region.
In the WHO-EMRO region, the observed RVGE burden varied between countries. In general, a low RVGE prevalence (<30%) was observed in Egypt, Saudi Arabia, and Tunisia, and a high prevalence (>30%) was reported in Iran, Lebanon, Morocco, Jordan, and Oman.Citation8 The annual proportion of RVGE among reported episodes of AGE in the under-five-years-of-age population in this region was 42%, and recent studies have estimated that about 65,000 children die each year from RV infections in the 22 WHO-EMRO countries.Citation9 This is likely to be an underestimation of the true prevalence, due to inadequate surveillance systems and lack of routine RV testing. Although a majority (81.8%) of EMRO countries have implemented RV vaccination, seven countries (Egypt, Iran, Lebanon, Oman, Somalia, Syria, and Tunisia) have not yet implemented RV vaccination in their NIPs. Furthermore, no RV vaccine is available in three (13.6%) EMRO countries (Iran, Somalia, and Syria).
RV was the most common AGE agent in the WHO-EMRO region in 76.3% of the reviewed studies. Our review also revealed that there is a wide variation (>20% difference between studies) in RV isolation rates in different studies conducted within the same countries; for example, in Iran,Citation40 Pakistan,Citation105,Citation114 Saudi ArabiaCitation132,Citation135 and Morocco,Citation89,Citation95 the difference of reported isolation rates was wide. However, in countries such as TunisiaCitation156,Citation163 and Jordan,Citation74,Citation78 the rate differences were smaller. Differences in study design, patient selection, and case definition could contribute to explain differences in observed incidences across studies.
This review also confirmed that as in Western Europe,Citation179 G1P[8] and G2P[4] are the predominant genotypes circulating in the WHO-EMRO region and that available RV vaccines can provide protection against them. It is apparent that the circulating RV strain distribution regularly changes, as well as the dominant genotype, even in the absence of vaccines. Interestingly, G12P[8], a recently emerging serotype previously detected in Europe, Asia, and the Americas, has also been reported in some studies in Saudi Arabia.Citation8,Citation139,Citation141 Unusual genotypes such as G1P[6], G2P[6], G3P[9], G4P[6], G9P[6] and G9P[8] have been detected in different countries,Citation118,Citation132,Citation148,Citation157 including the recent emergence of genotype G12P[6] in PakistanCitation115 and of genotype G12P[8] in Iran.Citation60 Data of genotyping diversity from the WHO-EMRO region confirm that these findings are not specific to a particular geographic location but a natural phenomenon worldwide.
Mixed genotype combinations were detected in Iran,Citation49,Citation60 Tunisia,Citation149,Citation150 and Pakistan.Citation112 These results are similar to recent findings in India, Indonesia, and Vietnam and are likely due to reassortments.Citation10 The proportion of non-typeable and partially typeable genotype combinations also varied widely across countries.Citation6,Citation48,Citation49,Citation100 Differences in laboratory techniques, as well as different geographic settings, can explain these findings. With the use of more appropriate genotyping primers and advanced molecular techniques, the proportion of non-typeable strains has decreased in recent studies.
A shift in genotype predominance and circulation before and after vaccine introduction is not unusual and cannot be attributed to available vaccines. Countries such as Australia and Brazil that have used different vaccines have also faced the re-emergence of G2 strains after vaccine introduction.Citation180,Citation181 These data simply reflect the normal fluctuation in RV genotype frequency. In order to substantiate any vaccine pressure on genotype shift, continued surveillance over time, detailed phylogenic analysis, and full RV genome sequencing are required. Although these measures are currently not in place in the WHO-EMRO region, policy measures aimed at implementing these measures would greatly enhance our understanding of changes in RV strain circulation in the region.
In general, RV infections peak in the cooler winter months, and this pattern is similar for all WHO-EMRO countries except Gulf region countries. Studies from Saudi Arabia and neighboring countries showed no distinct peak, as RV disease was observed throughout the year with occasional peaks irrespective of season.Citation132
Several countries that have implemented routine childhood vaccination against RV have documented a dramatic impact on severe diarrhea and RVGE requiring hospitalization.Citation182–185 In the WHO-EMRO region, a small number of studies also show the effectiveness of vaccination on RVGE hospitalization, as well as RV detection in AGE cases. The results of these studies reflect that vaccination can markedly reduce RVGE disease burden, especially hospitalization due to diarrhea.Citation99,Citation141,Citation173,Citation174
The WHO recommends sentinel surveillance before and after the introduction of vaccines to monitor vaccine impact. As financial resources may be limited in this region, regional governments should consider sharing their data with local laboratories or existing surveillance networks to help achieve this goal. Documentation of the burden of disease and of circulating strains is essential for decision-making on vaccine introduction and vaccine effectiveness assessments. The burden of RVGE and the circulation of different RV strains should be monitored in a surveillance system and through standardized methods, which would enable comparisons between countries.
We observed that no single standard technique was used to isolate and genotype RV from stool samples. This is likely to have led to a variation of estimates, not only in different countries but also across studies conducted within a country. Thus, we decided to present descriptive results instead of direct comparisons.
The various studies reported here included patients from different age strata in both in- and outpatient settings, which may potentially lead to misclassifications. It is easily conceivable that patients admitted as inpatients were suffering from more severe disease and were therefore more likely to yield a pathogen from their stool sample than outpatient stool samples originating from patients with less severe infections. This was evident from studies in Saudi Arabia,Citation127 where the RV detection rate was 5.9% in outpatient settings compared with 34.6% in inpatient settings; in IranCitation66 the rates were 20.9% and 79.1% in outpatient and inpatient settings, respectively.
Variations in the estimates between and within countries could also be due to different study populations, study time periods, RV isolation and detection techniques, and the definition of RVGE used as study inclusion criteria. In some countries such as Afghanistan, Bahrain, Iraq, Kuwait, Lebanon, Palestine, Qatar, Somalia, and Sudan, the number of studies and subjects enrolled was not adequate for accurately estimating the burden and strain distribution of RVGE.
Although our review is one of the first to explore the prevalence of RV in the WHO-EMRO region, it has several limitations. Firstly, there is also a possible bias in identifying a denominator for the estimates. In some studies, RV rates were detected over total stool samples collected, while in others, the RV proportion was reported among the cases where at least one pathogen was detected. Secondly, our search was limited to published studies with abstracts in English and French, which may have resulted in the exclusion of studies published only in local languages. Thirdly, gaps in reporting from several WHO-EMRO countries may be due to political instability and not a lack of RV infections, and political instability may also prevent the successful implementation of vaccination programs and longitudinal RV vaccine studies.
In conclusion, this review of 165 studies from WHO-EMRO countries revealed that RV was strongly associated with young age in children and remained the dominant etiology of diarrhea requiring hospitalization, even in countries that had recently introduced RV vaccination. The collated data in this study will serve as useful baseline RV epidemiological data for the planning and implementation of vaccination programs in the region. Although the burden of RVGE is likely to decrease following the successful introduction of RV vaccination programs, as exemplified by Yemen,Citation171 ongoing surveillance is needed to determine the residual burden of RV, the genotype distribution of RV, and to monitor the evolving etiology of diarrhea in these countries. Data compiled in this report highlights that nearly all serious infections due to diarrhea occurred in children under 2 years of age, which stresses the need for protection early in life, and advocates for RV vaccine schedule completion by 12 months of age.
Methods
The inclusion criteria for the search was any publication in English or French in a country of the WHO-EMRO region on RV diarrhea in children less than five years of age during the period of January 1990 to December 5, 2017. We used the keywords “rotavirus” and “[country name]” (for each individual country out of the 22 included in the WHO-EMRO) to search in The National Library of Medicine’s PubMed and grey literature (). We also included publications in local medical journals and in local languages for which abstracts in English or in French were available. We further reviewed the cross-referenced articles from the retrieved ones.
