ABSTRACT
The very recent US Supreme Court and Court of Appeals for Federal Circuit (CAFC) cases have dramatically changed the standard of patent eligibility. Several groundbreaking innovations were thus determined to be patent ineligible. The patent ineligibility would impact on the innovation s of the field of biomarkers, diagnostic methods and personalize cancer immunotherapy. To solve the thorny problem of eligibility, this study retrospectively analyzes all CAFC related cases and presents a flow chart determining patent eligibility based on the courts’ decisions. Our analysis indicates the best way to avoid eligible rejection or invalidation is that an invention cannot fall within the categories of natural law, natural phenomenon or abstract idea. Thus, claiming non-natural cDNA, involving a step to grow a transformed cell or adding a means clause in a method claim would be some possible solutions. Moreover, based on the flow chart, even though a claim with substantive limitation but not well-understood, routine or conventional activities would be patent eligible; no one has successfully made the argument in the CAFC so far. We believe that this flow chart can serve as a set of guidelines for determining patent eligibility.
KEYWORDS:
The patent system is organized to encourage innovation: a government grants the patentee the right to exclude others from practicing the technical development for a certain amount of time, in return for innovation disclosure.Citation1 The government hopes other inventors will learn from the disclosure and use the knowledge as a basis for further innovation.
To be patentable, an innovation needs to pass five patentability tests, including eligibility, utility, novelty, non-obviousness and adequate disclosure.Citation2 The patent office is required by statute to examine every patent application to be certain the application passes each of these tests. Among the tests, eligibility is the most fundamental requirement of patentability, as Section 101 of the US Patent Code defines that a person who “invents or discovers any new and useful process, machine, manufacture or any composition of matter or any new and useful improvement thereof, may obtain a patent.” To be eligible for a patent, an innovation must fall within one of the four above-mentioned statutory categories.
These four statutory categories are deliberately broad; thus previously, patent eligibility was seldom an obstacle during patent prosecution or litigation. However, very recent US Supreme CourtCitation3-Citation5and Court of Appeals for Federal Circuit (CAFC)Citation6cases have dramatically changed that situation. The up-to-date common law from the US courts, as well as Article 25 of China Patent Law, strongly indicates that a patent eligible innovation cannot be a natural law, natural phenomenon or abstract idea. These patent eligible exclusions guard against the wholesale preemption of fundamental principles. Granting patents on these principles would slow down technological progress and thus violate the patent doctrine in encouraging innovation.
Indeed, the recent court cases have further built the patent eligible barrier during prosecution or litigation. The US Supreme Court’s Mayo decisionCitation4 was even reported as a game changerCitation7 that could have dramatic and long-lasting effects on the protection of biotech-related inventions, especially in the areas of diagnostics, biomarkers and personalized medicine.Citation2,Citation8,Citation9 Fortunately, in a full court CAFC judges’s denial of rehearing case,Citation10 several judges indicated the right path of patent eligibility. These opinions serve as a predictor to the thorny question of patent eligibility and can therefore serve as guidelines for claim drafting.
The CAFC decisions on eligibility after mayo
More than three years have passed since the US Supreme Court’s Mayo Collaborative Services v. Prometheus Laboratories, Inc. case was decided on March 20, 2012. To identify the overall tendency of the CAFC decisions, we reviewed all 11356 CAFC cases decided from January 2001 to December 2018 (). Among these decisions, there were 3254 patent-related cases; we limited our analysis to the 100 cases in which the CAFC discussed the eligibility issue. We further classified the decisions of the CAFC as “eligible”, “non-eligible” and some were remanded for further proceedings. The eligible rulings included cases where the CAFC affirmed the district court’s eligibility judgments or reversed the lower court’s non-eligibility conclusions, while the non-eligible rulings entailed the CAFC affirming the district court’s non-eligibility judgments or reversing the lower court’s eligibility conclusions. It should be mentioned that six cases of the eligible rulings were further reversed, either by the CAFC or the Supreme Court.
