?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective: To delineate seroepidemiology of pertussis in Hangzhou, to evaluate the protection levels of pertussis among healthy populations, for improving prevention strategy of pertussis.Methods: During 2009–2017, a multistage stratified random sampling method was employed to select participants included via physical examination for subjects in several Community Health Centers in Hangzhou. Enzyme-Linked Immunosorbent Assay (ELISA) was used to detect Immunoglobulin G (IgG) antibodies against pertussis in serum samples. Results were compared among 11 age groups. Univariate and multivariate analysis were used to analyze the associations among the rates of pertussis IgG seropositivity and the geometric mean concentration (GMC) levels of pertussis IgG and the related factors.Results: A total of 3360 subjects with available information were included, with 1745 male and 1615 female. Of these, 59.6% subjects had a clear immunization history of diphtheria–tetanus–pertussis vaccine (DTP). The vaccination rates of DTP had a declined trend with older age. The rate of pertussis IgG seropositivity was 69.9% (95% confidence interval: 68.3–71.5) and the GMC for pertussis IgG was 48.46 U/ml. Significantly higher seropositivity and GMC for pertussis IgG were found in subjects that had inoculation vaccine history or unknown history when compared those without inoculation of vaccine, lower in age groups <10, 20–29, and 30–39 y when compared to the other age groups evaluated.Conclusions: There are different distribution profiles both of the seropositivity and GMC for pertussis IgG for different age groups and immunization history of vaccine groups. In order to prevent pertussis occurrence, it is important to employ a booster dose of pertussis vaccine in adolescents and adults.
1. Introduction
Pertussis is a potentially deadly bacterial infection that is a worldwide acute respiratory infectious disease transmitted through respiratory droplets generated by coughing or sneezing, which has a secondary attack rate of up to 80% among unvaccinated household contacts of infected individuals.Citation1 World Health Organization (WHO) estimates that there were 16 million pertussis cases and 195,000 childhood deaths worldwide in 2008.Citation2 Vaccination is the most effective and reliable strategy for preventing the morbidity of pertussis. According to the Hangzhou’s Vaccination Mandate record, children had been vaccinated by pertussis vaccine since 1954 in Hangzhou. Afterward, the main vaccines used for the prevention of pertussis are diphtheria–tetanus–pertussis (DTP), and the main components of which are diphtheria toxoid and tetanus toxoid combined with either whole-cell pertussis (DTwP) or acellular pertussis (DTaP). DTwP was introduced in 1978 in China, while in 1983 in Hangzhou. DTaP was introduced in 1999 in Hangzhou, replaced DTwP from 2008 and completely replaced in 2011. The vaccination rates achieved with four doses of the DTP vaccination in childhood have been more than 99% since 2011.Citation3 With the widespread use of DTP, the incidence and mortality associated with pertussis declined dramatically. In the United States, the incidence of pertussis once dropped from 157/100,000 to less than 1/100,000 in the 1970s.Citation4 Although China has implemented the expanded immunization program since 1978 to include the pertussis vaccine in children‘s planned immunization, the incidence of pertussis varies slightly from place to place.Citation3,Citation5 Under high vaccination rate of DTP, pertussis has begun to increase worldwide year by year, however.Citation6,Citation7 The United States, the United Kingdom, Australia, and other countries frequently appear pertussis outbreak, mainly in older children, adolescents, and adults.Citation5,Citation8,Citation9 Although pertussis is a vaccine-preventable infection, vaccine-induced immunity is not lifelong and booster doses are recommended according to national disease epidemiology.Citation10 The rapidly rising, known as “pertussis reproduction”, posed a huge challenge to the authorities in the prevention and control of infectious diseases. Domestic studies have shown that in the context of vaccination, combined with the abuse of antibiotics, clinical symptoms of pertussis are no longer typical, the rate of missed diagnosis by passive monitoring in hospitals is as high as 96%, the transmission patterns changes, and the epidemic intensity of pertussis is seriously underestimated.Citation6,Citation11
Immunoglobulin G (IgG) antibodies against pertussis is either a specific indicator of recent pertussis infection in general population or one of the indicators for surveillance of the effectiveness of the DTP vaccines in vaccinated population. In this study, we have previously measured the levels of IgG antibodies against pertussis. The aims were to delineate seroepidemiology of pertussis among ‘healthy’ population in Hangzhou; to gain an insight into seroepidemiology of pertussis to the improvement of the methods to prevent pertussis transmission in China. It plays an important role in formulating and improving control measures, adjusting immunization strategies, evaluating vaccine immunity effect, and predicting the incidence level, and it even helps to promote the development of new vaccines.