A total of 426 published articles were identified, 387 from PubMed and 39 from local journals. Articles studying animals or environment (97), reporting the clinical aspects of infection studies (116), studying vaccine implementation or cost-effectiveness (32), or using non-standard detection techniques (16) were excluded, and 165 articles were included for complete full-text review and qualitative assessment ().
Authors, year of publication, type of article, patient type and numbers were reported for each publication. The prevalence of RV infection mentioned in the articles was the main focus of this study, and we also retained articles mentioning RVGE mortality, or RV genotyping. In addition, and where available, data on nosocomial infections, seasonality, techniques used for the detection, RV genotype distribution, variation over time in the proportion of RVGE, and evolution of genotype distribution, presence of other enteric co-pathogens and the vaccination status of the country was also abstracted. Each datum was abstracted by one author, and crossed-check by another author. Next, we calculated the RVGE prevalence as the number of reported RVGE cases over the total number of samples included in each study, to use a standard measure of the RVGE prevalence. When possible (i.e. when three studies or more were retrieved for one country), the mean and median prevalence was given for each country. Data were stratified by country.
Trademark statement
Rotarix is a trademark of the GSK group of companies. Rotateq is a trademark of Merck and Co Inc.
Disclosure of potential conflicts of interest
All authors are employed by the GSK group of companies. SB, SO, PP, MAG, MK, YL, KH, and DS hold shares in the GSK group of companies.
Authors’ contribution
Selim Badur, Mohammad AbdelGhany, Mansour Khalaf, Youness Lagoubi, Onur Ozudogru, and Kashif Hanif performed the literature search. All authors contributed to acquisition of data, analysis and interpretation of data, and revised the article critically for important intellectual content. All authors provided final approval of the submitted version.
Supplemental Material
Download MS Word (223.2 KB)Acknowledgments
The authors thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Amandine Radziejwoski coordinated the manuscript development and editorial support. Stefan Amisten provided editorial support.
Supplemental Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;200(Suppl 1):S1–8. doi:10.1086/605061.
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72.
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi:10.1016/S1473-3099(11)70253-5.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD; World Health Organization-Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi:10.1093/cid/civ1013.
- Rota Council. Global introduction status. 2017. [accessed 2017 December 5]. http://rotacouncil.org/vaccine-introduction/global-introduction-status/.
- Agocs MM, Serhan F, Yen C, Mwenda JM, de Oliveira LH, Teleb N, Wasley A, Wijesinghe PR, Fox K, Tate JE, et al. WHO global rotavirus surveillance network: a strategic review of the first 5 years, 2008-2012. MMWR Morb Mortal Wkly Rep. 2014;63:634–37.
- United Nations, Population Division, Department of Economic and Social Affairs. World population prospects: the 2017 revision. 2017. [accessed 2017 December 5]. https://esa.un.org/unpd/wpp/Download/Standard/Population/.
- Khoury H, Ogilvie I, El Khoury AC, Duan Y, Goetghebeur MM. Burden of rotavirus gastroenteritis in the middle Eastern and North African pediatric population. BMC Infect Dis. 2011;11:9. doi:10.1186/1471-2334-11-208.
- Malek MA, Teleb N, Abu-Elyazeed R, Riddle MS, Sherif ME, Steele AD, Glass RI, Bresee JS. The epidemiology of rotavirus diarrhea in countries in the Eastern Mediterranean Region. J Infect Dis. 2010;202(Suppl):S12–22. doi:10.1086/653579.
- Kawai K, O‘Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30:1244–54. doi:10.1016/j.vaccine.2011.12.092.
- Dbaibo G, Tatochenko V, Wutzler P. Issues in pediatric vaccine-preventable diseases in low- to middle-income countries. Hum Vaccin Immunother. 2016;12:2365–77. doi:10.1080/21645515.2016.1181243.
- Elyan D, Wasfy M, El Mohammady H, Hassan K, Monestersky J, Noormal B, Oyofo B. Non-bacterial etiologies of diarrheal diseases in Afghanistan. Trans R Soc Trop Med Hyg. 2014;108:461–65. doi:10.1093/trstmh/tru096.
- Dutta SR, Khalfan SA, Baig BH, Philipose L, Fulayfil R. Epidemiology of rotavirus diarrhoea in children under five years in Bahrain. Int J Epidemiol. 1990;19:722–27.
- Ismaeel AY, Jamsheer AE, Yousif AQ, Al-Otaibi MA, Botta GA. Causative pathogens of severe diarrhea in children. Saudi Med J. 2002;23:1064–69.
- Musawi MA, Zainaldeen H, Shafi F, Anis S, Deantonio R. Rotavirus gastroenteritis in children under 5 years in the Kingdom of Bahrain: hospital-based surveillance. Clin Epidemiol. 2013;5:269–75. doi:10.2147/CLEP.S46822.
- Amer AA, el-Mougi M, Hughes J, el-Tayyeb S, el-Abhar A, el-Shafie A. Comparison of latex agglutination test with an ELISA to diagnose rotavirus-associated diarrhoea in infants and young children. J Diarrhoeal Dis Res. 1990;8:87–89.
- Pazzaglia G, Bourgeois AL, Araby I, Mikhail I, Podgore JK, Mourad A, Riad S, Gaffar T, Ramadan AM. Campylobacter-associated diarrhoea in Egyptian infants: epidemiology and clinical manifestations of disease and high frequency of concomitant infections. J Diarrhoeal Dis Res. 1993;11:6–13.
- Radwan SF, Gabr MK, El-Maraghi S, El-Saifi AF. Serotyping of group A rotaviruses in Egyptian neonates and infants less than 1 year old with acute diarrhea. J Clin Microbiol. 1997;35:2996–98.
- Naficy AB, Abu-Elyazeed R, Holmes JL, Rao MR, Savarino SJ, Kim Y, Wierzba TF, Peruski L, Lee YJ, Gentsch JR, et al. Epidemiology of rotavirus diarrhea in Egyptian children and implications for disease control. Am J Epidemiol. 1999;150:770–77.
- Holmes JL, Kirkwood CD, Gerna G, Clemens JD, Rao MR, Naficy AB, Abu-Elyazeed R, Savarino SJ, Glass RI, Gentsch JR, et al. Characterization of unusual G8 rotavirus strains isolated from Egyptian children. Arch Virol. 1999;144:1381–96.
- El-Mohamady H, Abdel-Messih IA, Youssef FG, Said M, Farag H, Shaheen HI, Rockabrand DM, Luby SB, Hajjeh R, Sanders JW, et al. Enteric pathogens associated with diarrhea in children in Fayoum, Egypt. Diagn Microbiol Infect Dis. 2006;56:1–5. doi:10.1016/j.diagmicrobio.2006.02.007.
- Wierzba TF, Abdel-Messih IA, Abu-Elyazeed R, Putnam SD, Kamal KA, Rozmajzl P, Ahmed SF, Fatah A, Zabedy K, Shaheen HI, et al. Clinic-based surveillance for bacterial- and rotavirus-associated diarrhea in Egyptian children. Am J Trop Med Hyg. 2006;74:148–53.
- Kamel AH, Ali MA, El-Nady HG, de Rougemont A, Pothier P, Belliot G. Predominance and circulation of enteric viruses in the region of Greater Cairo, Egypt. J Clin Microbiol. 2009;47:1037–45. doi:10.1128/JCM.01381-08.
