ABSTRACT
Background: Monoclonal antibody (mAb) drugs are increasingly important for the pharmaceutical industry across the globe. In China, mAb drug developments face many challenges. Multiple policies have been implemented recently to reinforce support in various areas. This study aims to investigate the latest landscape of mAb drugs in China from policy perspectives encompassing R&D, clinical trials, marketing approval, and talent pools.
Methods: Information about mAb drugs approved in the United States, the European Union, Japan, and China by 2017 and mAb-related policies in China were retrieved from government websites and third-party statistical databases for descriptive, statistical, and comparative analysis.
Results and discussion: In China, 21 mAb drugs (10 locally-developed and 11 imported) have so far been approved. For the 11 imported mAb drugs in China, the median drug lag in the marketing approval was estimated at 87.1 months, compared with the U.S. (0 months), the EU (8.9 months), and Japan (43.4 months). However, as far as the dramatically changing innovation supporting system in China is concerned, emergence of new biopharmaceutical companies, transformation of the current drug companies and their shift to antibody therapy, and the pooling of high-level talent contribute to mAb development in China. The number of clinical trials and marketing applications and approvals involving mAb drugs is also growing. Favorable policies will continue to play a role in the sustainable development of mAb drugs in China.
Conclusion: The research showed that the reform of multiple policies and incentives for attracting/retaining high-level talent has evidently been effective in addressing the drug lag of mAb drugs in China. In future development, China should actively monitor the global R&D outcomes and industrial development trends of mAb drugs and make the policy environment more attractive to enable more mAb drugs to be marketed in China as soon as possible.
Introduction
MAb drugs are important therapeutic biological products in the evolution of life-saving disease treatment. An antibody is an immunoglobulin (Ig) synthesized by plasma cells formed by the differentiation of B lymphocytes. Each B lymphocyte produces a unique antigen receptor gene by random rearrangement during maturation to plasma cells. After the body is stimulated by an antigen, a large number of B lymphocytes participate in the immune response, resulting in a variety of antibodies.Citation1 This “antibody population” is called a polyclonal antibody. In molecular biology, a highly uniform antibody produced by a single B cell clone can be obtained, also known as monoclonal antibodies (mAb).Citation2
Frank Macfarlane Burnet (FM Burnet), joint winner of the Nobel Prize in Physiology or Medicine in 1960, proposed the theory of clonal selection, predicting the birth of mAb. The research and application of mAb drugs arose in the 1980s following the approval of muromonab-CD3 (Ortholone OTK3) (a mouse mAb given for prevention of kidney transplant rejection) as the first marketed mAb drug. Unfortunately, the early results were disappointing due to the risks of anaphylactic reaction. The development of mAb drugs continued at a low ebb until 1997 when Genentech’s Rituxan (Rituximab, MabThera) was approved by the U.S. Food and Drug Administration (FDA) for treating non-Hodgkin lymphoma. Rituxan has since been widely applied as a genetically engineered mAb drug.
MAb drugs have now developed into a major branch of therapeutic agents as advances in biotechnology continue to reveal their potential for treating an expanding spectrum of diseases with high specificity and a sound safety profile. The global mAb drug market generated revenue of USD 110.1 billion in 2016. The share of mAb drugs in the global biopharmaceutical industry increased from 10.0% in 2000 to 42.0% in 2016, representing the largest and fastest-growing sub-industry in the modern biopharmaceutical industry. By December 31, 2017, the FDA and the European Medicines Agency (EMA) had approved 10 mAb drugs, resulting in a total of 83 mAb drugs around the world.Citation3
Despite the thriving development of mAb drugs, patients in China have not been able to gain access to all the mAb drugs available elsewhere. It was not until 1999 that the first mAb drug was introduced in the country. Mouse mAbs against Human CD3 Antigen of T Lymphocyte for Injection. Regarding anti-PD-1 or anti-PD-L1 mAb drugs, 5 have already been approved overseas, including 2 anti-PD-1 antibodies (KEYTRUDA [pembrolizumab] and OPDIVO [nivolumab]) and 3 anti-PD-L1 antibodies (TECENTRIQ [atezolizumab], BAVENCIO [avelumab], and IMFINZI [durvalumab]).Citation4 In China, however, only 1 of these mAb drugs has been approved, while 18 other similar mAb drugs have just been approved for clinical trials for cancer treatment. In 2017, only 11 of 83 innovative mAb drugs marketed in other countries were approved in China, indicating a huge drug lag between China and other developed countries. The drug lag of mAb drugs in China is multifaceted. The majority of mAb drugs in China were imported, and the local mAb industry was still in its infancy. The reliance on the imported mAb drugs was further complicated by the administrative burden of the drug approval process. Patients’ needs for appropriate clinical intervention were thus unmet, and their access to the most appropriate medicine was affected.