2. Results
2.1 Sociodemographic information and vaccination
Totally, 3360 subjects with available information were included in the current study. There were 1745 male and 1615 female. Samples were collected during 2009–2017; 11 various age groups were collected. The youngest was 2 months, the oldest was 83 y, and the average age was 25.70 y. There were 1159 subjects in urban while 2201 ones in rural.
Of these 59.6% (2001/3360) subjects had a clear immunization history of DTP, 5.2% (174/3360) subjects had no immunization history, while the remaining subjects’ vaccination history were unknown. The vaccination rate of DTP among 11 different age groups were statistically significance (χ2 = 252.458, Pfor trend<0.001), which had a lower trend in older age groups. There was no significant difference of the vaccination rate of DTP between the gender nor in various regions, which was 92.1% (1137/1235) in male while 91.9% (864/940) in female, and 92.5% (636/688) in urban while 91.8% (1365/1487) in rural. Among the subjects with unknown vaccination history, 43.0% (510/1185) were male and 57.0% (675/1185) were female, 39.8% (471/1185) lived in urban zones while 60.2% (714/1185) in rural and 73.0% (865/1185) were older than 15 y.
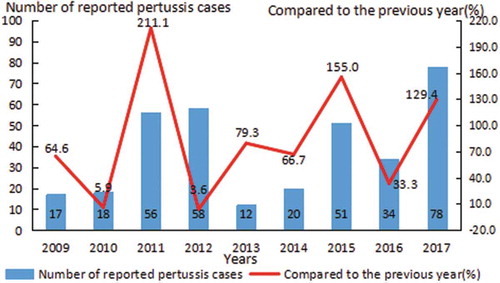
The number of reported pertussis cases once increased from 17 in 2009 to 78 in 2017, the ratio compared to the previous year increased from 64.6% in 2009 to 129.4 in 2017. During 2009–2017, the rapidly rising, known as “pertussis reproduction,” posed a huge challenge in the prevention and control of pertussis ().
2.2 Seroepidemiology of pertussis IgG among various groups via univariate analysis
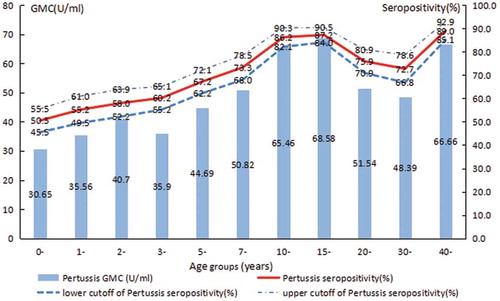
The rate of pertussis IgG seropositivity was 69.9% (95% confidence interval [CI]: 68.3–71.5) and the geometric mean concentration (GMC) for pertussis IgG was 48.46 U/ml. In univariate analysis, overall, significantly higher seropositivity and GMC for pertussis IgG were found in females compared with males, in urban compared with rural, in subjects that had inoculation DTP history or unknown history when compared those without inoculation of DTP () (all P-values <0.05).
Table 1. Seroepidemiology of pertussis IgG in population among various groups via univariate analysis.
In addition, in the present study, it is found that the pertussis IgG antibody was lowest in the age-group <1 y (50.5%), which was the highest in age-group >40 y (89.0%). Significantly lower seropositivity and GMC for pertussis IgG were found in age groups <10, 20–29, and 30–39 y when compared to the other age groups evaluated (χ2 = 275.90, P< .001; F = 26.35, P< .001) (, ).
