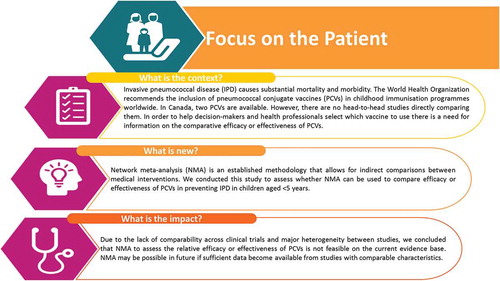
ABSTRACT
Background: No head-to-head studies are currently available comparing pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with 13-valent pneumococcal conjugate vaccine (PCV-13). This study explored the feasibility of using network meta-analysis (NMA) to conduct an indirect comparison of the relative efficacy or effectiveness of the two vaccines.
Methods: A systematic literature search was conducted for published randomized controlled trials (RCTs) and non-RCT studies reporting data on vaccine efficacy or effectiveness against invasive pneumococcal disease in children aged <5 years receiving 7-valent pneumococcal conjugate vaccine (PCV-7), PHiD-CV or PCV-13. Study quality was evaluated using published scales. NMA feasibility was assessed by considering whether a connected network could be constructed by examining published studies for differences in study or patient characteristics that could act as potential treatment effect modifiers or confounding variables.
Results: A total of 26 publications were included; 2 RCTs (4 publications), 7 indirect cohort studies, and 14 case-control studies (15 publications). Study quality was generally good. The RCTs could not be connected in a network as there was no common comparator. The studies differed considerably in design, dose number, administration schedules, and subgroups analyzed. Reporting of exposure status and subject characteristics was inconsistent.
Conclusion: NMA to compare the relative efficacy or effectiveness of PHiD-CV and PCV-13 is not feasible on the current evidence base, due to the absence of a connected network across the two RCTs and major heterogeneity between studies. NMA may be possible in future if sufficient RCTs become available to construct a connected network.
Introduction
Pneumococcal disease is caused by the bacterium Streptococcus pneumoniae. Over 90 serotypes of S. pneumoniae have been identified;Citation1 however, the 10 most common serotypes account for approximately 62% of invasive pneumococcal disease (IPD) worldwide.Citation2 The most common forms of IPD include pneumonia with empyema or bacteremia, meningitis and febrile bacteraemia.Citation3 Non-invasive diseases such as middle ear infections (acute otitis media [AOM]), sinusitis and bronchitis are less severe and more common manifestations of pneumococcal infection.Citation3 The World Health Organization (WHO) estimated in 2005 that pneumococcal disease was responsible for 1.6 million deaths annually worldwide, mainly concentrated in poorer countries.Citation3 In the developed world, serious pneumococcal disease occurs mainly in children aged <2 years and in elderly people.Citation4 IPD causes substantial mortality and morbidity, with 4,200 deaths and over 35,000 cases estimated in the USA in 2011.Citation2
Routine immunization with pneumococcal conjugate vaccines (PCVs) has been shown to reduce hospitalization of children for pneumoniaCitation5 and has substantially reduced the incidence of IPD in CanadaCitation6 and other countries.Citation7 The WHO recommends the inclusion of PCVs in childhood immunization programmes worldwide.Citation7 In Canada, 7-valent PCV (PCV-7) was first licensed for use in 2001 and the National Advisory Committee on Immunization (NACI) recommended routine infant vaccination in 2002.Citation6 NACI updated these recommendations to replace PCV-7 with pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV, Synflorix, GSK) in 2009 and the 13-valent PCV (PCV-13, Prevnar 13, Pfizer) in 2010.Citation8 PHiD-CV contains capsular polysaccharides of 10 pneumococcal serotypes: 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F and also induces protection against cross-reactive serotype 19A.Citation9 In Canada, PHiD-CV is licensed for immunization of infants and children from 6 weeks to 5 years of age,Citation10 and PCV-13 is licensed for immunization of infants and children from 6 weeks to 17 years of age.Citation11
There is a need for information on the comparative efficacy or effectiveness of PHiD-CV versus PCV-13 to help support decision-makers and health-care professionals selecting between available options. No head-to-head studies directly comparing PHiD-CV and PCV-13 are currently available.Citation12 A recently published meta-analysis of randomized controlled trials (RCTs) on the efficacy of all PCVs showed a significant reduction in risk for IPD and no significant reduction in risk for death over placebo.Citation13 This analysis did not attempt to compare between different PCVs. A recent systematic review of available evidence on the impact or effectiveness of PHiD-CV and PCV-13 in children aged <5 years in Latin American countries found that both PHiD-CV and PCV-13 were effective in reducing deaths or hospitalizations due to IPD, pneumonia, meningitis and sepsis, and there was no evidence of superiority of either vaccine over the other.Citation12 However, meta-analysis was considered inappropriate because the review included studies with a wide variety of designs, endpoints and age stratification.