In order to address the drug lag of mAb drugs in China, multiple policies that support the development of biopharmaceuticals, facilitate transformation of the local pharmaceutical industry, and untangle the administrative procedures of the marketing approval system are underway. However, these recent changes in the dynamics of mAb development in China have not been systematically analyzed and reported. Current literature on mAb drugs in China is limited and mainly reports the R&D updates of mAb. Wang ZhimingCitation5 (2012) introduced the basic characteristics of listed mAb drugs and corresponding targets and analyzed the product development prospects in China. Li MinCitation6 (2013) adopted a market perspective to compare mAb drugs developed locally and overseas and concluded that biosimilars might present a good opportunity for mAb development in China. Shao LimingCitation7 (2016) and Yao XuefangCitation8 (2017) conducted a preliminary analysis of the drug approval system and included mAb drugs in the discussion about the impact of registration fees, clinical data requirements, and conditional approval policies on overall drug innovation. The existing research mainly focused on the characteristics, research and development, market and industrial development of the mAb drugs in China. From the perspective of policy, research on the marketing and development of mAb drugs was more concerned with certain types of policies, such as registration and medical insurance, and systematic policy research on mAb drugs innovation and development is limited. This study aims to comprehensively analyze the current status and future perspectives of mAb drugs development in China in relation to the recent policy reforms. The findings will help to improve the understanding of the reasons for the current drug lag and delayed development of mAb drugs in China and will help navigate the innovation support system that is quickly taking shape. In addition, this research could help foreign scholars and pharmaceutical companies to fully understand the development and policy system of mAb drugs in China in order to develop a reasonable R&D investment and product marketing strategy.
Results and discussion
Inside the pipeline of mAb drugs for the Chinese market
Preclinical development
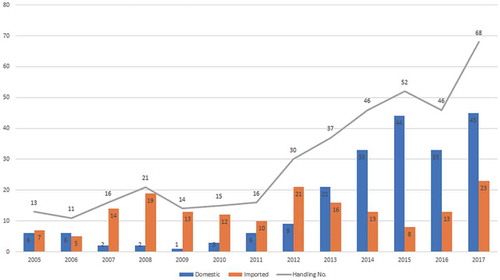
There has been a significant development in mAb drugs in China as R&D input increases and R&D technology matures. By December 2017, there were 385 applications for clinical trials of mAb drugs, of which 211 were developed locally and 174 were imported drugs, involving 144 applicants, of which 92 (64.0%) were domestic enterprises and 52 (36.1%) overseas-funded enterprises. Since 2013, the number of domestic enterprises applying for mAb drug clinical trials began to surpass that of overseas-funded enterprises, and the total number of clinical trial applications for mAb drugs in China has been on a rapidly increasing trend (see ).
Figure 1. Application situation on mAb drugs clinical trials in China. Data source: www.yaozh.com.
Clinical trials
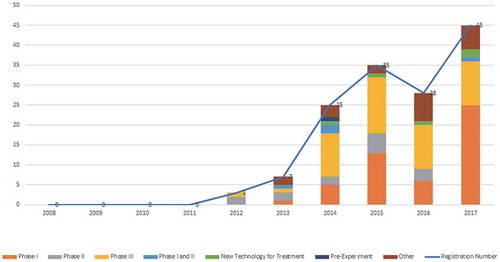
According to the information platform, the number of clinical trial registrations involving mAb drugs has increased by leaps and bounds since 2013, with a growth rate of 257.1%. Although there was a decrease in 2016, a large rebound was noted in 2017 with 45 registrations. Furthermore, the number of drug registrations in phase I clinical trials appeared to change greatly every year. The total number of registrations increased most significantly in 2017 (with a growth rate of 316.7%). The number of registrations for phase III of clinical trials was also significant and increased consistently (12 per year on average from 2014 to 2017). The number of clinical trials of new technology for treatment has also increased (see ).
Figure 2. Registration situation on mAbs drugs clinical trials in China. Data source: Registration of drug clinical trials and information publicity platform.
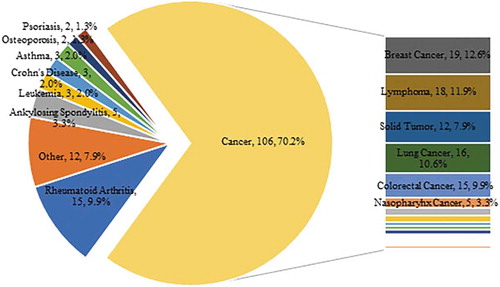
The clinical trials of mAb drugs mainly focused on the treatment of cancer (70.2%), rheumatoid arthritis (9.9%), and ankylosing spondylitis (3.3%). Among different types of cancer, the most mAb drugs on trial were indicated in breast cancer (12.6%), lymphoma (11.9%), and lung cancer (10.6%) (see ).
Marketing approval of mAb drugs in China
The number of mAb drugs approved for marketing in China has been increasing. The continuous rise of mAb drugs in China was partly related to patent expiration of super mAb drugs in the global market. In 2017, the number of new marketing applications reached its peak with a total of 7 antibody drugs from 5 enterprises. By December 2017, 21 antibody drugs had been approved to enter the market, of which 10 were locally developed and 11 were imported (excluding products that had been withdrawn from the market: muromonab-CD3 (Zenapax) (see ). Three of the local products were mouse mAb and had already been withdrawn from the market due to serious side effects. The number of locally developed antibody drugs accounted for less than half of the locally listed mAb drugs. The biotech drugs accounted for only 1.7% of sales, which was far below the global level (34.0%), and the market share of locally developed products was not highCitation9. Overall, the market of mAb drugs was basically monopolized by imported products.