2.3 Associations of seroepidemiology of pertussis IgG and related variables via multivariate analysis
In multivariate analysis, overall, significantly higher seropositivity and GMC for pertussis IgG were found in subjects that had inoculation DTP history (odds ratio [OR] = 2.957, 95% CI: 1.998–4.377; OR = 11.719, 95% CI: 4.124–19.313, all P-values <0.05) or unknown history when compared those without inoculation of DTP (OR = 2.904, 95% CI: 1.886–4.469; OR = 15.914, 95% CI: 7.618–24.209, all P-values <0.05) ().
Table 2. Association of Seroepidemiology of Pertussis IgG and related variables via multivariate analysis.
In addition, age is one of the important factors affecting the seroepidemiology of pertussis. Taking the <1-y-old group as a reference, the seropositivity and GMC for pertussis IgG in subjects aged 2, 5–6, 7–9, 10–14, 15–19, 20–19, 30–39, and ≥40 y were significantly higher (all P-values <0.05) ().
In short, both the rate of pertussis IgG seropositivity and the GMC for pertussis IgG were significantly influenced by age and the immunization history of DTP.
3. Discussion
Sero-surveillance is an essential tool to monitor vaccine-preventable diseases, as it can detect subgroups at risk and therefore deliver information which is often ignored in routine surveillance and vaccination rate studies.Citation12 It is important to investigate the seroepidemiology of pertussis in Hangzhou, for the occurrence of pertussis under high vaccination rate of DTP was increasing. In the present study, the rate of pertussis IgG seropositivity was 69.9% and the GMC for pertussis IgG was 48.46 U/ml. The “Regulations on the Management of Planned Immunization Technology” issued by the Ministry of Public Health proposed that the proportion of pertussis IgG to reach the level of protection was more than 75%. Results show that the pertussis IgG among ‘healthy’ population in Hangzhou was below the protection level. Factors that affect the level and duration of pertussis IgG antibodies including the infection of pertussis, the vaccination, the type of DTP vaccine, the age of the inoculator, the doses of inoculations, the interval between inoculations, etc.
Age is one of the important factors affecting the seroepidemiology of pertussis. Some domestic scholars have discovered that the positive rates of pertussis IgG increased with age, which were different across the provinces: 40.6% in Henan,Citation13 32.2% in Jiangsu,Citation14 46.3% in Shenzhen,Citation15 all of which were lower than 75.0% of the country‘s requirements.Citation16 While some other scholars have discovered that the positive rates of pertussis IgG decreased with age: such as in Dongyang, the positive rate of pertussis IgG is 89.7% in the <1-y group and 30.0% in the 20-y group, with the average protection rate is only 12.9%,Citation17 and the older age groups have a lower rate of protection and are more susceptible to pertussis infection. The immunity conferred by vaccines against pertussis changes with age.Citation18 In the present study, the seropositivity and GMC for pertussis IgG in the age-group <1 and 1–2 y were the lowest. It shows that the immunizations of children <1 y and 18 months of age have achieved good results, which was consistent with the results of Liu XL’s.Citation19 The results of age groups 3–4, 5–6 and 7–9 y also showed that pertussis IgG did not last long, which was consistent with the results of Shamsizadeh A’s.Citation10 Furthermore, although childhood vaccination series against pertussis exist, they are not routinely initiated until 3 months of age, leaving the newborn population unprotected. Maintaining high antibody levels in women of reproductive age helps to increase maternal antibody levels, thereby protecting newborns <3 months of age who were too young to be vaccinated from pertussis infection.Citation20,Citation21 The maternal immunization strategy with adult-formulated tetanus–diphtheria–pertussis (Tdap) is already included in some countries. In Australian, immunization changed from being permissive of maternal vaccination with Tdap to actively recommending pregnant woman be vaccinated in the third trimester of each pregnancy.Citation22 In the United States, 1 dose of Tdap should be given to women of childbearing age.Citation23 Significantly higher seropositivity and GMC for pertussis IgG were found in age groups 10–14, 15–19 and ≥40 y compared with other age groups, which was consistent with the results of foreign scholars‘ monitoring on the level of pertussis IgG antibody in healthy people aged 4–24 y. It is found that the pertussis IgG antibody is the highest in adolescents and adults, indicating the presence of recessive or natural infection in this group.Citation24 However, natural pertussis infection and vaccination do not confer lifelong immunity, and vaccine protection gradually diminishes after 4–12 y.Citation25 In the United States, it is recommended that 1 dose of Tdap should be given again at the age of 11–12, and 1 dose of Tdap should be given to adults aged 19, and 1 dose of Tdap should be given to women of childbearing age every time.Citation23 In China, primary vaccination with DTP is mandatory, while the diphtheriat–etanus vaccine booster doses are not. Primary vaccination of infants and young children consists of three doses of DTP in 3, 4, and 5 months old followed by a fourth dose at 18–24 months old. In the present survey, the vaccination rates of DTP among 11 age groups were statistically significant, which had a lower trend in older age groups. However, 73.0% among the subjects with unknown vaccination history were older than 15 y. This may account for the lower levels of seropositivity and GMC for pertussis IgG in age groups 20–29 and 30–39 y since the younger age groups were protected via vaccination and the older age groups were experienced the onset of pertussis. In short, the sero-surveillance of healthy population in Hangzhou shows that the immunity of adolescents and adults should be taken seriously, it is necessary to adopt corresponding immunization measures to reduce the incidence of pertussis.