Network meta-analysis (NMA) is an established technique that allows for indirect comparisons between multiple interventions in the absence of direct head-to-head studies. As long as all the available trials have at least one intervention in common with another, a network can be constructed to link the interventions tested across the different trials. It is then possible to estimate relative effects for all interventions included in the network. This approach is commonly used to compare health-care interventions and has recently been applied to vaccines. Recommending and decision-making bodies have used NMAs to base their treatments guidelines on, such as the World Health Organization’s updated guidelines for treatment of Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV).Citation14 More recently in vaccines, NACI commissioned an NMA in order to assess relative efficacy, effectiveness and safety of vaccines for herpes zoster,Citation15 and an NMA has been used to evaluate the comparative effectiveness of two rotavirus vaccines.Citation16 Given the complexity of the pediatric pneumococcal disease literature and the evolving landscape of new vaccine preparations, the use of NMA methodology to compare vaccines is a logical next step. The objective of this feasibility study was to assess whether NMA methodology could be used to evaluate the comparative efficacy or effectiveness of PHiD-CV and PCV-13 in preventing IPD in children aged <5 years, using RCTs and observational studies.
Results
Systematic literature review
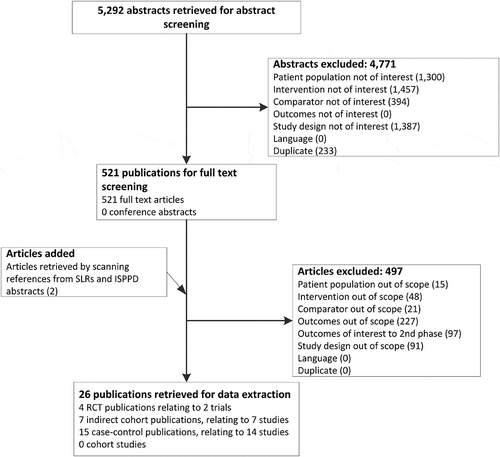
The literature search identified 5,292 publications, of which 521 were obtained for full-text review. Two additional publications were added from the International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD) 2016 conference abstracts and other systematic literature reviews. After full-text review, 26 publications met the inclusion criteria and were included in the review (). These consisted of 2 RCTs (4 publications),Citation17–Citation20 7 indirect cohort studiesCitation21-Citation27 and 14 case-control studies (15 publications).Citation28–Citation42
Figure 1. Flow diagram of search results and study selection.
ISPPD, International Symposium on Pneumococci and Pneumococcal Diseases; RCT, randomised controlled trial; SLR, systematic literature review.
The key characteristics of the included studies are summarised in . Both RCTs were double-blind and multicentred. Of the non-RCTs, seven were indirect cohort analyses, all derived from national surveillance centers. In this type of study, the subjects with vaccine-type IPD (VT-IPD) act as the cases, while all other subjects with non-VT-IPD act as the controls. Fourteen studies were case-control studies, in which IPD/VT-IPD cases are compared to controls who do not have IPD/VT-IPD. All except two of these studies used matched controls, and the other two studies controlled for confounding factors. The duration of follow-up ranged from 2.5 to 9 years, and the studies were conducted in North America, South America, Europe, and Asia.
Table 1. Key study characteristics.
All the included studies investigated the efficacy of a vaccine against IPD or VT-IPD. One RCT, the Kaiser Permanente trial, compared PCV-7 with the meningococcus type C conjugate vaccine (MCV).Citation17–Citation19 The other RCT, the COMPAS trial, compared PHiD-CV with the hepatitis B and hepatitis A vaccine.Citation20 The non-RCTs all studied the effect of PCV-7, PHiD-CV, PCV-13 or combinations of these vaccines. PCV-7 was studied in 12 case-control studies and 4 indirect cohort studies, PHiD-CV was studied in 3 case-control studies and 1 indirect cohort study, and PCV-13 was studied in 5 case-control studies and 3 indirect cohort studies. All cases and controls in the non-RCTs received ≥1 dose of PCV in an age-appropriate schedule, but the schedules varied between studies. There was little information available on co-administered vaccines, dose volume and the route of administration, and no information on vaccine preparation or storage temperature.
Feasibility of network meta-analysis for PCVs
Study quality assessment
The risk of bias assessment for RCT and non-RCT studies is summarised in .