Table 1. Domestically approved mAb drugs by 2017.
Globally, 83 innovative mAb drugs had received marketing approval by 2017. Among them, 78 (92.5%) were approved by the U.S., 71 (82.5%) by the EU, 50 (56.3%) by Japan, and 11 (12.5%) by China. Among the 11 mAb drugs approved in China, 10 were first approved in the U.S. and 1 in Japan. shows the drug lag between China and the country that granted the first approval of these 11 mAb drugs.
Figure 4. Drug lag of mAb drugs approved by China compared with the countries that granted the first approval (by 2017).
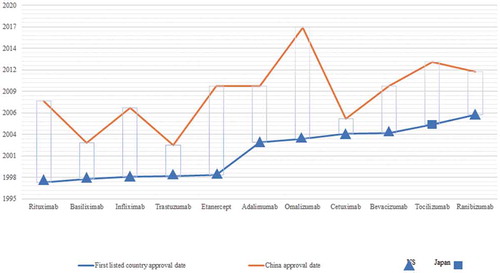
The drug lag in the marketing approval of mAb drugs in China compared with other countries was significant. According to the horizontal comparison of drug approval date in each region, the median approval lag time of Chinese mAb drugs (87.1 months) was far longer than that of the U.S. (0.0 months), the EU (8.9 months), and Japan (43.4 months) (see ).
Table 2. Approval lag time of mAb drugs in different regions.
The gap is going to be narrowed between China and the pharmaceutical developed countries in terms of mAb drugs marketing approval policies. Through the continuous improvement of the marketing approval system, 9 new mAb drugs were approved in 2018, and the drug lag compared with developed countries such as the U.S., the EU countries, and Japan was shrinking. From August 1, 2018 to December 31, 2018, 4 PD-1 mAb drugs were marketed in China, two of which were domestically produced and the other two imported. Taking two imported products as an example, the approval lag time of OPDIVO and KEYTRUDA between China and the exporting country is 48.1 and 47.2 months, respectively, which has been greatly reduced compared with the average approval lag time (90.4 months) above.
Infrastructure for mAb drugs development in China
The evolution of the infrastructure supporting the development of mAb drugs in China has seen a substantial growth in market share. The growth rate of mAb drugs in China has surpassed that in the global market and will continue to grow strongly. According to Frost & Sullivan, the market scale of China’s mAb drugs increased from USD 0.5 billion in 2012 to USD 1.3 billion in 2016 with a compound annual growth rate (CAGR) of 26.8%.Citation10 It is expected to continue to grow at a compound growth rate of 25.0% from 2016 to 2021, reaching a market scale of USD 4.0 billion in 2021. Some of the recent changes to the infrastructure include the following:
Rapid development of emerging biopharmaceutical companies
In recent years, the number of emerging local biopharmaceutical companies and enterprises has increased, with some enterprises reaching the scale of IPO launch within 3–5 years, and the financing speed is very fast. Within the first 6 months of 2018, a total of 11 biopharmaceutical enterprises, including CStone Pharmaceuticals, Innovent Biologics, and Hua Medicine, completed financing with a total scale of nearly 1.0 billion US dollars. Among these biopharmaceutical enterprises, 5 had a financing scale of more than USD 72 million.Citation11 On the other hand, nearly 15 enterprises, including Innovent Biologics, CStone Pharmaceuticals, and Genova Biotech have launched or revealed their launch plans. Applications of new enterprises constantly spring up, seeing Jiangsu Aosaikang Pharmaceuticals (VEGF), Zhejiang Teruisi Pharmaceutical (CD20), Hengkang Medical Group (ADC), and Yoko Pharmaceutical (CD20) successively enter the field of antibody R&D. The large financing scale and the high financing speed of biopharmaceutical enterprises reflected the recognition of the importance and potential of the biomedical field. This in turn fueled the R&D and production of Chinese mAb drugs so that a large number of new mAb drugs are currently being tested in clinical trials that will hopefully address patients’ needs in a timely fashion.
Rapid transition of large domestic companies
As of 2017, 277 enterprises in China engaged in the field of therapeutic antibodies with specific layouts, and the number of relevant biopharmaceutical firms had started to grow by approximately 20 per year since 2008. Subsequently, since 2012, the number of applications for new drugs started to grow exponentially. In addition to the emergence of new companies, local pharmaceutical giants also shifted rapidly from traditional drug manufacturing to developing therapeutic mAb, contributing to the fast-growing trend of mAb drugs in China.
North China Pharmaceutical Group Corp., Qilu Pharmaceutical Co., Harbin Pharmaceutical Group Co., Simcere Pharmaceutical Group, Jiangsu Hengrui Medicine Co., Zhejiang Hisun Pharmaceutical Co., and other pharmaceutical giants had submitted applications for their therapeutic antibody products. Sinopharm Group and Shanghai Pharmaceutical Group’s R&D for new therapeutic antibodies was underway. A subsidiary of North China Pharmaceutical Group Corp. had been equipped with a national key laboratory, a complete biotechnological drug development system, and a comprehensive biotechnological drug quality control and testing platform that conformed to international standards. The advancing infrastructure had also helped to attract, retain, and nurture a large number of professionals in R&D of therapeutic antibodies.