Vaccination is the most effective and reliable method to prevent pertussis, though they remain in the population. Subjects with inoculation DTP history compared those without or unknown history of DTP, both the IgG seropositivity and GMC for pertussis IgG were significantly higher, which was consistent with relevant literature reports.Citation26 Although many of the reported events occurred in vaccinated children, the risk of pertussis among a large number of unvaccinated children in a intra-region increased by 2.5 times.Citation27,Citation28 This situation of high vaccination rate and low protection rate has also been reported in China, which may be related to the characteristics of the vaccine, vaccination quality, and individual differences.Citation29 According to the record, DTaP was introduced in 1999 in Hangzhou, replaced DTwP from 2008 and completely replaced in 2011. The number of reported pertussis cases once increased from 17 in 2009 to 56 in 2011 and to 78 in 2017, the ratio compared to the previous year increased from 64.6% in 2009 to 211.1% in 2011. The rapidly rising, known as “pertussis reproduction”, posed a huge challenge in the prevention and control of pertussis. It is suggested to increase the dose and adjust immunization strategy to prevent the disease burden and consequence of pertussis recurrence. Surveys found that DTaP has a lower efficacy than DTwP against pertussis.Citation30,Citation31 It suggested that the conversion of DTwP to DTaP may be one of the factors contributing to the rebound of pertussis.Citation32,Citation33 After the introduction of DTaP, a worldwide efficacy evaluation of DTaP was conducted, but this focused only on the sero-conversion rate after vaccination. In China, little is known about the long-term changes in antitoxins against pertussis after the replacement of the DTwP with DTaP. Foreign studies have shown that after years of use of DTaP, natural pertussis bacillus has produced a mechanism to evade the body‘s complement system, leading to the increase of the incidence of pertussis, while the DTwP is not affected.Citation6 Studies in primate models have shown that the DTaP are not effective in preventing pertussis infection and blocking bacterial transmission.Citation34 A possible factor, recently proposed, has been demonstrated in mouse and baboon studies that DTwP enhances Th1 and Th2 responses, but DTaP only enhances Th2 cell responses, it means that DTaP prevents pertussis symptoms in baboon models, but do not protect against infection.Citation35,Citation36 If the same is true for humans, then human pertussis infections may be strengthened in countries where DTaP is used. Guidelines revised by the WHO for pertussis vaccine selection indicated the following:Citation37 Compared with DTwP, DTaP is weaker in preventing and reducing subsequent transmission; Mathematical model studies conducted in Australia, England, and the United States have found that conversion from DTwP to DTaP may cause pertussis resuscitation; In addition to taking into account the financial burden, countries that have replaced DTwP with DTaP and incorporated it into national immunization programmes are also advised to increase doses and adjust immunization strategies to prevent the burden and consequences of recurrent pertussis. Meanwhile, the development and promotion of new vaccines should be accelerated. Many countries have worked on genetically engineered pertussis vaccines, which is the challenge of the development of biology.Citation38 It is believed that in the near future, this vaccine will make a breakthrough and be applied on a large scale, which can effectively protect the susceptible population, reduce disease occurrence. In addition, inoculation doses, time intervals, and the presence or absence of enhanced immunity may also affect the IgG seropositivity and GMC for pertussis.