Figure 2. Summary of risk of bias assessment for (a) RCTs using the Cochrane risk of bias tool and (b) non-RCTs using the Newcastle-Ottawa scale.
RCT, randomized controlled trial.
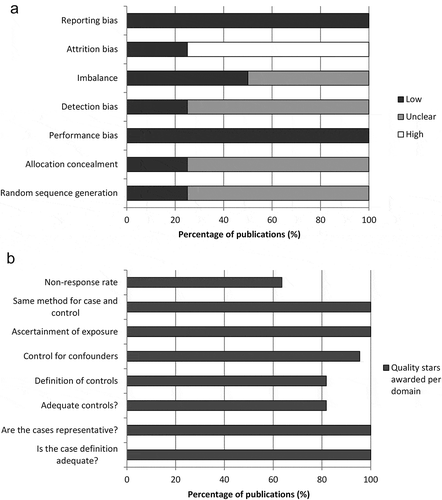
The results of the risk of bias assessment for the two RCTs are shown in Supplemental Material 1. Of the two RCTs included in the review, the reporting of the Kaiser Permanente trial was unclear about the randomization process, the concealment of treatment allocation, blinding of outcome assessment and the number of subjects included or excluded. The reporting of the other RCT, the COMPAS trial, was considered to be clear according to the Cochrane Risk of Bias Tool, perhaps reflecting differences in reporting guidelines between the dates at which the two trials were conducted.
Of the 22 non-RCT publications, all had case definitions adequate for informing NMA. All also had representative cases as defined by the Newcastle-Ottawa tool (all eligible cases with outcome of interest over a defined period of time; all cases in a defined catchment area; all cases in a defined hospital, clinic, group of hospitals or health maintenance organisation; or an appropriate sample of cases [e.g. random sample]). Four publications used hospital controls instead of community controls, meaning that it would be challenging to combine the studies in NMA. Four did not have a good definition of controls. In seven publications the non-response rate was different between groups (e.g. the percentage participating was very different between cases and controls) or was not described.
Network structure
The overall network structure for all the studies included in the review is shown in . There was no linked network for the two RCTs, as one compared PHiD-CV with hepatitis A/hepatitis B vaccine, and the other compared PCV-7 with MCV, so there was no common comparator between the two studies ().
Figure 3. Overall network for included studies.
PCV-7, 7-valent pneumococcal conjugate vaccine; PCV-13, 13-valent pneumococcal conjugate vaccine; PHiD-CV, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine; RCT, randomized controlled trial. *It cannot be assumed that exposure is solely PCV-13
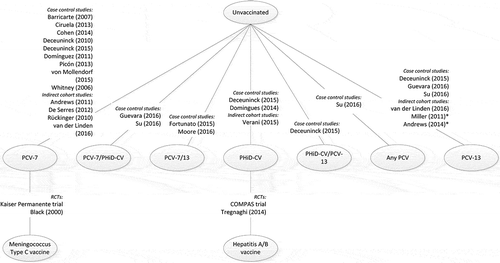
The evidence base for conducting an NMA should first be based on RCTs. Non-RCT studies can be used to extend the network as a secondary analysis, however, using non-RCTs as the base for analysis is not robust. Thus, the network structure from the available publications did not allow for NMA, as there was no link across the network for the two RCTs ().
Heterogeneity between studies
Heterogeneity between studies also precluded NMA. There was considerable heterogeneity in study designs, with three different types of studies identified (2 RCTs, 7 indirect cohort analyses and 14 case-control studies). In addition, the non-RCT studies also varied widely in location, date of publication, size, duration of follow-up and type of controls (17 studies used healthy community controls, and 4 studies used hospital controls). Combining evidence across heterogeneous designs has inherent difficulties due to differences in the measurement of vaccine efficacy or effectiveness. Indirect cohort studies and case-control studies have different types of controls (cases of non-VT-IPD, and controls without disease, respectively), and even within the group of case-control studies, different types of controls were used. There were also inconsistencies in the publications relating to one of the RCTs. These issues would introduce a high risk of bias if attempting to combine the studies in NMA.
There were large variations in dose number and administration schedules between studies (), with a different dose/schedule in almost every study. NMA relies on comparability of doses and schedules, so conducting an NMA using this heterogeneous evidence would only be possible if some of the disparate doses/schedules could be combined into fewer categories.