- Matson DO, Abdel-Messih IA, Schlett CD, Bok K, Wienkopff T, Wierzba TF, Sanders JW, Frenck RW Jr. Rotavirus genotypes among hospitalized children in Egypt, 2000-2002. J Infect Dis. 2010;202:S263–5. doi:10.1086/653581.
- Kamel AH, Ali MA, El-Nady HG, Aho S, Pothier P, Belliot G. Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. J Appl Microbiol. 2010;108:1620–29. doi:10.1111/j.1365-2672.2009.04562.x.
- El Sherif M, Esona MD, Wang Y, Gentsch JR, Jiang B, Glass RI, Abou Baker S, Klena JD. Detection of the first G6P[14] human rotavirus strain from a child with diarrhea in Egypt. Infect Genet Evol. 2011;11:1436–42. doi:10.1016/j.meegid.2011.05.012.
- Ahmed SF, Mansour AM, Klena JD, Husain TS, Hassan KA, Mohamed F, Steele D. Rotavirus genotypes associated with acute diarrhea in Egyptian infants. Pediatr Infect Dis J. 2014;33(Suppl 1):S62–8. doi:10.1097/INF.0000000000000052.
- El-Senousy WM, Ragab AM, Handak EM. Prevalence of rotaviruses groups A and C in egyptian children and aquatic environment. Food Environ Virol. 2015;7:132–41. doi:10.1007/s12560-015-9184-6.
- Shoeib AR, Hull JJ, Jiang B. Rotavirus G and P types in children with acute diarrhea in Cairo, Egypt, 2011-2012. J Egypt Public Health Assoc. 2015;90:121–24. doi:10.1097/01.EPX.0000470849.84604.13.
- Ibrahim SB, El-Bialy AA, Mohammed MS, El-Sheikh AO, Elhewala A, Bahgat S. Detection of rotavirus in children with acute gastroenteritis in zagazig university hospitals in Egypt. Electron Physician. 2015;7:1227–33. doi:10.14661/1227.
- El-Shabrawi M, Salem M, Abou-Zekri M, El-Naghi S, Hassanin F, El-Adly T, El-Shamy A. The burden of different pathogens in acute diarrhoeal episodes among a cohort of Egyptian children less than five years old. Prz Gastroenterol. 2015;10:173–80. doi:10.5114/pg.2015.51186.
- Saudy N, Elshabrawy WO, Megahed A, Foad MF, Mohamed AF. Genotyping and clinicoepidemiological characterization of rotavirus acute gastroenteritis in Egyptian Children. Pol J Microbiol. 2017;65:433–42. doi:10.5604/17331331.1227669.
- Amini S, Solati AA, Fayaz A, Mahmoodi M. Rotavirus infection in children with acute diarrhea in Tehran. Med J Islam Repub Iran. 1990;4:25–28.
- Khalili B, Cuevas LE, Reisi N, Dove W, Cunliffe NA, Hart CA. Epidemiology of rotavirus diarrhoea in Iranian children. J Med Virol. 2004;73:309–12. doi:10.1002/jmv.20092.
- Zarnani AH, Modarres S, Jadali F, Sabahi F, Moazzeni SM, Vazirian F. Role of rotaviruses in children with acute diarrhea in Tehran, Iran. J Clin Virol. 2004;29:189–93. doi:10.1016/S1386-6532(03)00123-9.
- Samarbafzadeh A, Tehrani EM, Makvandi M, Taremi M. Epidemiological aspects of rotavirus infection in Ahwaz, Iran. J Health Popul Nutr. 2005;23:245–49.
- Farahtaj F, Gallimore CI, Iturriza-Gomara M, Taremi M, Zali MR, Edalatkhah H, Fayaz A, Gray JJ. Rotavirus VP7, VP4 and VP6 genotypes co-circulating in Tehran, Iran, between 2003 and 2004. Epidemiol Infect. 2007;135:834–38. doi:10.1017/S0950268806007485.
- Kordidarian R, Kelishadi R, Arjmandfar Y. Nosocomial infection due to rotavirus in infants in Alzahra Hospital, Isfahan, Iran. J Health Popul Nutr. 2007;25:231–35.
- Modarres S, Rahbarimanesh AA, Karimi M, Modarres S, Motamedi-Rad M, Sohrabi A, Nasiri-Oskoii N. Electrophoretic RNA genomic profiles of rotavirus strains prevailing among hospitalized children with acute gastroenteritis in tehran, iran. Arch Iran Med. 2008;11:526–31.
- Eesteghamati A, Gouya M, Keshtkar A, Najafi L, Zali MR, Sanaei M, Yaghini F, El Mohamady H, Patel M, Klena JD, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in iran. J Infect Dis. 2009;200(Suppl 1):S244–7. doi:10.1086/605050.
- Alrifai SB, Alsaadi A, Mahmood YA, Ali AA, Al-Kaisi LA. Prevalence and etiology of nosocomial diarrhoea in children < 5 years in Tikrit teaching hospital. East Mediterr Health J. 2009;15:1111–18.
- Sadeghian A, Hamedi A, Sadeghian M, Sadeghian H. Incidence of rotavirus diarrhea in children under 6 years referred to the pediatric emergency and clinic of ghaem hospital, Mashhad, Iran. Acta Med Iran. 2010;48:263–65.
- Hamkar R, Yahyapour Y, Noroozi M, Nourijelyani K, Jalilvand S, Adibi L, Vaziri S, Poor-Babaei A, Pakfetrat A, Savad-Koohi R. Prevalence of rotavirus, adenovirus, and astrovirus infections among patients with acute gastroenteritis in, Northern Iran. Iran J Public Health. 2010;39:45–51.
- Ghorashi Z, Behbahan AG, Oskouei SA. Rotavirus enteric infection in children of northwest Iran. Pediatr Infect Dis J. 2011;30:616–18. doi:10.1097/INF.0b013e31820a45cb.
- Modaress S, Rahbarimanesh AA, Edalat R, Sohrabi A, Modarres S, Gomari H, Motamedirad M, Sayari AA. Human rotavirus genotypes detection among hospitalized children, a study in Tehran, Iran. Arch Iran Med. 2011;14:39–45. doi: 1011141/AIM.009.
- Kargar M, Zare M, Najafi A. Molecular epidemiology of rotavirus strains circulating among children with gastroenteritis in Iran. Iran J Pediatr. 2012;22:63–69.
- Najafi A, Kargar M, Jafarpour T. Burden and typing of rotavirus group a in children with acute gastroenteritis in shiraz, southern iran. Iran Red Crescent Med J. 2012;14:531–40.
- Shoja Z, Jalilvand S, Mokhtari-Azad T, Nategh R. Epidemiology of cocirculating human rotaviruses in Iran. Pediatr Infect Dis J. 2013;32:e178–81. doi:10.1097/INF.0b013e31827ee392.
- Kargar M, Akbarizadeh AR. Prevalence and molecular genotyping of group a rotaviruses in Iranian children. Indian J Virol. 2012;23:24–28. doi:10.1007/s13337-012-0070-7.
- Motamedifar M, Amini E, Talezadeh Shirazi P. Frequency of rotavirus and adenovirus gastroenteritis among children in shiraz, iran. Iran Red Crescent Med J. 2013;15:729–33. doi:10.5812/ircmj.4415.
- Najafi A, Najafi S, Vahdat K, Kargar M, Javdani N. Importance of viral pathogens in children with acute gastroenteritis in the south of Iran. Ann Saudi Med. 2013;33:124–29. doi:10.5144/0256-4947.2013.124.