In conclusion, the rate of pertussis IgG seropositivity and the GMC for pertussis were significantly influenced by age and the immunization history of DTP. In order to prevent pertussis transmission, we should stress the importance of employment of booster dose of pertussis vaccine in adolescents and adults, it is necessary to adopt corresponding immunization measures in population with low antibody level in Hangzhou; in addition, the development and promotion of new vaccines should be accelerated.
With study subjects covering 11 various age groups, study districts including rural and urban areas, and the span of study time as long as 9 y, there were some unavoidable limitations in this study: First, vaccination history of all subjects included in the current study was derived from Zhejiang Information System for Immunization Program, which was used since 2004. Thus, vaccination history of subjects in older age groups were not complete. Second, although ELISA is the most frequently used method to assess the anti-pertussis antibodies in population-based studies, there is no definite protective titer of pertussis IgG. Moreover, the kits used in this study using mixed antigens are less specific due to cross-reactivity. Third, the collection and storage requirements of serum samples are very strictly monitored and there will be slight difference in various regions, for the span of study time is long. Lastly, the impact of differences in vaccine types such as the PNTAXIM (diphtheria, tetanus, pertusis [acellular, component], poliomyelitis [inactivated] vaccine [absorbed] and Hemophilus influenzae type b conjugate vaccine), the domestic quadruple vaccine (diphtheria, tetanus, acellular pertusis and Hemophilus influenzae type b combined vaccine), DTwP, and DTaP has not been thoroughly analyzed in this study; the differences in vaccine types can be analyzed in subsequent studies to further improve the development and the use of vaccines.
4. Materials and methods
4.1 Subjects and data collection
The present sero-surveillance of healthy population was performed in Hangzhou during 2009–2017, which was carried out once a year. The seroepidemiology of pertussis IgG was compared among 11 various age groups: <1; 1; 2; 3–4; 5–6; 7–9; 10–14; 15–19; 20–29; 30–39; and ≥40 y. A multistage stratified random sampling method was employed to select the participant. A sample size of subjects for each group was included for cross-sectional investigation based on the formula: (p-pertussis = 93.9%, δ = 3%, α = 0. 05, p is the rates of pertussis IgG seropositivity).Citation39 The samples used were residual specimens via regular physical examination for subjects from kindergarten, primary school, and secondary school students and in the Community Health Centre in Hangzhou, China. Basic sociodemographic information and vaccination history of all subjects were derived from Zhejiang Information System for Immunization Program, respectively. The epidemiology of pertussis was derived from China Information System for Diseases Control and Prevention. Meanwhile, blood samples were collected and serums were isolated for antibody detection. In total, 3360 subjects with available information were included in the present study. All of the participants were given a brief oral description of the aims of the present study, and verbal consent was obtained from the participants. This program was approved by the ethics committee of the Hangzhou Center for Disease Control and Prevention.
4.2 Experimental approach and results calculation
ELISA was used to detect pertussis IgG antibody quantitatively by commercial ELISA kits (IBL: RE56141 for pertussis, IBL International GmbH, Germany) according to the manufacturer’s instructions. Two levels of immunity to pertussis were defined: pertussis antibody levels of <16 U/mL = ‘sero-negativity’, ≥16 U/ml = ‘sero-positivity’. Pertussis IgG antibody positive means past pertussis infection, successful vaccination or immunoglobulin recipient.
4.3 Statistical analysis
Quantitative variables with normal distributions were expressed as mean ± standard deviation. Statistical significance between groups was examined by two independent variable t-test or one-way ANOVA for the GMC for pertussis IgG level. The prevalence rates of pertussis IgG were expressed as frequencies (percentages), and Pearson’s chi-square test or Fisher‘s exact test was used to determine group differences. Logistic regression was used to analyze the associations between the rate of pertussis IgG seropositivity and the related factors. Linear regression was used to analyze the associations between the changes of the GMC levels and the related factors. The 95% CIs were calculated. Data were double entered independently and checked for accuracy using Epidata software (version 3.1, Denmark). All statistical analyses were performed and graphs created, using SPSS statistical software for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). A value of P< .05 (two-sided) was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Hamborsky J, Kroger A, Wolfe S. Epidemiology and prevention of vaccine-preventable diseases (the pink book). Washington (DC): Public Health Foundation; 2015.