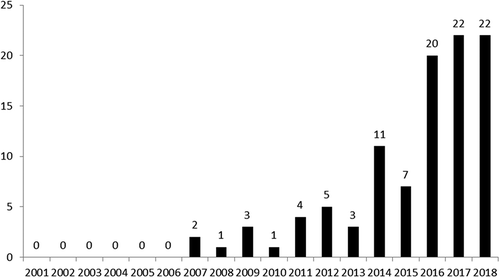
Figure 1. The outcomes of the US court of appeals for the federal circuit rulings decided from January 2001 to 2018.
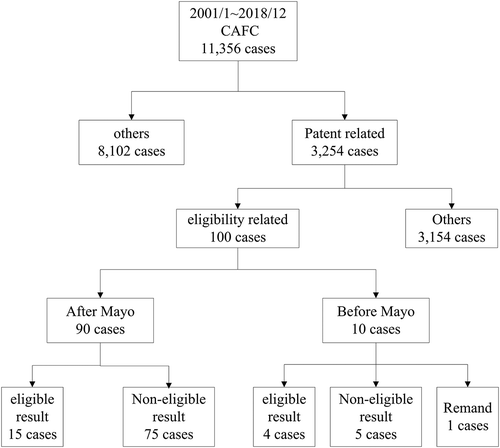
To demonstrate the influence of the Mayo case, we selected the date of the case, March 20, 2012, as a cutoff point. Excluding those six eligible result cases further reversed either by the CAFC or the Supreme Court, only 13 cases really survived by eligible challenge in the CAFC from January 2001 to December 2018. Moreover, our analysis showed that the eligible ruling rate decreased from 40% (4 out of 10 cases) to 17% (15 out 90 cases) and the non-eligible ruling rate increased from 50% (5 out of 10 cases) to 83% (75 out of 90 cases) after the Mayo case. Based on the precedent of Mayo,Citation4 the trends for eligible and non-eligible rulings in terms of the CAFC decisions decreased and increased dramatically, respectively. The differences in the rate were statistically significant when we applied Fisher’s exact test for association.
Furthermore, from the years of 2001 to 2018, the first eligible related case disputed in the CAFC was on December 2007 (). Before 2011, the yearly eligible case numbers were all below three. The numbers suddenly increased in 2014, which would indicate that the US Supreme Court’s precedentsCitation4,Citation5 did affect the patent litigation strategy in the CAFC.
The CAFC’s reasons for patent ineligibility
The majority of ineligible CAFC rulings have been related to software and biotech technologies. Only one case,Citation11 claiming transitory propagating signal, was determined to be not a process, machine, manufacture or composition of matter under 35 U.S.C. § 101. All software claims disputed in the CAFCCitation12-Citation30 were directed to an abstract idea. Thus, the claim should contain an inventive concept to transfer the claimed abstract idea into a patent eligible application. However, appending purely conventional steps or adding generic computer components such as, “interface,” “network,” and “database” to an abstract idea, would not supply a sufficiently inventive concept.
Moreover, to build an inventive concept by transferring the claimed abstract idea into a patent eligible application would be very difficult. For example, a patent claiming a method for distributing copyrighted products (e.g., songs, movies, books) over the Internet, where the consumer receives a copyrighted product for free in exchange for viewing an advertisement, and the advertiser pays for the copyrighted content. The method claim comprised 11 steps requiring intricate and complex computer programming as well as a specific application to the Internet and a cyber-market environment. A panel of CAFC judges determined the claim was patent eligible twice.
However, the US Supreme Court vacated these two CAFC’s eligible decisions. Following the second vacating, the CAFC panel, with another judge to replace a retired one, determined the claim did not transfer the claimed abstract idea into a patent eligible application. Moreover, the CAFC indicated that even though “some of the eleven steps were not previously employed in this art, it is not enough to confer patent eligibility upon the claims at issue.”
It should be stressed that only two CAFC software casesCitation31,Citation32 have survived patent eligible challenges. One case disclosed a system that generates and directs the visitor to a composite web page that displayed product information from the third-party merchant and retained the host website’s style. The majority of a CAFC panel determined that the claim recited a specific way to automate the creation of a composite web page and thus provided an inventive concept to render the claim patent eligible. However, the other judge dissented the decision.