- Shokrollahi MR, Noorbakhsh S, Monavari HR, Ghavidel Darestani S, Vosoughi Motlagh A, Javadi Nia S. Acute nonbacterial gastroenteritis in hospitalized children: a cross sectional study. Jundishapur J Microbiol. 2014;7:e11840. doi:10.5812/jjm.
- Moradi-Lakeh M, Shakerian S, Yaghoubi M, Esteghamati A, Shokraneh F, Baradaran HR, Ghanaee RM. Rotavirus infection in children with acute gastroenteritis in Iran: a systematic review and meta-analysis. Int J Prev Med. 2014;5:1213–23.
- Khoshdel A, Parvin N, Doosti A, Eshraghi A. Prevalence and molecular characterization of rotaviruses as causes of nosocomial diarrhea in children. Turk J Pediatr. 2014;56:469–74.
- Kargar M, Khodadadi P, Najafi A, Ansari H. Predominance of rotavirus G8 genotype in hospitalized children with acute gastroenteritis in Yasuj, Iran. Eur Rev Med Pharmacol Sci. 2014;18:699–702.
- Sharifi-Rad J, Hoseini Alfatemi SM, Sharifi-Rad M, Miri A. Frequency of adenoviruses, rotaviruses and noroviruses among diarrhea samples collected from infants of zabol, southeastern iran. Jundishapur J Microbiol. 2015;8:e15440. doi:10.5812/jjm.
- Azaran A, Makvandi M, Samarbafzadeh A, Neisi N, Hoseinzadeh M, Rasti M, Teymurirad M, Teimoori A, Varnaseri M, Makvandi K. Study on Rotavirus Infection and Its Genotyping in Children Below 5 Years in South West Iran. Iran J Pediatr. 2016;26:e2080.
- Mousavi Nasab SD, Sabahi F, Makvandi M, Mirab Samiee S, Nadji SA, Ravanshad M. Epidemiology of rotavirus-norovirus co-infection and determination of norovirus genogrouping among children with acute gastroenteritis in Tehran, Iran. Iran Biomed J. 2016;20:280–86.
- Monavari SHR, Hadifar S, Mostafaei S, Miri A, Keshavarz M, Babaei F, Moghoofei M. Epidemiology of rotavirus in the iranian children: a systematic review and meta-analysis. J Glob Infect Dis. 2017;9:66–72. doi:10.4103/0974-777X.205173.
- Azaran A, Makvandi M, Teimoori A, Ebrahimi S, Heydari F, Nikfar R. Distribution of rotavirus genotypes ccirculating in Ahvaz, Iran in 2016. Iran Biomed J. 2018;22:107–16.
- Modarres S, Modarres S, Oskoii NN. Rotavirus infection in infants and young children with acute gastroenteritis in the islamic republic of Iran. La Revue de Santé de la Méditerranée Orientale. 1995;1:210–14.
- Shahrzad M, Rahbarimanesh A, Shahab M, Faghihzadeh S, Jamafzon F, Karimi M. The role of Rotavirus in acute gastroenteritis and molecular epidemiology patterns of Rotavirus infection in hospitalized children in Tehran. Iranian J Infect Dis Trop. 2005;10:21–29.
- Taremi M, Farahtaj F, Gachkar L, Adalatkhah H, Zali MR, Fayaz A. Epidemiological survey of Rotavirus infection among children less than 5 years with acute diarrhea admitted in markaz tebbi pediatric hospital, Tehran, 2003-4. Iranian J Infect Dis Trop. 2005;10:13–22.
- Kazemi A, Tabatabaie F, Agha-Ghazvini MR, Kelishadi R. The role of Rotavirus in acute pediatric diarrhea in Isfahan, Iran. Pak J Med Sci. 2006;22:282–85.
- Yahyapour Y, Savadkouhi R, Hajian KA, Jalilvand S, Hamkar R. Prevalence of rota, adeno and astrovirus in children with acute gastroenteritis in Babol, Iran. J Gorgan Univer Med Sci. 2008;10:65–70.
- Zaraei-Mahmoodabadi B, Kargar M, Tabatabaei H, Saedegipour S, Ghaemi A, Nategh R. Determination of annual incidence, age specific incidence rate and risk of rotavirus gastroenteritis among children in Iran. Iranian J Virol. 2009;3:39–42.
- Ataei-Pirkooh A, Shamsi-Shahrabadi M, Haghi-Ashtiani MT. Incidence of coinfection between rotavirus and some enteropathogenic agents in children referred to children medical center hospital, Tehran, 2009. Iranian J Virol. 2011;5:23–27. doi:10.21859/isv.5.1.23.
- Kangar M, Najafi A, Zandi K, Hashemizadeh Z. Genotypic distribution of rotavirus strains causing severe gastroenteritis in children under 5 years old in Borazjan, Iran. Afr J Microbiol Res. 2011;5:2936–41. doi:10.5897/AJMR11.347.
- Ahmed HM, Coulter JB, Nakagomi O, Hart CA, Zaki JM, Al-Rabaty AA, Dove W, Cunliffe NA. Molecular characterization of rotavirus gastroenteritis strains, Iraqi Kurdistan. Emerg Infect Dis. 2006;12:824–26. doi:10.3201/eid1205.051422.
- Ahmed S, Klena J, Albana A, Alhamdani F, Oskoff J, Soliman M, Heylen E, Teleb N, Husain T, Matthijnssens J. Characterization of human rotaviruses circulating in Iraq in 2008: atypical G8 and high prevalence of P[6] strains. Infect Genet Evol. 2013;16:212–17. doi:10.1016/j.meegid.2012.12.003.
- Nimri LF, Hijazi S. Rotavirus-associated diarrhoea in children in a refugee camp in Jordan. J Diarrhoeal Dis Res. 1996;14:1–4.
- Meqdam MM, Youssef MT, Nimri LF, Shurman AA, Rawashdeh MO, al-Khdour MS. Viral gastroenteritis among young children in northern Jordan. J Trop Pediatr. 1997;43:349–52. doi:10.1093/tropej/43.6.349.
- Youssef M, Shurman A, Bougnoux M, Rawashdeh M, Bretagne S, Strockbine N. Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan. FEMS Immunol Med Microbiol. 2000;28:257–63. doi:10.1111/j.1574-695X.2000.tb01485.x.
- Battikhi MN. Epidemiological study on Jordanian patients suffering from diarrhoea. New Microbiol. 2002;25:405–12.
- Nimri LF, Elnasser Z, Batchoun R. Polymicrobial infections in children with diarrhoea in a rural area of Jordan. FEMS Immunol Med Microbiol. 2004;42:255–59. doi:10.1016/j.femsim.2004.05.014.
- Khuri-Bulos N, Al Khatib M. Importance of rotavirus as a cause of gastroenteritis in Jordan: a hospital based study. Scand J Infect Dis. 2006;38:639–44. doi:10.1080/00365540600606515.
- Nafi O. Rotavirus gastroenteritis among children aged under 5 years in Al Karak, Jordan. East Mediterr Health J. 2010;16:1064–69.
- Kaplan NM, Kirby A, Abd-Eldayem SA, Dove W, Nakagomi T, Nakagomi O, Cunliffe NA. Detection and molecular characterisation of rotavirus and norovirus infections in Jordanian children with acute gastroenteritis. Arch Virol. 2011;156:1477–80. doi:10.1007/s00705-011-0996-x.
- Salem K, Bdour S, Zeller M, Van Ranst M, Matthijnssens J. Genotypes of rotavirus strains circulating in Amman, Jordan, in 2006/07 and their significance for the potential effectiveness of future rotavirus vaccination. Arch Virol. 2011;156:1543–50. doi:10.1007/s00705-011-1028-6.