- WHO.Immunization surveillance, assessment and monitoring [EB/OL]. (2008) [2010]. http://www.Wuo.int/immunization-monitoring/diseases/pertussis/en.
- Zhang Q, Zheng H, Liu M,Han K, Shu J, Wu C, Xu N, He Q, Luo H. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis. 2012;12:138. doi:10.1186/1471-2334-12-166.
- Winter K, Glaser C, Watt J, Harriman K; Centers for Disease C, Prevention. Pertussis epidemic–california, 2014. MMWR Morb Mortal Wkly Rep. 2014;63(48):1129–32.
- CHEN Tao, XIE Na, FUERHATI W,Epidemiology of pertussis in Xinjiang uyghur autonomous region, 2004–2012 [J]. Dis Surveillance. 2013;28(11):911–13. (In Chinese).
- Huang H. Research progress on re-emergence of pertussis and its control strategy [J]. Med Recapitulate. 2015;21(9):1630–32. (in Chinese)
- Hu W, Zhang S. Epidemiological characteristic of pertussis and sero-epidemiology investigation of the population in Shanxi Province, 2006–2015 [J]. Mod Prev Med. 2017;44(1):163–66. (In Chinese)
- Williamson YM, Moura H, Whitmon J, Woolfitt AR, Schieltz DM, Rees JC, Guo S, Kirkham H, Bouck D, Ades EW, et al. A proteomic characterization of Bordetella pertussis clinical isolates associated with a California State pertussis outbreak. Int J Proteomics. 2015;2015:536537. doi:10.1155/2015/647408.
- Paradowska-Stankiewicz I, Rudowska J. Pertussis in Poland in 2012. Przegl Epidemiol. 2014;68:205–07.
- Shamsizadeh A, Nikfar R, Yusefi H, Abbasi-Montazeri E, Cheraghian B. Seroprevalence of pertussis antibodies in 6–17-year-old students in Ahvaz, south-west Islamic Republic of Iran. East Mediterr Health J. 2014;20(10):623–26.
- ZHANG Ying, HUANG Hai-Tao, LIU Yong, et al. Incidence surveillance of pertussis based on community and analysis of its transmitted features in Tianjin [J]. Chinese J Vaccines Immunization. 2011;17(3):209–12. (In Chinese).
- Theeten H, Hutse V, Hens N, Yavuz Y, Hoppenbrouwers K, Beutels P, Vranckx R, Van Damme P. Are we hitting immunity targets? The 2006 age-specific seroprevalence of measles, mumps, rubella, diphtheria and tetanus in Belgium. Epidemiol Infect. 2011;139(4):494–504. doi:10.1017/S0950268810001536.
- Liu Q, Feng D, Zhao S, et al. Antibody levels of pertussis, diphtheria and tetanus in healthy population in Henan province [J]. Contemp Med. 2013;19(31):160–61. (In Chinese).
- Su F, Ge B, Liang F, et al. Monitoring the immunity level of diphtheria in healthy population in Jiangyin city,in 2011[J]. J Med Pest Control. 2012;28(9):1047–48. (In Chinese).
- Liu G, Lu Z, Lin Y, et al. Antibody level and epidemiological character of pertussis in Shenzhen from 2010 to 2013 [J]. Prog Microbiol Immunol. 2015;43(4):40–43. (In Chinese).
- Zhan J, Huo X, Yang B, et al. Surveillance on immuning levels against pertussis, diphtheria and tetanus among healthy population in some areas of Hubei Province in 2010 [J]. Practi Prev Med. 2011;18(9):1647–49. (In Chinese).
- Wu A, Guo B, Chen M, et al. Detection and analysis of antibody level of pertussis in healthy population [J]. Zhejiang Preventive Med. 2013;25(6):35–36. (In Chinese).
- Lang PO, Govind S, Michel JP, Aspinall R, Mitchell WA. Immunosenescence: implications for vaccination programmes in adults. Maturitas. 2011;68(4):322–30. doi:10.1016/j.maturitas.2011.01.011.