Moreover, the other caseCitation32 was a data storage and retrieval system with a self-referential table to improve the way a computer stores and retrieves data in memory. The CAFC determined that the claim reciting a self-referential table did not include any other form of storing tabular data. The claim with a specific type of data structure was not directed to an abstract idea; thus, it was patent eligible.
Regarding the ineligible biotech cases,Citation10,Citation25,Citation30,Citation33-Citation38 most of the claims were related to naturally occurring phenomena with no “markedly different characteristics from any found in nature.”Citation39 Moreover, the method claims,Citation10,Citation25,Citation33-Citation38 similar to most of those used in medical practice, involved conventional, routine or well-understood steps. Thus, the claims did not provide an inventive concept to meet the eligible standard.
Before the US Supreme Court’s Myriad case,Citation5 the CAFC had held the belief that isolated DNAs, having a markedly different chemical structure compared to native DNAs, were patent eligible.Citation38 Furthermore, the CAFC stressed that patent eligible decision comported with the longstanding practice of the US Patent and Trademark Office (USPTO) and the courts. Significantly, the USPTO had issued patents relating to DNA molecules for almost 30 years before 2012. However, the US Supreme Court reversed the decisionCitation5 because the patentee did not create or alter either the genetic information encoded in the genes or the genetic structure of the DNA. The Supreme Court further notes that a groundbreaking, innovative, or even brilliant discovery, such as finding an important and useful gene, does not by itself satisfy the patent eligible inquiry.
Moreover, the Supreme Court’s Mayo caseCitation4 also impacts the CAFC’s decisions related to biotech and immunotherapy method claims. For example, a method claim of screening a tumor sample for a somatic alternation in a specific gene comprised comparing or analyzing the gene sequence. A panel of CAFC judges determined that the comparing or analyzing step was an abstract mental process indeed and thus fell outside the scope of patent eligibility.Citation38 For an immunotherapy case, by preventing Programmed cell death protein 1 (PD-1) ligands from binding the PD-1receptor, the anti-PD-1 antibodies preventing the pathway from suppressing the immune system and then killing cancer cells would be a patent ineligible natural phenomenon.
Until now, the CAFC has affirmed two biotech claims disputed to be patent eligible. One was complementary DNA (cDNA), which was synthesized from mRNA using complementary base pairing in a manner analogous to transcription. The other one was a method claim for screening potential cancer therapeutics, which involved a step to grow a transformed eukaryotic host cell. It would be more than clear that a patent eligible biotech innovation shall be either significantly different from nature or with substantive limitations that are not well-understood, routine or conventional activities previously engaged in with the technology.Citation2
A process for determining patent eligibility
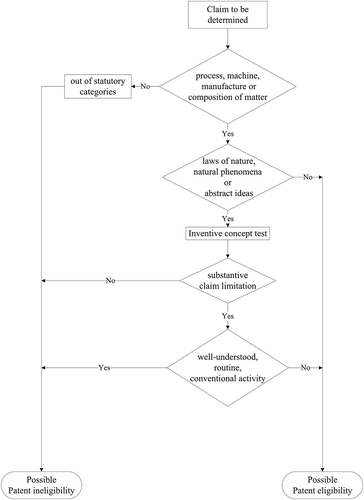
Based on the above analysis and flow chart previously developed,Citation2 we here revise the chart of determining patent eligibility (). To be patent eligible, a claim must belong to one of the following four statutory categories: process, machine, manufacture or composition of matter. Furthermore, a patent eligible claim cannot fall within the following three exceptions to § 101’s broad patent-eligibility principles: laws of nature, natural phenomena and abstract ideas. Thus, the second inquiry of the flow chart is to determine whether the claim is one of the exceptions.