The number of vaccinated and unvaccinated subjects was not consistently reported across the included studies. Some studies reported only partial information on exposure (e.g. exposure information was missing for some outcomes or some study arms), while other studies did not report any information on exposure at all. Of the two RCTs, one reported complete exposure information together with the number of cases in each arm, while for the other RCT two of the three publications reported only the total number included across both arms. Of the seven indirect cohort studies, exposure was reported for both arms in five studies (however, two reported exposure data only for some outcomes), one study reported exposure only for one arm, and the other reported no exposure data at all. Of the 15 case-control publications, only seven reported full exposure data, two reported partial data on exposure (for one arm only), and the other six reported no exposure data. Overall, only about half of the included studies clearly reported the numbers of vaccinated and unvaccinated subjects for each analysis. It is essential to be certain about the numbers of vaccinated and unvaccinated subjects to conduct a valid NMA, and therefore the inconsistent exposure reporting makes the current evidence base unsuitable for NMA.
Outcome definitions for IPD and VT-IPD were highly comparable across studies.
There was substantial heterogeneity and little overlap across subgroups between the studies. Inconsistent categorization by age, dose/schedule, and serotypes between the different studies meant that over 750 different subgroups were analyzed or reported on across the studies, making it impractical to compare outcomes across the studies in an NMA. A robust NMA requires that similar subgroups are compared.
There was also heterogeneity in the risk of bias across the studies (, Supplemental Material 1).
Patient characteristics should be similar across a network to minimize the risk of bias. The information reported on subject characteristics was limited, and inconsistent between the studies (). Age, sex and comorbidities were the most frequently reported, but even these parameters were not available in all studies. This lack of information on subject characteristics would make it difficult to assess or control for potential confounders in an NMA.
Table 2. Subject characteristics reported across studies.
Overall, the results of the feasibility assessment indicated that it was not appropriate to use NMA to conduct an indirect comparison between PHiD-CV and PCV-13, due to the absence of a network connecting the RCTs and major heterogeneity between the available studies.
Discussion
To our knowledge, this is the first attempt to explore the feasibility of using NMA techniques to investigate the relative efficacy or effectiveness of PHiD-CV compared with PCV-13. We identified 26 publications in a systematic literature review, relating to 2 RCTs, 7 indirect cohort studies and 14 case-control studies. These publications were assessed to determine the feasibility of conducting a NMA using them as an evidence base. presents a summary of the outcomes and the impact of this study for health-care providers. While there is a need for information on the comparative efficacy or effectiveness of PHiD-CV versus PCV-13, the current data do not support the use of NMA methodology.
The overall quality of the evidence base was good, with reporting quality assessed as relatively high for the non-RCTs, high for one RCT and low for the other RCT. However, the results of the feasibility analysis showed that an NMA would not be appropriate based on the publications identified. The first limiting factor is the absence of a network connecting the two RCTs. Although non-RCTs can be added to the network in a secondary step to strengthen the evidence base and improve generalisability, this is only relevant if there is a connected network of RCTs to build upon. The two RCTs included in the literature search were evaluating PHiD-CV and PCV-7, but there was no RCT found evaluating PCV-13. Licensure requirements set forth by the WHO did not require an RCT, but instead proof of non-inferiority by immunogenicity, demonstration of functional antibody response and induction of immunological memory were considered sufficient.Citation43 As such, no RCT was required for the licensure of PCV-13 in the pediatric population, so the two RCTs in this analysis were for PCV-7 and PHiD-CV.
The second reason why NMA is not feasible is due to substantial heterogeneity across the studies in many dimensions. Outcome definitions for IPD and VT-IPD are comparable across studies. However, there are many differences in other dimensions such as study design (2 RCTs, 7 indirect cohort studies and 14 case-control studies), dose number, administration schedules and subgroups analyzed, and inconsistent reporting of exposure status and subject characteristics.
The risk of bias quality assessment indicated that the non-RCTs are of relatively high quality, so there would be little concern over using these studies in NMA. However, the two RCTs differ considerably in their risk of bias score, with the COMPAS study scoring highly and the reporting of the Kaiser Permanente study achieving only a low score. To conduct a robust NMA, the quality of the RCTs would need to be tested in scenario analysis, for which the evidence base is currently insufficient with only two RCTs.
As the different studies were conducted over a period of 16 years, it is not unusual that there would be substantial heterogeneity between them. There are other vaccine-preventable diseases for which the RCTs and other studies were conducted more similarly to one another, allowing for NMA to synthesize the comparable efficacy or effectiveness.Citation15,Citation16 While use of an NMA to evaluate the comparative efficacy or effectiveness of PCV-13 and PHiD-CV would allow for a relatively simple statistical analysis of existing data to address an important data gap, the current evidence base is insufficient to conduct and NMA. In the absence of head-to-head studies, public health decision-makers evaluating PCVs will need to rely on real-world evidence such as the reviews conducted by the WHO Strategic Advisory Group of Experts on ImmunizationCitation1 and the Comité sur l’immunisation du Québec (CIQ),Citation44 together with cost considerations.