- Liu XL, Wang YJ, Li GP, et al. Serological surveillance on antibody levels to pertussis, diphtheria and tetanus among healthy people in Tongchuan [J]. Dis Surveillance. 2012;27(7):516–19. (In Chinese).
- Saul N, Wang K, Bag S, Baldwin H, Alexander K, Chandra M, Thomas J, Quinn H, Sheppeard V, Conaty S. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: the NSW public health network case-control study. Vaccine. 2018;36(14):1887–92. doi:10.1016/j.vaccine.2018.02.047.
- Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015;60(3):333–37. doi:10.1093/cid/ciu821.
- Australian Technical Advisory Group on Immunisation (ATAGI). The Australian immunisation handbook. 10th ( (2015 update)). Canberra, Australia: Australian Government Department of Health; 2015.
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–19. doi:10.1056/NEJMoa1200850.
- Cevik M, Beyazova U, Aral AL, Camurdand AD, Camurdan AD, Ozkane S, Sahinb F, Aybay C. Seroprevalence of IgG antibodies against Bordetella pertussis in healthy individuals aged 4–24 years in Turkey. Clin Microbiol Infect. 2008;14(4):388–90. doi:10.1111/j.1469-0691.2007.01926.x.
- Who. Pertussis vaccines: WHO position paper, August 2015–recommendations. Vaccine. 2016;34(12):1423–25. doi:10.1016/j.vaccine.2015.10.136.
- Wang Q, Sun M, Shi S. Analysis of diphtheria antibody level in healthy population in Xicheng district of Beijing. Capital J Public Health. 2013;7(5):226–28. (In Chinese)
- Glanz JM, Narwaney KJ, Newcomer SR, Daley MF, Hambidge SJ, Rowhani-Rahbar A, Lee GM, Nelson JC, Naleway AL, Nordin JD, et al. Association between undervaccination with diphtheria, tetanus toxoids, and acellular pertussis (DTaP) vaccine and risk of pertussis infection in children 3 to 36 months of age. JAMA Pediatr. 2013;167(11):1060–64. doi:10.1001/jamapediatrics.2013.2353.
- Atwell JE, Van Otterloo J, Zipprich J, Winter K, Harriman K, Salmon DA, Halsey NA, Omer SB. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics. 2013;132(4):624–30. doi:10.1542/peds.2013-0878.
- Wu J, Chen Q, Zhang W. Antibody levels of pertussis, diphtheria and tetanus in healthy population in Shaoguan city [J]. South China J Prev Med. 2006;32(4):45–46. (In Chinese)
- Witt MA, Katz PH, Witt DJ. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis. 2012;54(12):1730–35. doi:10.1093/cid/cis287.
- Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308(5):454–56. doi:10.1001/jama.2012.6364.
- Jongerius I, Schuijt TJ, Mooi FR, Pinelli E. Complement evasion by Bordetella pertussis: implications for improving current vaccines. J Mol Med (Berl). 2015;93(4):395–402. doi:10.1007/s00109-015-1259-1.
- Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015;13:146. doi:10.1186/s12916-015-0382-8.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111(2):787–92. doi:10.1073/pnas.1314688110.
- Higgs R, Higgins SC, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. 2012;5(5):485–500. doi:10.1038/mi.2012.54.
- Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K,, Misiak A, Lavelle EC, McLoughlin RM, Mills KH. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013;9(4):e1003264. doi:10.1371/journal.ppat.1003264.
- WHO. Guidelines for pertussis vaccine selection (revised)[J]. Int J Biol. 2015;38(1):46–47.
- Kazunar I, Kamachi K. DNA vaccine encoding pertussis toxin S1 Subuntinduces protection against Bordetella pertussis in mice [J]. Vaccine. 2003;21(31):4609. doi:10.1016/S0264-410X(03)00441-9.
- Huang L, Xu E, Yang L, et al. Observation of immunity level of pertussis-diphtheria-tetanus in healthy people in Hangzhou City during 1995–2006. Chinese J Vaccines Immunization. 2009;15(1):68–71. (In Chinese).