According to the chart, a claim that is not within the exceptions, such as cDNA mentioned above, would be patent eligible. On the other hand, when a claim is within the exceptions, the next step is to look for the inventive concept test. An exceptional claim shall need a substantive claim limitation to meet the standard of inventive concept and thus to be patent eligible. For example, a method claim presented a screening method premised on the use of transformed host cells, which became non-naturally occurring cells because they included a foreign gene. The CAFC determined that the method claim included more than the abstract mental step of looking at two numbers and comparing two host cells’ growth rates. Thus, the claim had a substantive claim limitation to being patent eligible. However, the CAFC judgesCitation38 also held that other method claims of only analyzing and comparing certain NDA sequences were patent ineligible because the claims did not have a substantive limitation to transfer the abstract mental process into patent eligibility in light of the Supreme Court’s decision in Mayo.Citation4
In sum, the very basic process for determining patent eligibility is to require a claim be one of the four statutory categories. In addition, the query is to check whether the claim falls within three exceptions to patent eligible categories. If a claim is neither a law of nature, natural phenomenon nor abstract idea, the claim would be patent eligible. However, when a claim is within the exceptions, the third interrogation is to determine whether the claim includes a substantive limitation to meet the standard of being an inventive concept. Moreover, the last query of the flow chart is to confirm that a substantive limitation of an exceptional claim cannot be well-understood, routine or conventional activities previously engaged in with the technology.Citation2
Possible solutions to patent eligibility
According to the flow chart developed above, the only two principles to draft patent eligible claims are either to make them outside the three exceptions to patent eligible categories or to meet the standard of an inventive concept by adding substantive limitation but not well-understood, routine or conventional activities previously engaged in with the technology. With these two principles in mind, we will now discuss possible solutions for two patents disputed in previous CAFC cases.
Case 1: prenatal diagnosis method
The first case provided for making a diagnosis of certain fetal characteristics based on the detection of paternally inherited cell-free fetal DNA (cffDNA). The specification explained that the analysis of cffDNA permitted more efficient determination of genetic defects and that a pregnant woman carrying a fetus with certain genetic defects would have more cffDNA in her blood than would a woman with a normal fetus. However, the CAFC affirmed district court’s finding that the claim () was not directed to patent eligible subject matter.
Table 1. the original claim and possible solution of case 1.
The CAFC [35] determined that the original claimed method began and ended with a naturally occurring phenomenon. Later, the CAFC examined the elements of the claim to determine whether the claim contained an inventive concept sufficient to transform the claimed naturally occurring phenomenon into a patent eligible application. Unfortunately, the CAFC concluded that the claim did not result in an inventive concept that transforms the natural phenomenon of cffDNA into a patentable invention because the amplifying and detecting steps were well-understood, conventional and routine activities.
Moreover, a CAFC judge joined another judge in concurring in the denial of rehearing the case en banc [10] and indicated that the original claim was overbroad. In another concurring opinion, a judge also called attention to the claim “broadly encompassed any diagnosis of any disease, disorder or condition.” In other words, the claim would be too broad to be patent eligible.
A CAFC judge suggested drafting the claim in Jepson-Type (), which recites all of the elements of known process in the preamble to the claim, includes a transition that states “wherein the improvement comprises or an improvement” and then describes in the body of the claim only the new or modified elements. The interpretation of a Jepson-Type claim includes all the elements or steps recited in the preamble as a part of the claimed combinations.
We may not be that optimistic in the drafting the claim in Jepson-Type because the claim still encompasses the diagnosis of any disease, disorder or condition. To be conservative, we would suggest narrowing the claim covering application actually reduced to practice, such as adding a limitation of a specific disorder and detail procedures (), as a CAFC judge has indicated [10] and skillful claim drafters have suggested [7].
Case 2: method for screening genes
The US Supreme Court has determined that isolated DNA is not markedly different from nature and thus is not patent eligible. Furthermore, the CAFC also determined a method claim, which mainly compared or analyzed certain isolated sequences, to be an ineligible abstract mental process. The claim () only recited a mental step, comparing, without including any other step related to the structure of physical DNA molecules.
Table 2. the original claim and possible solution of case 2.