Future research such as direct head-to-head studies or impact studies would be valuable to compare the two vaccines. For example, the Swedish health system allows the 21 counties to select either PCV-13 or PHiD-CV for their local immunization programmes, and approximately half the counties use each vaccine. Researchers in Sweden have recently used this unique opportunity to conduct an impact study comparing the two vaccines, which found no statistically significant difference in overall IPD incidence between counties using PCV-13 or PHiD-CV.Citation45 This study is the nearest available to a head-to-head comparison; however, further studies are needed to corroborate the results.
If further RCTs using comparators common to the two existing RCTs are published in the future, a small NMA may become possible provided the studies are not too heterogeneous. Given that there are two pneumococcal conjugate vaccines currently in development,Citation46,Citation47 there may be RCTs evaluating these products in a pediatric setting that could be added to the network. However, it is unlikely that the addition of these potential studies would change the conclusion that an NMA is not feasible to evaluate the comparative efficacy/effectiveness of PHiD-CV and PCV-13. Emerging techniques, such as estimating effects based on similar comparators or the inclusion of single-arm studies,Citation48 may also permit NMA if RCTs using different comparators or single-arm studies become available in the future. Should new-published evidence make a connected RCT base network possible in the future, adding evidence from non-RCTs could be considered to broaden the evidence from a small RCT network. If the heterogeneity is too great for this to be practical, the non-RCT evidence might be used to build information to conduct a Bayesian NMA.
Conclusion
In conclusion, this feasibility study showed that NMA to compare the relative efficacy or effectiveness of PHiD-CV and PCV-13 is not appropriate using the current evidence base. The absence of a connected network across the two RCTs was a key limiting factor. However, even with a robust RCT base, the major heterogeneity between studies in design, dose, schedules, and subgroups analyzed, together with the inconsistent and limited reporting of subject characteristics and exposure status, makes synthesizing the current evidence impractical.
Materials and methods
Systematic literature review
The study investigated two research questions:
What is the comparative efficacy of PCVs in preventing IPD in children?
What is the comparative effectiveness of PCVs in preventing IPD in children?
A literature search was conducted on 17 May 2017 in OVID (including MEDLINE, MEDLINE in Process, EMBASE, and Cochrane Central Registry of Controlled Trials (CENTRAL)). Details of the search strategy used are provided in Supplemental Material 2. To ensure no studies were missed, the Cochrane Database of Systematic Reviews (CDSR) was also searched for existing systematic literature reviews on clinical efficacy of PCVs, and the abstracts from the 2016 ISPPD conference were manually searched, as this is the largest conference in the field of pneumococcal disease. The reference lists of two recent systematic literature reviewsCitation1,Citation12 were also scanned.
Studies were selected for inclusion based on Population, Intervention, Comparators, Outcomes and Study design (PICOS) criteria as follows:
Population: Healthy male and female children aged <5 years;
Interventions: PCV-7, PHiD-CV, and PCV-13, regardless of the dose or schedule;
Comparators: Any vaccine, placebo or unexposed cohort;
Outcomes: IPD of all serotypes, and IPD of VT serotypes (VT-IPD);
Study design: RCTs with vaccine efficacy data, and observational studies with vaccine effectiveness data (nested case-control studies and cohort studies were included if they reported individual comparative data). Vaccine impact studies were excluded.
Full details of the PICOS criteria are provided in Supplemental Material 3. The search was limited to human studies published from 1990 to the present. There were no language restrictions.
Publications identified by the search were initially screened against the PICOS criteria by two independent researchers using the title and abstract. For publications assessed as potentially relevant, full-text articles were obtained and evaluated against the same criteria. Any conflicts were resolved by a third independent researcher.