- Marmash RW, Dalwai AK, Szucs G, Molla AM, Pacsa AS, Al-Nakib W, Albert MJ. Genotypic characterization of rotaviruses and prevalence of serotype-specific serum antibodies in children in Kuwait. Epidemiol Infect. 2007;135:1331–37. doi:10.1017/S0950268807007868.
- Albert MJ, Rotimi VO, Iqbal J, Chehadeh W. Evaluation of the xTAG gastrointestinal pathogen panel assay for the detection of enteric pathogens in Kuwait. Med Princ Pract. 2016;25:472–76. doi:10.1159/000447698.
- Al-Ali RM, Chehadeh W, Hamze M, Dabboussi F, Hlais S, Mallat H. First description of gastroenteritis viruses in Lebanese children: a pilot study. J Infect Public Health. 2011;4:59–64. doi:10.1016/j.jiph.2011.01.002.
- Ali Z, Harastani H, Hammadi M, Reslan L, Ghanem S, Hajar F, Sabra A, Haidar A, Inati A, Rajab M, et al. Rotavirus genotypes and vaccine effectiveness from a sentinel, hospital-based, surveillance study for three consecutive rotavirus seasons in Lebanon. PLoS One. 2016;11:e0161345. doi:10.1371/journal.pone.0161345.
- Dbaibo G, Rajab M, Inati A, Mikhael R, Choueiry E, Al-Tannir M, Salam O, Ramakrishnan G, DeAntonio R. Hospital-based surveillance study of Rotavirus gastroenteritis in children under 5 years of age in Lebanon. Trials Vaccinol. 2013;2:25–30. doi:10.1016/j.trivac.2013.08.002.
- Kalaf RN, Elahmer OR, Zorgani AA, Ghenghesh KS. Rotavirus in children with diarrhea in Tripoli, Libya. Libyan J Med. 2011;6:6041. doi:10.3402/ljm.v6i0.6041.
- Rahouma A, Klena JD, Krema Z, Abobker AA, Treesh K, Franka E, Abusnena O, Shaheen HI, El Mohammady H, Abudher A, et al. Enteric pathogens associated with childhood diarrhea in Tripoli-Libya. Am J Trop Med Hyg. 2011;84:886–91. doi:10.4269/ajtmh.2011.11-0116.
- Alkoshi S, Ernst K, Maimaiti N, Dahlui M. Rota viral infection: a significant disease burden to libya. Iran J Public Health. 2014;43:1356–63.
- Abugalia M, Cuevas L, Kirby A, Dove W, Nakagomi O, Nakagomi T, Kara M, Gweder R, Smeo M, Cunliffe N. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J Med Virol. 2011;83:1849–56. doi:10.1002/jmv.22141.
- Alkoshi S, Leshem E, Parashar UD, Dahlui M. Anticipating rotavirus vaccines–a pre-vaccine assessment of incidence and economic burden of rotavirus hospitalizations among children <5 year of age in Libya, 2012-13. BMC Public Health. 2015;15:26.
- Benhafid M, Youbi M, Klena JD, Gentsch JR, Teleb N, Widdowson MA, Elaouad R. Epidemiology of rotavirus gastroenteritis among children <5 years of age in Morocco during 1 year of sentinel hospital surveillance, June 2006-May 2007. J Infect Dis. 2009;200(Suppl 1):S70–5. doi:10.1086/605048.
- Benhafid M, Rguig A, Trivedi T, Elqazoui M, Teleb N, Mouane N, Maltouf AF, Parashar U, Patel M, Aouad RE. Monitoring of rotavirus vaccination in Morocco: establishing the baseline burden of rotavirus disease. Vaccine. 2012;30:6515–20. doi:10.1016/j.vaccine.2012.08.058.
- Bentama I, Soussi I, Ghanimi Z, Riane S, Tligui H, Mdaghri Alaoui A, Izgua AT. [Epidemic of nosocomial infection by rotavirus in a neonatology service]. Rev Med Brux. 2012;33:519–24.
- Benhafid M, Elomari N, Elqazoui M, Meryem AI, Rguig A, Filali-Maltouf A, Elaouad R. Diversity of rotavirus strains circulating in children under 5 years of age admitted to hospital for acute gastroenteritis in Morocco, June 2006 to May 2009. J Med Virol. 2013;85:354–62. doi:10.1002/jmv.23445.
- El Qazoui M, Oumzil H, Baassi L, El Omari N, Sadki K, Amzazi S, Benhafid M, El Aouad R. Rotavirus and norovirus infections among acute gastroenteritis children in Morocco. BMC Infect Dis. 2014;14:300. doi:10.1186/1471-2334-14-300.
- Benmessaoud R, Jroundi I, Nezha M, Moraleda C, Tligui H, Seffar M, Alvarez-Martínez MJ, Pons MJ, Chaacho S, Hayes EB, et al. Aetiology, epidemiology and clinical characteristics of acute moderate-to-severe diarrhoea in children under 5 years of age hospitalized in a referral paediatric hospital in Rabat, Morocco. J Med Microbiol. 2015;64:84–92. doi:10.1099/jmm.0.079830-0.
- Benhafid M, Elomari N, Azzouzi Idrissi M, Rguig A, Gentsch JR, Parashar U, Elaouad R. Effect of monovalent rotavirus vaccine on rotavirus disease burden and circulating rotavirus strains among children in Morocco. J Med Virol. 2015;87:944–53. doi:10.1002/jmv.24122.
- Aouad FZ. Diarrhées à Rotavirus - Etude rétrospective à l’hôpital militaire d’instruction Mohammed V de Rabat. 2011. ( PhD thesis).
- Aghoutane M. Evaluation du système de surveillance des gastroentérites à Rotavirus chez les enfants de moins de 5 ans au Maroc. 2012. ( PhD thesis).
- Aithala G, Al Dhahry SH, Saha A, Elbualy MS. Epidemiological and clinical features of rotavirus gastroenteritis in Oman. J Trop Pediatr. 1996;42:54–57. doi:10.1093/tropej/42.1.54.
- Al Awaidy SA, Bawikar S, Al Busaidy S, Baqiani S, Al Abedani I, Varghese R, Abdoan HS, Al Abdoon H, Bhatnagar S, Al Hasini KS, et al. Considerations for introduction of a rotavirus vaccine in Oman: rotavirus disease and economic burden. J Infect Dis. 2009;200(Suppl 1):S248–53. doi:10.1086/605339.
- Al Baqlani S, Peenze I, Dewar J, Al Lawati Z, Pearson L, Rupa V, Mothokoa C, Al Awaidy S, Al Busaidy S, Steele AD. Molecular characterization of rotavirus strains circulating in Oman in 2005. J Infect Dis. 2010;202(Suppl):S258–62. doi:10.1086/653582.
- Al Baqlani S, Al Awaidy S, Al Lawati Z, Al Toubi M, Sajina F, Teleb N, Al Busaidy S. Molecular characterization of group A Rotavirus genotypes circulating in Oman between 2009 and 2013. J Virol Emerg Dis. 2016;2:1–6.
- Abu-Elamreen FH, Abed AA, Sharif FA. Viral, bacterial and parasitic etiology of pediatric diarrhea in Gaza, Palestine. Med Princ Pract. 2008;17:296–301. doi:10.1159/000129609.
- Laham NA, Elyazji M, Al-Haddad R, Ridwan F. Prevalence of enteric pathogen-associated community gastroenteritis among kindergarten children in Gaza. J Biomed Res. 2015;29:61–68. doi:10.7555/JBR.29.20130108.