Qilu Pharmaceutical Co. has also made significant progress in expanding their product categories to include therapeutic mAb. With their mAb products introduced to the market, the monopoly over the Chinese market by foreign companies has been terminated. According to the statistics issued by db.yaozh.com, Qilu Pharmaceutical submitted applications for 6 biological products in 2017. Five of them are considered highly promising and have been approved for clinical trials.Citation12 Hengrui Medicine Co. and other leading local pharmaceutical companies with antibody products, such as PD-1mAbs, PD-L1 mAbs, and c-Met ADC, are also actively planning for biopharmaceutical innovations.
Venture capital objects focusing on the field of mAb
The number of companies engaged in the antibody industry in China has increased significantly since 2008 and numbered at nearly 300 by 2017. In terms of capital, traditional drug manufacturing companies relied on their mature markets to provide funds, while start-ups were also supported by a series of high-quality investment companies. Therefore, the development of local antibody companies is on a clear upward trend.
At present, the proportion of financing in the biomedical sector has remained at around 2/3, and biotechnology has become the main driver of financing in the pharmaceutical field. In November 2016, Innovent Biologics, Inc. announced the successful completion of a USD 260 million D-round financing. This is among the largest financing in China’s entire pharmaceutical industry and even in global non-IPO financing.Citation13 In May 2018, CStone Pharmaceuticals announced the completion of B-round financing. This USD 260 million financing set a new record for B-round financing among China’s bio-pharmaceutical innovation companies. PD-1 mAbs were one of its core R&D varieties.Citation14 In June 2018, Livzon Pharmaceutical Group was awarded USD 148 million financing in the A round. In July 2018, Tasly Biomedical Co., the innovative biopharmaceutical company that possesses the listed blockbuster product, Pro-UK, and the most popular R&D pipeline in the cardiovascular and cerebrovascular field, announced the completion of its pre-IPO financing. Its post-investment valuation will reach approximately 1.9 billion US dollars. Antibody pharmaceutical companies are gaining more and more capital market concerns. Especially in the context of asset shortages, antibody pharmaceutical companies as a whole have gained significant valuation improvements.
Local enterprise-led drug R&D
With the development of biotechnology in China and the efforts of many companies, the R&D pipelines of biotechnology companies are attracting more and more attention from companies overseas. Advanced technology is being steadily transferred to foreign companies for mutual benefit. In October 2015, Innovent and Eli Lilly and Company (Lilly for short) reached a global development cooperation agreement for three tumor-immunotherapy bispecific antibody drugs. The total amount of milestone payments exceeded USD 1 billion. All three antibody drugs used the PD-1 mAbCitation15 independently developed by Innovent. In September 2015, Hengrui Medicine and Incyte Corp. reached an agreement to license the PD-1 mAb project for tumor immunotherapy with independent intellectual property rights to Incyte. This sublicense transfer will bring up to USD 795 million in revenue to the company.Citation16 In addition, in the past two years, Lilly, Merck, and other pharmaceutical companies have signed multi-million dollar agreements with Chinese companies to sell biotechnological medicines developed in China overseas.
Taking the data in 2017 as an example, the research and development expenses of 5 biopharmaceutical companies accounted for more than 10% of the operating income in the same period. Fosun Pharma’s R&D investment exceeded 1 billion yuan. The research and development investment of 9 biopharmaceutical companies, including Hualan Biological Engineering, Walvax Biotechnology Co., and ShuangLu Pharmaceutical Co., was between 100 million and 500 million yuan.Citation17 As another example, Henlius Biotech, Inc. has been focusing on the research and development of mAb drugs for 8 years, since its establishment. It has invested more than 800 million yuan.Citation18
Based on the unremitting efforts of domestic R&D personnel and the country’s strong support for the biomedical industry, drug R&D led by domestic enterprises has begun to continuously transfer technology to foreign companies. With the gradual development of international cooperation and advancement of China’s innovation, the R&D strength and level of China’s bio-innovative drugs have been recognized by international pharmaceutical companies.
The return of high-level talent
In light of attractive policies targeting high-level talent, namely the “Recruitment Program of Global Experts,” or the “Thousand Talents Plan,” and “The Strategy of Reinvigorating China Through Human Resource Development,” more and more Chinese students who studied abroad are willing to return to and work in China. The vitality of emerging companies, the support of national policies and treatment, and the continuous improvement of the local research atmosphere together provide a good platform for these high-level talents to continue to thrive and realize their endeavors after their return. Many investors and entrepreneurs believe that this growing pool of talent, both returnees and foreign experts, will help continue the nurturing of newer generations to support the sustainable development of R&D work in the country.Citation19
At present, there are two main types of local mAb companies: large pharmaceutical companies and returnee startups. Continuous technology in R&D, capital investment, and in particular, the connection of the mAb teams overseas have injected a booster into the development of Chinese mAb drugs. According to some reports, the returnee entrepreneurial sectors are mainly concentrated in the fields of new bioengineering and new pharmaceutical, accounting for 18.6%.Citation20 The large number of elite talents who return as entrepreneurs will also help establish new industry standards that are more in line with international standards.