The patent owner argued that there should be steps of extracting DNA from a human sample and sequencing the DNA molecule before the comparing procedure. Moreover, the assignee noted that the patent specification showed that the claim term sequence did not refer to information, but rather to a physical DNA molecule, whose sequence had to be determined before it could be compared. However, the claim itself included neither of these two steps to make it patent eligible. In other words, adding these two physical steps in the claim would take it out of the category of abstract idea.
To make the claim be patent eligible, we would confine the screening method on breast or ovarian cancer, but not to other possible cancer associated to BRAC1 mutation. Moreover, we also add extracting, sequencing and detecting means in the claim. The statutory claim language “means” is for performing a specific act or operation. Thus, a claim with specific acts would certainly not be an abstract idea, as experienced claim drafters suggested [7]. Furthermore, the means claim would be interpreted to cover the corresponding structure, material, or acts described in the specification and equivalents. The interpretation would limit the claim so as not to cover later techniques developed. As a CAFC judge indicated, limiting the scope of a claim covering applications actually reduced to practice would allow the inventor to enjoy an exclusive right, but not to prevent new applications of the natural law by others.
Conclusion
Eligibility is the most fundamental patentability requirement. Failing to meet the eligible requirement, even groundbreaking, innovative or brilliant discovery, such as finding an important and useful gene, cannot obtain patent protection. Significantly, the very recent US Supreme CourtCitation3-Citation5 and CAFCCitation6 cases strongly stressed that the patent eligible exclusions, such as a natural law, natural phenomenon or abstract idea, guard against the wholesale preemption of fundamental principles. Our analysis of the CAFC cases indicated that these courts’ cases have also built the patent eligible barrier during prosecution or litigation.
Based on this analysis, we drew a flow chart for determining patent eligibility. As indicated in the chart, the easiest way to escape the patent ineligible barrier is drafting the claim to be neither a law of nature or natural phenomena nor an abstract idea. For example, claiming non-natural cDNA would not be a natural phenomenon, and a method claim involving a step to grow a transformed cell would not be an abstract idea. It follows that we suggest adding a means clause in a method claim to avoid mental process ineligible rejection or invalidation.
Moreover, the flow chart for determining patent eligibility also suggests that a claim with substantive limitation but not well-understood, routine or conventional activity, would be patent eligible. However, our analysis found that no case has successfully made this argument in the CAFC so far.
Finally, the patent system is designed to encourage innovation and to anticipate further technological development for the society. Granting patents on a natural law, natural phenomenon or abstract idea would certainly slow down technological progress and thus violate the patent doctrine to encourage innovation. The US common law and the patent laws of other countries all have the patent eligible exclusions to guard against the wholesale preemption of fundamental principles.
As a CAFC judge stated in the denial of rehearing a case en banc,Citation10 “the major defect is not the claims lack inventive concept but rather they are overbroad.” The judge further noted that the claim “broadly covered any method of detecting paternally inherited cffDNA from material serum or plasma via amplification and detection of that cffDNA.” Capturing the entire natural phenomenon of cffDNA rather than a particular application would not be permissible. Thus, the judge suggested narrowing a claim to cover an application actually reduce to practice. The implication is exactly the same as we propose in the article, adding specific means in a method claim to avoid mental process ineligible rejection or invalidation.
Disclosure of potential conflicts of interest
The author has no conflicts of interest to report.
Additional information
Funding
References
- Wang S-J. Designing around patents: a guideline. Nat Biotechnol. 2008;26(5):519–22. doi:10.1038/nbt0508-519.
- Chang BC, Wang S-J. The impact of patent eligibility on biotech patents A flow chart for determining patent eligibility and an immune therapy case study. Hum Vaccin Immunother. 2015;11(3):798–794. doi:10.1080/21645515.2015.1009344.
- Bilski v. Kappos, (The US Supreme Court 2010).
- Mayo Collaborative Services v. Prometheus Laboratories, Inc., (The US Supreme Court 2012).
- Association for Molecular Pathology v. Myriad Genetics, (US Supreme Court 2013).
- CLS Bank International v. Alice Corporation Pty. Ltd, (Federal Circuit Court of Appeals 2013).