- Mubashir M, Khan A, Baqai R, Iqbal J, Ghafoor A, Zuberi S, Burney MI. Causative agents of acute diarrhoea in the first 3 years of life: hospital-based study. J Gastroenterol Hepatol. 1990;5:264–70.
- Huilan S, Zhen LG, Mathan MM, Mathew MM, Olarte J, Espejo R, Khin Maung U, Ghafoor MA, Khan MA, Sami Z, et al. Etiology of acute diarrhoea among children in developing countries: a multicentre study in five countries. Bull World Health Organ. 1991;69:549–55.
- Agboatwalla M, Isomura S, Akram DS, Isihara Y, Sakae K, Yamashita T, Nishuo O. Enteric viral infections in pre-school children in Karachi, Pakistan. Indian J Pediatr. 1995;62:345–51.
- Nishio O, Matsui K, Oka T, Ushijima H, Mubina A, Dure-Samin A, Isomura S. Rotavirus infection among infants with diarrhea in Pakistan. Pediatr Int. 2000;42:425–27.
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–S15. doi:10.1086/605025.
- Iftikhar T, Butt A, Nawaz K, Sarwar Y, Ali A, Mustafa T, Haque A. Genotyping of rotaviruses detected in children admitted to hospital from Faisalabad Region, Pakistan. J Med Virol. 2012;84:2003–07. doi:10.1002/jmv.23402.
- Alam MM, Khurshid A, Shaukat S, Suleman RM, Sharif S, Angez M, Malik SA, Ahmed TM, Aamir UB, Naeem M, et al. Epidemiology and genetic diversity of rotavirus strains in children with acute gastroenteritis in Lahore, Pakistan. PLoS One. 2013;8:e67998. doi:10.1371/journal.pone.0067998.
- Tamim S, Hasan F, Matthijnssens J, Sharif S, Shaukat S, Alam MM, Angez M, Suleman Rana M, Khurshid A, Zaidi SS. Epidemiology and phylogenetic analysis of VP7 and VP4 genes of rotaviruses circulating in Rawalpindi, Pakistan during 2010. Infect Genet Evol. 2013;14:161–68. doi:10.1016/j.meegid.2012.10.009.
- Habib MI, Kazi SG, Ahmed Khan KM, Zia N. Rota virus Diarrhea in Hospitalized Children. J Coll Physicians Surg Pak. 2014;24:114–17. doi: 02.2014/JCPSP.114117.
- Alam MM, Khurshid A, Shaukat S, Rana MS, Sharif S, Angez M, Nisar N, Aamir UB, Naeem M, Zaidi SSZ. Viral etiologies of acute dehydrating gastroenteritis in pakistani children: confounding role of parechoviruses. Viruses. 2015;7:378–93. doi:10.3390/v7010378.
- Umair M, Abbasi BH, Nisar N, Alam MM, Sharif S, Shaukat S, Rana MS, Khurshid A, Mujtaba G, Aamir UB, et al. Molecular analysis of group A rotaviruses detected in hospitalized children from Rawalpindi, Pakistan during 2014. Infect Genet Evol. 2017;53:160–66. doi:10.1016/j.meegid.2017.05.009.
- Shah Y, Syed KRR, Hafız A. Detection of Rotavirus from diarrheal stool specimens. J Surgery Pakistan. 1999;4:20–23.
- Qazi R, Sultana S, Sundar S, Warraich H, un-Nisa T, Rais A, Ak Z. Population-based surveillance for severe rotavirus gastroenteritis in children in Karachi, Pakistan. Vaccine. 2009;27(Suppl 5):F25–30. doi:10.1016/j.vaccine.2009.08.064.
- Kazi AM, Warraich GJ, Qureshi S, Qureshi H, Khan MM, Zaidi AK, members of the Pakistan Rotavirus Study Group. Sentinel hospital-based surveillance for assessment of burden of rotavirus gastroenteritis in children in Pakistan. PLoS One. 2014;9:e108221. doi:10.1371/journal.pone.0108221.
- Afzal A, Tariq PA, Choudhry S. Rota virus gastroenteritis in children upto five years of age. J Rawapindi Med Coll. 2010;14:33–35.
- Al-Thani A, Baris M, Al-Lawati N, Al-Dhahry S. Characterising the aetiology of severe acute gastroenteritis among patients visiting a hospital in Qatar using real-time polymerase chain reaction. BMC Infect Dis. 2013;13:329. doi:10.1186/1471-2334-13-329.
- El Assouli SM, Banjar ZM, Mohammed KA, Ft Z. Rotavirus infection in children in Saudi Arabia. Am J Trop Med Hyg. 1992;46:272–77.
- Mohammed KA, El Assouli SM, Banjar ZM. Human rotavirus subgroups and serotypes in children with acute gastroenteritis in Saudi Arabia from 1988 to 1992. J Med Virol. 1994;44:237–42.
- Milaat WA, Elassouli SM. Epidemiology of diarrhoea in two major cities in Saudi Arabia. J Commun Dis. 1995;27:84–91.
- El Assouli SM, Mohammed KA, Banjar ZM. Human rotavirus genomic RNA electropherotypes in Jeddah, Saudi Arabia from 1988 to 1992. Ann Trop Paediatr. 1995;15:45–53.
- elAssouli SM, Banjar ZM, Mohammed KA, Milaat WA, Mz E. Genetic and antigenic analysis of human rotavirus prevalent in Al-Taif, Saudi Arabia. J Trop Pediatr. 1996;42:211–19. doi:10.1093/tropej/42.4.211.
- el-Assouli SM. Inter-relationships among subgroups, serotypes, and electropherotypes of rotaviruses isolated from humans. J Diarrhoeal Dis Res. 1996;14:201–06.
- el-Sheikh SM, el-Assouli SM. Prevalence of viral, bacterial and parasitic enteropathogens among young children with acute diarrhoea in Jeddah, Saudi Arabia. J Health Popul Nutr. 2001;19:25–30.
- Ghazi HO, Khan MA, Telmesani AM, Idress B, Mahomed MF. Rotavirus infection in infants and young children in Makkah, Saudi Arabia. J Pak Med Assoc. 2005;55:231–34.
- Kheyami AM, Cunliffe NA, Hart CA. Rotavirus infection in Saudi Arabia. Ann Saudi Med. 2006;26:184–91.
- Kheyami AM, Nakagomi T, Nakagomi O, Dove W, Hart CA, Cunliffe NA. Molecular epidemiology of rotavirus diarrhea among children in Saudi Arabia: first detection of G9 and G12 strains. J Clin Microbiol. 2008;46:1185–91. doi:10.1128/JCM.02244-07.
- Kheyami AM, Areeshi MY, Dove W, Nakagomi O, Cunliffe NA, Anthony Hart C. Characterization of rotavirus strains detected among children and adults with acute gastroenteritis in Gizan, Saudi Arabia. Saudi Med J. 2008;29:90–93.
- Tayeb HT, Dela Cruz DM, Al-Qahtani A, Al-Ahdal MN, Carter MJ. Enteric viruses in pediatric diarrhea in Saudi Arabia. J Med Virol. 2008;80:1919–29. doi:10.1002/jmv.21291.
- Kheyami AM. Rotavirus gastroenteritis and strain diversity in Saudi Arabia. Current status and future prospects. Saudi Med J. 2010;31:276–79.
- Johargy A, Ghazi H, Mumenah A. Frequency of viral, bacterial and parasitic enteropathogens among young children with acute diarrhoea in Saudi Arabia. J Pak Med Assoc. 2010;60:456–59.
- Tayeb HT, Balkhy HH, Aljuhani SM, Elbanyan E, Alalola S, Alshaalan M. Increased prevalence of rotavirus among children associated gastroenteritis in Riyadh Saudi Arabia. Virol J. 2011;8:548. doi:10.1186/1743-422X-8-548.