Policy updates
Supporting and guiding policies for R&D
At present, China has promulgated a series of industrial development plans and policies to support independent R&D and to accelerate the industrialization of biopharmaceuticals (see ). The research of mAb has been listed on the 863 Program and national key research projects, and a number of projects involving mAb have received national key funding.
Table 3. Supports of R&D and guidance policies for Chinese biological drugs.
In 2012, the 12th Five-Year Plan of Biotechnology Development promulgated by the National Medical Products Administration (NMPA, formerly CFDA), emphasized strengthening the application of life sciences and biotechnology in 4 important sectors, including the health sector, representing a breakthrough for mAb drugs development in China. The number of mAb clinical trial applications has increased dramatically since then.
In addition, the Guidelines for the Development and Evaluation of Biosimilars (Trial) issued by the NMPA in February 2015 was a turning point in the regulation of biosimilars in terms of application procedures, registration categories, and application requirements. The vigorous encouragement for enterprises to develop high-level and high-quality biosimilars catalyzed the rapid growth of biosimilars in China. While no real biosimilars have been approved in China, mAb drugs developed in China and for which clinical trials have been applied are predominantly biosimilars.
Moreover, three other important documents were issued at the Central Committee level in 2016 in favor of the development of mAb-related biopharmaceuticals. The “Guiding Opinions of the General Office of the State Council on Promoting the Healthy Development of the Pharmaceutical Industry,” issued in March of that year, demanded an acceleration in R&D and industrialization of new antibodies, proteins, and polypeptides. The “Outline of the National Strategy of Innovation-Driven Development,” issued jointly by the Central Committee of the Communist Party of China and the State Council in May, emphasized the importance of the R&D of innovative drugs, new vaccines, advanced medical equipment, and biological treatment technologies. The “13th Five-Year Plan for the Country’s Scientific and Technological Progress,” issued by the State Council, demanded a national drug innovation system with an advanced level and provided support for the development of drugs that were innovative, efficacious, clinically important, and with associated major industrialization prospects. All these policies played an important role in accelerating the transformation of China from a large to a strong medical and pharmaceutical country.
The Chinese government has strategically planned for the development of innovative biopharmaceuticals at the national level. Financial and policy incentives are in place to promote the R&D of biopharmaceuticals within local enterprises and scientific research institutes. More and more local companies have successfully developed mAb drugs through independent or cooperative R&D projects. R&D investments and the number of drug approval applications have increased year by year. Some mAb drugs produced by companies such as Sunshine Guojian Pharmaceutical, Celgen Biopharma, Biotechpharma, and Zhejiang Hisun Pharmaceutical Co. have successfully received market approval. However, the support of the Chinese government mainly comes in the form of financial assistance. The industrialization cooperation mechanism among universities, research institutes, and enterprises still needs to be reinforced to allow the development of scientific research results into products. More high-level biomedical talents are also essential to the support of continuous innovation.
After the issuance of a series of favorable policies, the domestic biopharmaceutical R&D inputs and the number of clinical trial applications increased significantly. By December 2017, there were 385 applications for clinical trials of mAb drugs, of which 54.8% were domestically produced. As for annual R&D investment, there are more than 10 pharmaceutical companies whose input exceed 100 million yuan in the field of mAb drugs.
Clinical trial management policy
China has made great progress in reforming its clinical trial management system and implementing specific supporting measures (see ). Considering that approximately 70% of mAb drugs are indicated for treating cancer, 4 guidelines specific to clinical trials of anti-tumor drugs were implemented in 2012 that provided methodological guidance on clinical trial design, execution, and data evaluation.
Table 4. China’s bio-pharmaceutical clinical trial policy.
The accreditation process for clinical trial institutions and the requirements of clinical trial data also underwent drastic changes. Before 2017, clinical trial institutions in China were required to seek accreditation from the drug authority before being authorized to conduct clinical trials. An approval document was also needed for each clinical trial, and the application process was time-consuming. Moreover, clinical trial data submitted for drug evaluation must be originated from phase I, phase II, and phase III trials conducted in China for both local and imported mAb drugs. The above policies not only affected the R&D of domestic mAb drugs but also caused a serious lag in the launch of foreign mAb drugs in China. The average drug lag was estimated at 7.5 years, compared with the U.S., the EU, and Japan.
Acknowledging the impact of drug lag on the overall development of mAb drugs, policies regarding clinical trials underwent several adjustments in recent years. On October 8, 2017, the General Office of the CPC Central Committee and the General Office of the State Council promulgated the “Opinions on Deepening the Reform of the Examination, Evaluation, and Approval System to Encourage the Innovation in Drugs and Medical Equipment.” Accordingly, the approval system for accrediting clinical trial institutions was then replaced by a filing system leading to a default licensing system, and clinical trial data collected overseas would also be considered. Moreover, on October 10, 2017, the NMPA promulgated “Decisions on Changes in Terms of the Registration Administration of Imported Drugs,” which simplified the application for clinical trials of imported drugs and allowed international multi-center clinical trials. The NMPA also promulgated “Announcement on Adjusting the Examination, Evaluation, and Approval Procedures for Drug Clinical Trials” in July 2018, which changed the clinical trial application process to a 60-day default licensing system.