For each publication that met the selection criteria, data extraction was performed by one researcher and checked against the original study by an independent researcher. Data on study and patient characteristics were extracted to evaluate the comparability of the studies and patients. For outcomes, data on the incidence of IPD and/or VT-IPD were extracted from the text or tables in the publication where available. Data from figures were extracted using the Digitize-it software.Citation49
RCTs included were assessed for internal (amount of selection, information and confounding bias) and external (generalisability of study results) validity, using the Cochrane Risk of Bias Tool, which has been tested for internal consistency, reliability and validity.Citation50 Non-RCTs were assessed using the Newcastle-Ottawa tool for observational studies, which judges each study on three broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest.Citation51
Feasibility assessment for network meta-analysis
When considering the feasibility of using NMA as an appropriate evidence synthesis tool for a clinical field, the availability of data to create a network for each outcome, the quality of any included data, and any potential study heterogeneity need to be assessed. The feasibility of conducting a valid NMA was evaluated using a standardized approach.Citation52 The first step was to assess whether there was a network of interlinked studies to allow comparisons between the vaccines, considering each study type and the combined studies for the overall network and for each outcome. The second step was to assess whether there were differences within or between direct treatment comparisons in study or patient characteristics that could act as potential treatment effect modifiers or confounding variables. Patient characteristics identified included age, sex, race, comorbidities, premature birth, immunocompromised status and area (urban versus rural). Study characteristics identified included study design, doses and schedules, number exposed (vaccinated versus unvaccinated), subgroups compared, outcome definitions and reporting, study quality, baseline risk, and relative vaccine efficacy. The results of the feasibility assessment determined whether it was feasible to conduct an NMA with the evidence available.
Abbreviations
Disclosure of potential conflicts of interest
AM, SI, CT, and PI are employees of the GSK group of companies. JL, JR, and RSN report financial support from the GSK group of companies to their employer for the study conduct.
Trademark statement
Synflorix is a trademark of the GSK group of companies. Prevnar and Prevnar 13 are trademarks of Pfizer Canada Inc.
Author contributions
All authors participated in the design or implementation or analysis, and interpretation of the study.
Supplemental Material
Download MS Word (69.2 KB)Acknowledgments
The authors thank Dominique Luyts for discussion during study development. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination. Pierre-Paul Prevot coordinated publication development and editorial support. Carole Nadin (Fleetwith Ltd, on behalf of GSK) provided medical writing assistance.
Supplementary materials
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Cohen O, Knoll M, O‘Brien K, Ramakrishnan M, Constenla D, Privor-Dumm L, Buss-Younkin J, Farrar J, Pilishvili T, Whitney C. et al. Pneumococcal Conjugate Vaccine (PCV) product assessment; 2017 [accessed 2017 Sept 30]. https://www.jhsph.edu/ivac/wp-content/uploads/2018/05/pcv-product-assessment-april-25-2017.pdf.
- Centers for Disease Control. Pneumococcal disease; 2015 [accessed 2017 Sept 30]. https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html.
- World Health Organization. Pneumococcal disease; 2016 [accessed 2017 Sept 30]. http://www.who.int/ith/diseases/pneumococcal/en/.
- World Health Organization. Immunization, vaccines and biologicals. pneumococcal disease; 2011 [accessed 2017 Sept 30]. http://www.who.int/immunization/topics/pneumococcal_disease/en/.
- Afonso ET, Minamisava R, Bierrenbach AL, Escalante JJ, Alencar AP, Domingues CM, Morais-Neto OL, Toscano CM, Andrade AL. Effect of 10-valent pneumococcal vaccine on pneumonia among children, Brazil. Emerg Infect Dis. 2013;19:589–97. doi:10.3201/eid1904.121198.
- Kellner J. Update on the success of the pneumococcal conjugate vaccine. Paediatr Child Health. 2011;16:233–40.
- World Health Organization. Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec. 2012;87:129–44.
- National Advisory Committee on Immunization. Statement on recommended use of pneumococcal conjugate vaccine. Can Commun Dis Rep. 2002 January 15;28:1–32.
- European Medicines Agency. Synflorix, INN-Pneumococcal polysaccharide conjugate vaccine (adsorbed)-annex1; 2018 [accessed 2017 Sept 30]. https://www.ema.europa.eu/en/documents/product-information/synflorix-epar-product-information_en.pdf.
- GlaxoSmithKline Inc. Synflorix product monograph, Canada; 2018 [accessed 2017 Sept 30]. https://ca.gsk.com/media/591956/synflorix.pdf.
- Pfizer Canada Inc. Prevnar 13 product monograph, Canada; 2015 [accessed 2017 Sept 30]. https://www.pfizer.ca/sites/g/files/g10028126/f/201601/Prevnar_13_PM_189931_22Dec2015_E.pdf.
- de Oliveira LH, Camacho LA, Coutinho ES, Martinez-Silveira MS, Carvalho AF, Ruiz-Matus C, Toscano CM. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American Countries: a systematic review. PLoS One. 2016;11:e0166736. doi:10.1371/journal.pone.0166736.
- Ewald H, Briel M, Vuichard D, Kreutle V, Zhydkov A, Gloy V. The clinical effectiveness of pneumococcal conjugate vaccines: a systematic review and meta-analysis of randomized controlled trials. Dtsch Arztebl Int. 2016;113:139–46. doi:10.3238/arztebl.2016.0139.