- Afifi R, Nabiha M. The burden of Rotavirus gastroenteritis among hospitalized pediatric patients in a tertiary referral hospital in Jeddah. Ann Saudi Med. 2013;33:241–46. doi:10.5144/0256-4947.2013.241.
- Abdel-Moneim AS, Al-Malky MI, Alsulaimani AA, Abuelsaad AS, Mohamed I, Ismail AK. Sequence diversity of VP4 and VP7 genes of human rotavirus strains in Saudi Arabia. Foodborne Pathog Dis. 2015;12:937–44. doi:10.1089/fpd.2015.1990.
- Khalil M, Azhar E, Kao M, Al-Kaiedi N, Alhani H, Al Olayan I, Pawinski R, Gopala K, Kandeil W, Anis S, et al. Gastroenteritis attributable to rotavirus in hospitalized Saudi Arabian children in the period 2007-2008. Clin Epidemiol. 2015;7:129–37. doi:10.2147/CLEP.S69502.
- Aly M, Al Khairy A, Al Johani S, Balkhy H. Unusual rotavirus genotypes among children with acute diarrhea in Saudi Arabia. BMC Infect Dis. 2015;15:192. doi:10.1186/s12879-015-0923-y.
- Hegazi MA, Sayed MH, Sindi HH, Bekhit OE, El-Deek BS, Alshoudri FM, Noorelahi AK. Is rotavirus still a major cause for diarrheal illness in hospitalized pediatric patients after rotavirus vaccine introduction in the Saudi national immunization program? Medicine (Baltimore). 2017;96:e6574. doi:10.1097/MD.0000000000006574.
- Al-Ayed MS, Asaad AM, Qureshi MA, Hawan AA. Epidemiology of group A rotavirus infection after the introduction of monovalent vaccine in the national immunization program of Saudi Arabia. J Med Virol. 2017;89:429–34. doi:10.1002/jmv.24664.
- Gargano LM, Tate JE, Parashar UD, Omer SB, Cookson ST. Comparison of impact and cost-effectiveness of rotavirus supplementary and routine immunization in a complex humanitarian emergency, Somali case study. Confl Health. 2015;9:5. doi:10.1186/s13031-015-0032-y.
- Ope M, Nyoka R, Unshur A, Oyier FO, Mowlid SA, Owino B, Ochieng SB, Okello CI, Montgomery JM, Wagacha B, et al. Evaluation of the field performance of immunoCard STAT !® rapid diagnostic test for rotavirus in dadaab refugee camp and at the kenya-somalia border. Am J Trop Med Hyg. 2017;96:1302–06. doi:10.4269/ajtmh.16-0885.
- Elhag WI, Saeed HA, Omer El FE, Ali AS. Prevalence of rotavirus and adenovirus associated with diarrhea among displaced communities in Khartoum, Sudan. BMC Infect Dis. 2013;13:209. doi:10.1186/1471-2334-13-209.
- Mustafa A, Makki A, Siddig O, Haithami S, Teleb N, Trivedi T, Parashar U, Patel M. Baseline burden of rotavirus disease in Sudan to monitor the impact of vaccination. Pediatr Infect Dis J. 2014;33(Suppl 1):S23–7. doi:10.1097/INF.0000000000000095.
- Magzoub MA, Bilal NE, Bilal JA, Osman OF. Rotavirus infection among Sudanese children younger than 5 years of age: a cross sectional hospital-based study. Pan Afr Med J. 2013;16:88. doi:10.11604/pamj.2013.16.88.2519.
- Saeed A, Abd H, Sandstrom G. Microbial aetiology of acute diarrhoea in children under five years of age in Khartoum, Sudan. J Med Microbiol. 2015;64:432–37. doi:10.1099/jmm.0.000043.
- Bouanane I Surveillance de gastroentérites à Rotavirus chez les enfants de moins de 5 ans en Tunisie. 2011. ( PhD thesis).
- Moalla H, Fendri C. [Etiology of acute diarrhea in children]. Tunis Med. 1994;72:25–28.
- Trabelsi A, Peenze I, Pager C, Jeddi M, Steele D. Distribution of rotavirus VP7 serotypes and VP4 genotypes circulating in Sousse, Tunisia, from 1995 to 1999: emergence of natural human reassortants. J Clin Microbiol. 2000;38:3415–19.
- Fodha I, Chouikha A, Peenze I, De Beer M, Dewar J, Geyer A, Messaadi F, Trabelsi A, Boujaafar N, Taylor MB, et al. Identification of viral agents causing diarrhea among children in the Eastern Center of Tunisia. J Med Virol. 2006;78:1198–203. doi:10.1002/jmv.20681.
- Al-Gallas N, Bahri O, Bouratbeen A, Ben Haasen A, Ben Aissa R. Etiology of acute diarrhea in children and adults in Tunis, Tunisia, with emphasis on diarrheagenic Escherichia coli: prevalence, phenotyping, and molecular epidemiology. Am J Trop Med Hyg. 2007;77:571–82.
- Chouikha A, Fodha I, Noomen S, Bouzid L, Mastouri M, Peenze I, De Beer M, Dewar J, Geyer A, Sfar T, et al. Group A rotavirus strains circulating in the eastern center of Tunisia during a ten-year period (1995-2004). J Med Virol. 2007;79:1002–08. doi:10.1002/(ISSN)1096-9071.
- Sdiri-Loulizi K, Gharbi-Khelifi H, de Rougemont A, Chouchane S, Sakly N, Ambert-Balay K, Hassine M, Guédiche MN, Aouni M, Pothier P. Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J Clin Microbiol. 2008;46:1349–55. doi:10.1128/JCM.02438-07.
- Tinsa F, Brini I, Yahyaoui S, Bousenna O, Bousetta K, Trabelsi A, Bossina S. [Infectious diarrhoea in children under five years]. Tunis Med. 2009;87:599–602.
- Chouikha A, Fodha I, Bouslama L, Ben Hadj Fredj M, Jaoua S, Boujaafar N, Trabelsi A, Steele AD. Emergence and characterization of human rotavirus g9 strains in Tunisia. J Infect Dis. 2009;200(Suppl 1):S239–43. doi:10.1086/605029.
- Trabelsi A, Fodha I, Chouikha A, Ben Hadj Fredj M, Mastouri M, Abdelaziz AB, Sfar T, Essoussi AS, Jaoua S, Steele AD. Rotavirus strain diversity in the centre coast of Tunisia from 2000 through 2003. J Infect Dis. 2010;202(Suppl):S252–7. doi:10.1086/653580.
- Sdiri-Loulizi K, Ambert-Balay K, Gharbi-Khelifi H, Hassine M, Chouchane S, Sakly N, Neji-Guédiche M, Pothier P, Aouni M. Molecular epidemiology and clinical characterization of group A rotavirus infections in Tunisian children with acute gastroenteritis. Can J Microbiol. 2011;57:810–19. doi:10.1139/w11-074.
- Hassine-Zaafrane M, Sdiri-Loulizi K, Ben Salem I, Kaplon J, Ayouni S, Ambert-Balay K, Sakly N, Pothier P, Aouni M. The molecular epidemiology of circulating rotaviruses: three-year surveillance in the region of Monastir, Tunisia. BMC Infect Dis. 2011;11:266. doi:10.1186/1471-2334-11-208.
- Chouikha A, Fredj MB, Fodha I, Mathlouthi I, Ardhaoui M, Teleb N, Brini I, Messaadi F, Mastouri M, Sfar T, et al. [Evolution of group A Rotavirus strains circulating in Tunisia over a 3-year period (2005-2007)]. Pathol Biol (Paris). 2011;59:e79–83. doi:10.1016/j.patbio.2009.05.007.