The adjustment of drug clinical trial policies, especially the acceptance of overseas clinical trial data, made it possible for China to participate in international multicenter clinical trials. This was conducive to the first launch or simultaneous launch of new mAb drugs in China. Moreover, the improved communication by the NMPA with the applicants during the preclinical stage has also helped facilitate the successful execution of clinical trials of mAb drugs. All these measures have played a positive role in shortening the time lag for the launch of mAb drugs in China.
Marketing approval management policies
Imported mAb drugs account for the majority of mAb drugs approved in China but experience significant drug lag due to stringent clinical trial requirements and marketing approval policies. Similarly, the marketing approval policies also affect the speed of marketing domestic mAb drugs in China. In order to speed up the marketing process for innovative drugs, China has made efforts to reform the existing special approval channels for drug registration (see ). In 2015, a priority review and approval system for new drugs was established for innovative drugs that demonstrated clinical value in treating major diseases. Since then, innovative drugs that have not been listed in China and abroad or that have been transferred to China, are involved in major special research projects, or are designed for the prevention and treatment of major diseases such as AIDS, may be given top priority for approval and review.
Table 5. China bio-medicine marketing approval policy.
As of April 2018, out of the 520 innovative drugs granted priority by the Center for Drug Evaluation (CDE), 22 were mAb drugs, and the time taken to launch these products was thus shortened. For example, with HUMIRA (adalimumab injection), the CDE made a decision on November 3, 2016 to prioritize it for the review process on the grounds that “there is a clear therapeutic advantage over existing treatments.” The approval for its third indication of treating psoriasis was subsequently granted on May 19, 2017, taking only 6 months. Similarly, the time taken for Avastin (bevacizumab) to receive approval for a new indication had been shortened to less than 22 months.
In addition, China’s drug R&D and registration system is expected to become more in line with international standards since the NMPA became a member of the International Council for Harmonization (ICH) in June 2017 and the NMPA became an elected member of the ICH Management Committee at the first Congress of ICH 2018 on June 7, 2018. Gradual implementation of ICH guidelines in China will play a positive role in promoting the launch of innovative drugs.
The authority has also stepped up its drug evaluation capacity by increasing the number of drug evaluators from fewer than 200 to more than 800 and by reorganizing the team so that it adopts an indication-based and a project-management approach. By prioritizing the innovative drugs for evaluation, enhancing communication with the applicants, formation of expert committees for consultations and disclosing drug evaluation information in a seamless manner, the backlog of drug registration applications will be reduced and the evaluation process of innovative drugs will become more efficient. However, there are other issues hindering the innovation and development of mAb drugs in China to be resolved: a lack of supporting policies for the reimbursement of mAb drugs by insurers, the lack of a compensation system for drug patent deadlines, and the lack of a protection system to ensure data exclusivity.
The reform and improvement of the marketing approval policy has accelerated the marketing process of new mAb drugs in China. In 2017, the number of new marketing applications reached its peak with a total of 7 antibody drugs from 5 enterprises. By April 2018, the CDE had included 22 mAb drugs in the list of top priority for approval and review, which provides an opportunity to shorten the time for the marketing approval of mAb drugs.
The supporting system for the innovation of mAb drugs in China
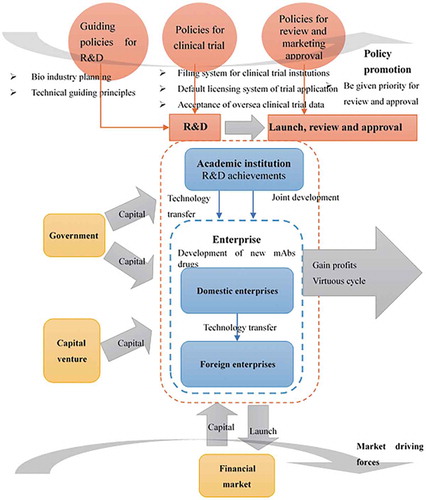
The innovation supporting system refers to the organic combination of various factors favorable for regional innovation, including policy environment; market environment; legal environment; resource environment; international environment; regional enterprises; cooperative projects of manufacturers, universities and research departments; and government and market mechanisms.Citation21 Over the course of developing mAb drugs in China, the key players include the government, pharmaceutical companies, research institutes, financial markets, and venture capital. In particular, the market and the government are the two major forces behind the supporting system for innovation of mAb drugs. The high returns of the mAb industry drive the development of pharmaceutical enterprises, which are then incentivized to invest further in mAb R&D, which attracts the support of the financial market at the same time. Together with the favorable and supportive policies implemented by the government, the innovation of mAb drugs and the development of the industry in China will continue to thrive (see ).