- Kanters S, Ford N, Druyts E, Thorlund K, Mills EJ, Bansback N. Use of network meta-analysis in clinical guidelines. Bull World Health Organ. 2016;94:782–84. doi:10.2471/BLT.16.174326.
- Tricco AC, Zarin W, Cardoso R, Veroniki AA, Khan PA, Nincic V, Ghassemi M, Warren R, Sharpe JP, Page AV, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. Bmj. 2018;363:k4029. doi:10.1136/bmj.k4029.
- Takeuchi M. Bayesian network meta-analysis suggests a similar effectiveness between a monovalent and a pentavalent rotavirus vaccine: a preliminary report of re-analyses of data from a Cochrane database systematic review. Hum Vaccin Immunother. 2014;10:1421–24. doi:10.4161/hv.28284.
- Black S, Shinefield H. Safety and efficacy of the seven-valent pneumococcal conjugate vaccine: evidence from Northern California. Eur J Pediatr. 2002;161(Suppl 2):S127–31. doi:10.1007/s00431-002-1064-z.
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser permanente vaccine study center group. Pediatr Infect Dis J. 2000;19:187–95.
- Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J. 2002;21:182–86.
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11:e1001657. doi:10.1371/journal.pmed.1001657.
- Andrews N, Waight PA, Borrow R, Ladhani S, George RC, Slack MP, Miller E. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS One. 2011;6:e28435. doi:10.1371/journal.pone.0028435.
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14:839–46. doi:10.1016/S1473-3099(14)70822-9.
- De Serres G, Pilishvili T, Link-Gelles R, Reingold A, Gershman K, Petit S, Farley MM, Harrison LH, Lynfield R, Bennett NM, et al. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine. 2012;30:4067–72. doi:10.1016/j.vaccine.2012.04.017.
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Effectiveness of the new serotypes in the 13-valent pneumococcal conjugate vaccine. Vaccine. 2011;29:9127–31. doi:10.1016/j.vaccine.2011.09.112.
- Ruckinger S, van der Linden M, Reinert RR, von Kries R. Efficacy of 7-valent pneumococcal conjugate vaccination in Germany: an analysis using the indirect cohort method. Vaccine. 2010;28:5012–16. doi:10.1016/j.vaccine.2010.05.021.
- van der Linden M, Falkenhorst G, Perniciaro S, Fitzner C, Imohl M. Effectiveness of Pneumococcal Conjugate Vaccines (PCV7 and PCV13) against invasive pneumococcal disease among children under two years of age in Germany. PLoS One. 2016;11:e0161257. doi:10.1371/journal.pone.0161257.
- Verani JR, Domingues CM, de Moraes JC. Brazilian pneumococcal conjugate vaccine effectiveness study group. Indirect cohort analysis of 10-valent pneumococcal conjugate vaccine effectiveness against vaccine-type and vaccine-related invasive pneumococcal disease. Vaccine. 2015;33:6145–48. doi:10.1016/j.vaccine.2015.10.007.
- Barricarte A, Castilla J, Gil-Setas A, Torroba L, Navarro-Alonso JA, Irisarri F, Arriazu M. Effectiveness of the 7-valent pneumococcal conjugate vaccine: a population-based case-control study. Clin Infect Dis. 2007;44:1436–41. doi:10.1086/516779.
- Ciruela P, Soldevila N, Hernandez S, Selva L, de Sevilla MF, Garcia-Garcia JJ, Moraga F, Planes AM, Munoz-Almagro C, Dominguez A. Risk factors for invasive pneumococcal disease in a community with a high proportion of non vaccine serotypes. Vaccine. 2013;31:960–66. doi:10.1016/j.vaccine.2012.11.102.
- Cohen C, von Mollendorf C, de Gouveia L, Naidoo N, Meiring S, Quan V, Nokeri V, Fortuin-de Smit M, Malope-Kgokong B, Moore D, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in HIV-infected and -uninfected children in South Africa: a matched case-control study. Clin Infect Dis. 2014;59:808–18. doi:10.1093/cid/ciu431.
- Deceuninck G, De Wals P, Boulianne N, De Serres G. Effectiveness of pneumococcal conjugate vaccine using a 2+1 infant schedule in Quebec, Canada. Pediatr Infect Dis J. 2010;29:546–49. doi:10.1097/INF.0b013e3181cffa2a.
- Deceuninck G, De Serres G, Boulianne N, Lefebvre B, De Wals P. Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine. 2015;33:2684–89. doi:10.1016/j.vaccine.2015.04.005.