- Chouikha A, Fodha I, Ben Hadj Fredj M, Ardhaoui M, Teleb N, Brini I, Messaadi F, Mastouri M, Sfar T, Hachicha M, et al. Relationship between electropherotypes and VP7/VP4 genotypes of group A rotaviruses detected between 2000 and 2007 in Tunisian children. Pathol Biol (Paris). 2011;59:e43–8. doi:10.1016/j.patbio.2009.04.008.
- Soltani M, Bouanene I, Trabelsi A, Harbi A, Hachicha M, Amri F, Boussnina S, Gueddiche MN, Sfar MT, Teleb N, et al. [Epidemiology of rotavirus gastroenteritis among children under 5 years of age in Tunisia - results of sentinel hospital surveillance 2009 to 2011]. Rev Epidemiol Sante Publique. 2012;60:473–80. doi:10.1016/j.respe.2012.04.005.
- Ben Salem-Ben Nejma I, Hassine ZM, Hassine F, Sdiri-Loulizi K, Ben Said M, Aouni M, Mzoughi R. Etiology of acute diarrhea in tunisian children with emphasis on diarrheagenic escherichia coli: prevalence and identification of E. coli virulence markers. Iran J Public Health. 2014;43:947–60.
- Soltani MS, Salah AB, Bouanene I, Trabelsi A, Sfar MT, Harbi A, Gueddiche MN, Farhat EB. Epidemiology and medical cost of hospitalization due to rotavirus gastroenteritis among children under 5 years of age in the central-east of Tunisia. East Mediterr Health J. 2015;21:584–90.
- Ayouni S, Sdiri-Loulizi K, de Rougemont A, Estienney M, Ambert-Balay K, Aho S, Hamami S, Aouni M, Neji-Guediche M, Pothier P, et al. Rotavirus P[8] infections in persons with secretor and nonsecretor phenotypes, Tunisia. Emerg Infect Dis. 2015;21:2055–58. doi:10.3201/eid2111.141901.
- Moussa A, Ben Hadj Fredj M, Fodha I, BenHamida-Rebai M, Kacem S, Argoubi A, Bennour H, Boujaafar N, Trabelsi A. Distribution of rotavirus VP7 and VP4 genotypes circulating in Tunisia from 2009 to 2014: emergence of the genotype G12. J Med Microbiol. 2016;65:1028–37. doi:10.1099/jmm.0.000305.
- Moussa A, Fredj MB, BenHamida-Rebai M, Fodha I, Boujaafar N, Trabelsi A. Phylogenetic analysis of partial VP7 gene of the emerging human group A rotavirus G12 strains circulating in Tunisia. J Med Microbiol. 2017;66:112–18. doi:10.1099/jmm.0.000420.
- Ijaz MK, Alharbi S, Uduman SA, Cheema Y, Sheek-Hussen MM, Alkhair AR, Shalabi AG, Ijaz SS, Bin-Othman SA, Sattar SA. Seasonality and prevalence of rotavirus in Al-Ain, United Arab Emirates. Clin Diagn Virol. 1994;2:323–29.
- Howidi M, Balhaj G, Yaseen H, Gopala K, Van Doorn LJ, DeAntonio R. Burden and genotyping of rotavirus disease in the United Arab Emirates: a multicenter hospital-based surveillance. Hum Vaccin Immunother. 2014;10:2284–89. doi:10.4161/hv.29386.
- Cheriathu J, Jenny John L, Ignatius Dsouza E, Shamseldeen M, Mathur A. Rotavirus gastroenteritis and nosocomial rotavirus gastroenteritis among children aged under 5 years in United Arab Emirates: epidemiology, clinical profile, demographic characteristics and severity. Arch Dis Child. 2014;99:317–18. doi:10.1136/archdischild-2014-307384.876.
- Kirby A, Al-Eryani A, Al-Sonboli N, Hafiz T, Beyer M, Al-Aghbari N, Al-Moheri N, Dove W, Cunliffe NA, Cuevas LE, et al. Rotavirus and norovirus infections in children in Sana‘a, Yemen. Trop Med Int Health. 2011;16:680–84. doi:10.1111/j.1365-3156.2011.02756.x.
- Al-Badani A, Al-Areqi L, Majily A, Al-Sallami S, Al-Madhagi A, Amood Al-Kamarany M. Rotavirus diarrhea among children in taiz, yemen: prevalence-risk factors and detection of genotypes. Int J Pediatr. 2014;2014:928529. doi:10.1155/2014/928529.
- Banajeh SM, Abu-Asba BA. The epidemiology of all-cause and rotavirus acute gastroenteritis and the characteristics of rotavirus circulating strains before and after rotavirus vaccine introduction in Yemen: analysis of hospital-based surveillance data. BMC Infect Dis. 2015;15:418. doi:10.1186/s12879-015-1165-8.
- Amood Al-Kamarany M, Al-Areqi L, Mujally A, Alkarshy F, Nasser A, Jumaan AO. Diarrheal diseases hospitalization in yemen before and after rotavirus vaccination. Scientifica (Cairo). 2016;2016:8485417.
- Wagner AL, Mubarak MY, Johnson LE, Porth JM, Yousif JE, Boulton ML. Trends of vaccine-preventable diseases in Afghanistan from the Disease Early Warning System, 2009-2015. PLoS One. 2017;12:e0178677. doi:10.1371/journal.pone.0178677.
- Miftah A, Alkoshi SIM, Ernst KC, Nagib SM. Frequency of rota virus infection among children in North-Eastern Region of Libya: A hospital-based study from Almarj. Libyan J Med Sci. 2017;1:76–79. doi:10.4103/LJMS.LJMS_17_17.
- Rennert WP, Hindiyeh M, Abu-Awwad FM, Marzouqa H, Ramlawi A. Introducing rotavirus vaccine to the Palestinian territories: the role of public-private partnerships. J Public Health 2019; 41 (1): e78–e83.
- Health Authority-Abu Dhabi. Vaccination is essential. 2014. [accessed 2018 November 2]. https://www.haad.ae/HAAD/LinkClick.aspx?fileticket=iN8vjsU-LJU%3d&tabid=1329.
- Van Damme P, Giaquinto C, Maxwell M, Todd P, Van der Wielen M, on behalf of the RSG. Distribution of Rotavirus Genotypes in Europe, 2004–2005: the REVEAL Study. J Inf Dis. 2007;195:S17–S25. doi:10.1086/516715.
- Kirkwood CD, Cannan D, Boniface K, Bishop RF, Barnes GL, Australian Rotavirus Surveillance Group. Australian Rotavirus Surveillance Program annual report, 2007/08. Commun Dis Intell Q Rep. 2008;32:425–29.
- Patel MM, de Oliveira LH, Bispo AM, Gentsch J, Parashar UD. Rotavirus P[4]G2 in a vaccinated population, Brazil. Emerg Infect Dis. 2008;14:863–65. doi:10.3201/eid1405.071440.
- Hungerford D, Vivancos R, Read JM, Iturriza-Gόmara M, French N, Cunliffe NA. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med. 2018;16:10. doi:10.1186/s12916-017-0989-z.
- Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006–2014. Vaccine. 2015;33:2097–107. doi:10.1016/j.vaccine.2015.03.016.
- Soares‐Weiser K, MacLehose H, Bergman H, Ben‐Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Syst Rev. 2012;11:CD008521.
- Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Inf Dis. 2014;59:1291–301. doi:10.1093/cid/ciu564.