Conclusion
MAb drugs have become the fastest growing field in biopharmaceuticals. Due to the high specificity, established curative effects, and sound safety profile, mAb drugs are widely used in cancer treatment and autoimmune diseases and show great potential in new therapeutic areas, such as infectious diseases, cardiovascular disease, and diabetes. Reform of multiple policies encompassing the pharmaceutical industry, R&D, clinical trials, marketing approval, and incentives for attracting/retaining high-level talents has evidently been effective in addressing the drug lag of mAb drugs in China. Although the technical difficulties in developing mAb drugs and the gaps between the domestic industry and international counterparts remain, it is anticipated that, as the biotechnology advances and the investment in R&D continues, the development of mAb drugs in China will soon enter a new era.
In the future development, from the perspective of R&D supporting policy, we suggest that the NMPA/CDE should formulate specific technical guidelines in view of the characteristics of mAb drugs pharmacology, clinical research, and quality management; and provide diverse communication channels for applicants during the registration and review process. In addition, it is recommended to explore the new drug market monopoly and patent term compensation system in order to play a role in the market incentives for research and development in the innovation supporting system. A well-established medical insurance payment policy should also be matched to provide a good guiding environment for the innovation and development of mAb drugs. At the same time, the government ought to actively pay attention to the global R&D outcomes and industrial development trends of mAb drugs, and make the policy environment more attractive to enable more mAb drugs to be marketed in China as soon as possible.
This study is not without limitations. Due to the limited data sources, this study was unable to collect more data to conduct an in-depth analysis of the mAb drugs industry development environment. In the mAb drug innovation supporting system, policy analysis such as investment management, results transformation, and market incentive were not involved and should be specifically studied in future research.
Data retrieval and analysis methods
Data sources
An investigation of the new mAb drugs approved in the U.S., the EU, Japan, and China by 2017 was conducted using information publicly available on official websites: the U.S. FDA,Citation22 the EU European Public Assessment Report (EPAR), the Committee for Medicinal Products for Human Use (CHMP), the European Medicines Agency (EMA),Citation23 Japan’s Pharmaceuticals and Medical Devices Agency (PMDA),Citation24 and China’s National Medical Products Administration (NMPA).Citation25 New mAb drugs were defined as drugs having an active ingredient that has never before been marketed in any form in the U.S., the EU, Japan, or China. In addition, information about mAb drugs in China (including the number of applications, the number of approvals, the registration types, and the therapeutic areas of clinical trials involving mAb drugs developed locally and overseas by 2017) was retrieved from the Yaozh databaseCitation26 and the Drug Clinical Trials and Information Publicity PlatformCitation27 (hereinafter referred to as “information platform”). Yaozhi database is a professional third-party platform specializing in big data services in China‘s health industry. At present, the official source of the drug registration and approval database is CDE drug review management database, but it is not disclosed to the public. The Yaozhi database has been widely recognized in academic and industrial circles in China with the high reliability and reputation. Meanwhile, all authors in this research work have no any potential conflict of interests that could influence or bias the work. Thus, the data from the third-party data provider and the analysis by the third-party academic units can avoid bias effectively. This independent information platform, which was developed in 2012 and fully launched in 2013, provides public information about clinical trials and drug registration approved by the NMPA.
Data analysis
This study adopted descriptive statistical analysis and comparative analysis. In the comparative analysis, the drug lags of the 11 innovative mAb drugs approved in China, the U.S., the EU, and Japan were analyzed and evaluated. First, the number of mAb drugs approved for market in each country/region was compared against the mAb drugs approved in China. Second, the drug lag was assessed using statistical analysis of the drug lag (30 days per month) using the country/region granting the first approval as the baseline. For example, if a drug was first approved for marketing on January 1, 2015 in the EU, on June 1, 2015 in the U.S., and on January 1, 2016 in Japan, the drug lag is 0 months in the EU, 5 months in the U.S., and 12 months in Japan.
Abbreviations
| mAb | = | monoclonal antibody |
| R&D | = | research and development |
| Ig | = | immunoglobulin |
| FM Burnet | = | Frank Macfarlane Burnet |
| FDA | = | Food and Drug Administration |
| EMA | = | European Medicines Agency |
| EPAR | = | European Public Assessment Report |
| CHMP | = | Committee for Medicinal Products for Human Use |
| PMDA | = | Pharmaceuticals and Medical Devices Agency |
| NMPA | = | National Medical Products Administration |
| CAGR | = | compound annual growth rate |
| CFDA | = | China food and drug administration |
| CDE | = | Center for Drug Evaluation |
| ICH | = | International Council for Harmonization |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the National Office for Philosophy and Social Sciences for financial support for this research by the project 15ZDB167; and the University of Macau for financial support for this research by the project MYRG2016-00144-ICMS-QRCM. The authors have no conflicts of interest to disclose and no financial conflicts with the subject matter or materials discussed in the manuscript.
Additional information
Funding
References
- Geng XM, Kong XJ, Hu H. Research and development of therapeutic mAbs: an analysis based on pipeline projects. Hum Vac Immunother. 2015;11(12):2769–72. doi:10.1080/21645515.2015.1074362.
- Guo JJ, Wang LL, Zhang Q, Zhang AB, Zhu J, Zhao YY, Zhang BX, Bu HZ. Advances in pharmacokinetics of MAbs drugs. Chin Pharmacol Bull. 2016;32(2):172–76. doi:10.3969/j.issn.1001-1978.2016.02.006.