- Domingues CM, Verani JR, Montenegro Renoiner EI, de Cunto Brandileone MC, Flannery B, de Oliveira LH, Santos JB, de Moraes JC. Brazilian pneumococcal conjugate vaccine effectiveness study group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014;2:464–71. doi:10.1016/S2213-2600(14)70060-8.
- Dominguez A, Ciruela P, Garcia-Garcia JJ, Moraga F, de Sevilla MF, Selva L, Coll F, Munoz-Almagro C, Planes AM, Codina G, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine in the prevention of invasive pneumococcal disease in children aged 7–59 months. A matched case-control study. Vaccine. 2011;29:9020–25. doi:10.1016/j.vaccine.2011.09.034.
- Fortunato F, Martinelli D, Cappelli MG, Cozza V, Prato R. Impact of Pneumococcal Conjugate Universal Routine Vaccination on Pneumococcal Disease in Italian Children. J Immunol Res. 2015;2015:206757. doi:10.1155/2015/206757.
- Guevara M, Barricarte A, Torroba L, Herranz M, Gil-Setas A, Gil F, Bernaola E, Ezpeleta C, Castilla J. Working group for surveillance of the pneumococcal invasive disease in Navarra. Direct, indirect and total effects of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children in Navarra, Spain, 2001 to 2014: cohort and case-control study. Euro Surveill. 2016;21.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4:399–406. doi:10.1016/S2213-2600(16)00052-7.
- Picon T, Alonso L, Garcia Gabarrot G, Speranza N, Casas M, Arrieta F, Camou T, Rosa R, De Oliveira LH, Verani JR. Effectiveness of the 7-valent pneumococcal conjugate vaccine against vaccine-type invasive disease among children in Uruguay: an evaluation using existing data. Vaccine. 2013;31(Suppl 3):C109–13. doi:10.1016/j.vaccine.2013.01.059.
- Su WJ, Lo HY, Chang CH, Chang LY, Chiu CH, Lee PI, Lu CY, Hsieh YC, Lai MS, Lin TY. Effectiveness of pneumococcal conjugate vaccines of different valences against invasive pneumococcal disease among children in taiwan: a nationwide study. Pediatr Infect Dis J. 2016;35:e124–33. doi:10.1097/INF.0000000000001054.
- von Mollendorf C, Cohen C, de Gouveia L, Naidoo N, Meiring S, Quan V, Lindani S, Moore DP, Reubenson G, Moshe M, et al. Risk factors for invasive pneumococcal disease among children less than 5 years of age in a high HIV prevalence setting, South Africa, 2010 to 2012. Pediatr Infect Dis J. 2015;34:27–34. doi:10.1097/INF.0000000000000484.
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–502. doi:10.1016/S0140-6736(06)69637-2.
- Pilishvili T, Zell ER, Farley MM, Schaffner W, Lynfield R, Nyquist AC, Vazquez M, Bennett NM, Reingold A, Thomas A, et al. Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics. 2010;126:e9–17. doi:10.1542/peds.2009-2150.
- WHO/World Health Organization. Recommendations for the production and control of pneumococcal conjugate vaccines. Technical Report Series; 2005:35.
- Comité sur l’immunisation du Québec (CIQ). Scientific advisory on the optimal schedule for childhood immunization against pneumococcal disease in Québec; 2017 [accessed 2017 Sept 30]. https://www.inspq.qc.ca/sites/default/files/publications/2379_opinion_optimal_schedule_childhood_immunization_pneumococcal_disease.pdf.
- Naucler P, Galanis I, Morfeldt E, Darenberg J, Ortqvist A, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65:1780–89. doi:10.1093/cid/cix685.
- Merck. Press release. [accessed 2017 Sept 30]. https://investors.merck.com/news/press-release-details/2018/Merck-Announces-First-Phase-Three-Studies-for-PCV-15-V114-Its-Investigational-Pneumococcal-Disease-Vaccine/default.aspx.
- Merck. pipeline. [accessed 2017 Sept 30]. https://www.pfizer.com/science/vaccines/pipeline
- Jansen JP, Leahy J Disconnected or limited evidence in network meta-analysis: what else can be done? Presented at ISPOR 2017, Glasgow, Scotland 2017.
- Digitizeit (Computer software); 2018 [accessed 2017 Sept 30]. https://www.digitizeit.de/.
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]; 2011 [accessed 2017 Sept 30]. http://handbook.cochrane.org.
- Wells GA, Shea B, O‘Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 2018 [accessed 2017 Sept 30]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Cope S, Zhang J, Saletan S, Smiechowski B, Jansen JP, Schmid P. A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med. 2014;12:93. doi:10.1186/s12916-014-0141-2.