- Haanes E, Cànaves J. Stealing fire: a retrospective survey of biotech patent claims in the wake of Mayo v. Prometheus. Nat Biotech. 2012;30(8):758–60. doi:10.1038/nbt.2318.
- Rantanen J Teva, Nautilus, and Change Without Change Stanford Technology Law Review. 2015.
- Rai A, Js S. The changing life science patent landscape. Nat Biotech. 2016;34(3):292–94. doi:10.1038/nbt.3504.
- Ariosa Diagnostics, Inc v. Sequenom, Inc., 14-1139 (Federal Circuit Court of Appeals 2015).
- In re Petrus A.C.M. Nuijten, 06-1371 (Federal Circuit Court of Appeals 2007).
- Mortgage Grader, Inc. v. First Choice Loan Services, 15-1415 (Federal Circuit Court of Appeals 2016).
- In Re Smith, 15-1664 (Federal Circuit Court of Appeals 2016).
- Allvoice Developments US, LLC v. Microsoft Corp., 14-1258 (Federal Circuit Court of Appeals 2015).
- Internet Patents Corporation v. Active Network, Inc., 14-1048 (Federal Circuit Court of Appeals 2015).
- OIP Technologies, Inc. v. Amazon.com, Inc., 12-1696 (Federal Circuit Court of Appeals 2015).
- Versata Development Group v. Sap America, Inc., 14-1194 (Federal Circuit Court of Appeals 2015).
- Intellectual Ventures I LLC v. Capital One Financial, 14-1506 (Federal Circuit Court of Appeals 2015).
- Vehicle Intelligence v. Mercedes-Benz USA, LLC, 15-1411 (Federal Circuit Court of Appeals 2015).
- Buysafe, Inc. v. Google, Inc., 13-1575 (Federal Circuit Court of Appeals 2014).
- Ultramercial, Inc. v. Hulu, LLC 10-1544 (Federal Circuit Court of Appeals 2014).
- Planet Bingo, LLC v. VKGS LLC, 13-1663 (Federal Circuit Court of Appeals 2014).
- Digitech Image Technologies v. Electronics for Imaging, Inc., 13-1600 (Federal Circuit Court of Appeals 2014).
- Cyberfone Systems v. CNN Interactive Group, 12-1673 (Federal Circuit Court of Appeals 2014).
- Smartgene, Inc. v. Advanced Biological Lab, 13-1186 (Federal Circuit Court of Appeals 2014).
- Content Extraction v. Wells Fargo Bank, 13-1588 (Federal Circuit Court of Appeals 2014).
- CLS Bank International v. Alice Corporation, 11-1301 (Federal Circuit Court of Appeals 2013).
- In Re Ferguson, 07-1232 (Federal Circuit Court of Appeals 2009).
- Bancorp Services, L.L.C. v. Sun Life Assurance Co. of Canada (U.S.), 11-1467 (Federal Circuit Court of Appeals 2012).
- Bancorp Services, L.L.C. v. Sun Life Assurance Co. of Canada (U.S.), 07-1130 (Federal Circuit Court of Appeals 2008).
- DDR Holdings, LLC v. Hotels.Com, L.P., 13-1505 (Federal Circuit Court of Appeals 2014).
- Enfish, LLC v. Microsoft Corporation 15-1244 (Federal Circuit Court of Appeals 2016).
- In re Bentwich, 13-1460 (Federal Circuit Court of Appeals 2014).
- In re Roslin Institute(Edinburgh), 13-1407 (Federal Circuit Court of Appeals 2014).
- University of Utah Research v. Ambry Genetics Corporation 14-1361 (Federal Circuit Court of Appeals 2014).
- Perkinelmer, Inc. v. Intema LTD 11-1577 (Federal Circuit Court of Appeals 2012).
- The Association for Molecular Pathology v. U.S. Patent and Trademark Office, 10-1406 (Federal Circuit Court of Appeals 2011).
- The Association for Molecular Pathology v. U.S. Patent and Trademark Office, 10-1406 (Federal Circuit Court of Appeals 2012).
- Diamond v. Chakrabarty, (The US Supreme Court 1980).