- CNII. Market data analysis of mAb drugs industry in China; 2017 Oct 20 [accessed 2019 Mar 6]. http://www.chyxx.com/industry/201710/574555.html.
- Hui X. Domestic PD-1 drugs achieve zero breakthrough; 2018 Jun 19 [accessed 2019 Mar 6]. http://www.xinyaohui.com/news/201806/19/10319.html.
- Wang ZM, Gao J, Li G. Current status and development trend of MAbs drugs. China Biotechnol. 2013;33(6):117–24. doi:10.13523/j.cb.20130619.
- Li M, Wu RW. Overview of MAbs drug markets at home and abroad. China Biotechnol. 2017;37(3):106–14. doi:10.13523/j.cb.20170315.
- Shao L, Xu L, Li Q, Chakravarthy R, Yang Z, Kaitin KI. Regulatory watch: innovative drug availability in China. Nat Rev Drug Discov. 2016;15(11):739. doi:10.1038/nrd.2016.200.
- Yao XF, Ding JX, Liu YF, Li PH. The new drug conditional approval process in China: challenges and opportunities. Clin Ther. 2017;39(5):1040. doi:10.1016/j.clinthera.2017.03.016.
- Y-LP. Only 10 kinds of domestic monoclonal antibodies approved by the CFDA; 2015 Oct 19 [accessed 2018 Dec 23]. http://www.sohu.com/a/36466861_126503.
- Bioclub. Top 10 global sales: the pharmaceutical market segment with the most investment value in the next decade has emerged; 2017 Oct 10 [accessed 2018 Dec 23]. https://www.sohu.com/a/196968056_397362.
- Casting net. Touwho, Cstonepharma, Innoventbio, huamedicine and other 11 companies raised more than 6 billion RMB! Listing plans of 15 companies were exposed; 2018 Jun 4 [accessed 2018 Dec 23]. http://baijiahao.baidu.com/s?id=1602346080335027025&wfr=spider&for=pc.
- Quxuedong. Case analysis and enlightenment of domestic and foreign pharmaceutical companies entering the field of biotechnology drugs; 2015 Jul 31 [accessed 2018 Dec 23]. https://wenku.baidu.com/view/9b8c4fe725c52cc58ad6be85.html.
- Pharmcube. Innovent biologics, Inc. Completed the largest financing in the domestic biomedical field: D round of 1.7 billion yuan; 2016 Nov 29 [accessed 2018 Dec 23]. https://xueqiu.com/8965749698/78221065.
- Sina News. CSTONE pharmaceuticals completed a B round financing of $260 million; 2018 May 9 [accessed 2018 Dec 23]. http://finance.sina.com.cn/7x24/2018-05-09/doc-ihaichqy7825943.shtml.
- China Securities Journal. Transfer of new drug rights to foreign companies at skyrocketing price, the research and development of domestic new drugs have gradually entered blissful circumstances; 2016 Jan 22 [accessed 2018 Dec 23]. http://www.chinanews.com/cj/2016/01-22/7728217.shtml.
- China Pharmaceutical Network. Hengrui medicine new drug technology exports to the United States for the first time; 2015 Sept 14 [accessed 2018 Dec 23]. http://www.zyzhan.com/news/detail/49337.html.
- Biosino. 81 pharmaceutical companies have invested more than 100 million in R&D, biopharmaceutical field is significant; 2018 May 03 [accessed 2019 Mar 8] http://www.biotech.org.cn/information/154921.
- Henlius Biotech. Let your compatriots use better anticancer drugs; 2018 May 11 [accessed 2019 Mar 8] http://www.henlius.com/NewsD-810.html.
- Pharmadl. Three secrets for the rise of China’s biotechnology industry: talents, talents, talents; 2017 Jun 30 [accessed 2018 Dec 23]. http://www.sohu.com/a/153234222_481782.
- Southcn. Report for 2015 employment and entrepreneur of Chinese returnees: big data detailed current situation of returnees; 2015 Sept 6 [accessed 2018 Dec 23]. http://www.360doc.com/content/15/0906/22/26887393_497358949.shtml.
- Wei XZ, Chen JF. Research on support system for innovation and development of regional technology. J East China Shipbuild Inst. 2005;5(2):25–29. doi:10.16148/j.cnki.cn32-1743/c.2005.02.007.
- Food and Drug Administration. FDA approved drug products; 2018 Dec 23 [accessed 2018 Dec 23]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- European Medicines Agency. European public assessment report; 2018 Dec 23 [accessed 2018 Dec 23]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124.
- Pharmaceuticals and Medical Devices Agency. Search for medical drug information; 2018 Dec 23 [accessed 2018 Dec 23]. http://www.pmda.go.jp/PmdaSearch/iyakuSearch/.
- YaoDu. R&D database; 2018 Dec 23 [accessed 2018 Dec 23]. https://www.pharmacodia.com/.
- Yaozh. Drug data expy; 2018 Dec 23 [accessed 2018 Dec 23]. https://db.yaozh.com/.
- Center for Drug Evaluation. Drug clinical trial registration and information publicity platform; 2018 Dec 23 [accessed 2018 Dec 23]. http://www.chinadrugtrials.org.cn/.