ABSTRACT
Background: Broadly protective, long-lasting universal influenza vaccines are under development in response to low-moderate seasonal vaccine effectiveness, frequent genetic changes in circulating viruses and extended turnaround for vaccine manufacture. Because a long-lasting vaccine might be less effective than a seasonal vaccine that has been matched to current circulating strains, the public health impact of its introduction should be evaluated.
Methods: A modified agent-based model (ABM) examined multi-year effects of a universal vaccine among 18 to 49-year-olds, given in Year 1 only. The proportion of vaccinated 18 to 49-year-olds who received universal vaccine was varied from 0% to 100%. Model parameters were drawn from US databases and the medical literature. Outcomes were 4-year cumulative and annual influenza cases as well as annual cases averted/100,000 population for 3 age groups, 0–17 years, 18–49 years and 50+ years.
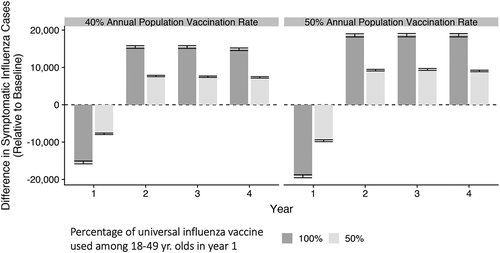
Results: In Year 1 when universal vaccine was given to 50% or 100% of all vaccinated 18 to 49-year-olds, more influenza cases occurred, compared to no universal vaccine, but fewer cases occurred in Years 2–4 as overall protection increased. Cumulative averted cases over 4 years in 18 to 49-year-olds were 892/100,000 and 1,687/100,000 population for the 50% and 100% universal vaccine for 18 to 49-year-olds scenarios, respectively, with additional benefits to children and older adults through indirect effects.
Conclusions: In ABM, the universal vaccine with a conservative VE estimate given once to 18 to 49-year-olds reduced influenza cases among all age groups in Years 2–4 following its introduction. Reduced influenza burden may occur sooner if VE of universal vaccines exceeds that assumed in these models.
Introduction
An array of universal influenza vaccines that would replace annual seasonal influenza vaccine is currently under development.Citation1 An influenza vaccine that reduces the frequency of recommended vaccination is a potential solution for those who resist or delay vaccination because of needle aversion,Citation2,Citation3 or who are not vaccinated because they “forgot” or “were too busy,”Citation4 or who believe that the seasonal vaccine is not safe,Citation5,Citation6 presumably because of frequent reformulation and inadequate testing. Furthermore, influenza vaccines of various types are being developed to address the modest vaccine effectiveness (VE) of the currently available seasonal vaccines and the variations in VE resulting from genetic drift of the highly variable hemagglutinin (HA) portions of the influenza virus.Citation7 The cost of a broadly protective universal influenza vaccine that requires administration only 2–3 times in a lifetime could be significantly less than annual receipt of seasonal influenza vaccine that currently costs an estimated $3,500/person/lifetime or more.Citation8 Moreover, the public health benefits of higher influenza vaccine coverage resulting from the use of a universal vaccine, even one with lower VE, could be extensive.
Until effective universal vaccines come widely onto the market, little can be known about their impact on influenza disease burden and epidemiology. Although a recent definition of a universal vaccine proposed a 75% VE,Citation9 concern has been raised that it might be less effective, particularly against influenza type B.Citation10 Indeed, a specific concern is that focusing on a single-conserved epitope may be problematic if immune pressure on that conserved epitope results in mutations that allow eventual escape from this vaccination scheme.Citation11 Thus, the first generation of universal vaccines may have long-lasting duration but be of modest effectiveness, which we explore in this paper. This level of VE would be a clear improvement over recent seasonal influenza VE that has ranged from 19% to 54% in recent years.Citation12–Citation16 Computational modeling can provide insights into the potential public health benefits of a universal influenza vaccine long before effectiveness studies can be conducted and use estimates for lower universal VE. We compared a long-lasting, moderately effective universal vaccine and currently available seasonal influenza vaccine that needs to be updated annually, using inputs from known parameters and reasonable estimates of vaccine uptake, effectiveness and other factors.
This study describes an expanded use of the Framework for Reconstructing Epidemic Dynamics (FRED).Citation17 FRED, an agent-based modeling (ABM) framework, was originally calibrated to model pandemic influenza over a single season with virus-naïve persons, no available vaccine and place-specific attack rates.Citation17 Building on the FRED modeling framework we developed an extended model of influenza transmission that is focused on the impact of vaccination on the seasonal transmission of influenza. It was recalibrated to use age-specific attack rates in the context of background immunity and was expanded from a single year to multiple years to enable a comparison of universal vaccine capable of protection over multiple influenza seasons with seasonal influenza vaccine given annually. The new software is called Public Health Influenza Laboratory (PHIL). Using this expanded modeling platform, we then examined the potential public health consequences of introducing a universal influenza vaccine among adults 18–49 years of age because this age group is least likely to receive annual influenza vaccination and may be the first for whom a broadly protective universal influenza vaccine is licensed. Specifically, vaccination strategies were tested under various scenarios in which universal vaccine replaced a) none; b) half; and c) all seasonal vaccine for all vaccinated 18 to 49-year-olds. Three research questions were explored: 1) Does offering universal influenza vaccine to adults 18–49 years of age decrease their influenza cases over a 4-year time horizon? 2) How does this public health benefit of universal influenza vaccine vary with different levels of vaccine coverage? 3) Do the age groups who are more vulnerable to influenza (children 6 months to 17 years and older adults ≥50 years) benefit from indirect or herd immunity resulting from universal influenza vaccination of adults 18–49 years of age?
Results
To determine the public health impact of vaccination strategies that include a universal influenza vaccine, the model calculated the annual influenza cases and the cumulative number of influenza cases among 18 to 49-year-olds during four seasons.
In the base case simulation, where total population vaccine coverage was 50% in Year 1, seasonal VE was 60% and universal VE was 30%, more influenza cases occurred in the first year, due to decreased universal VE compared to the seasonal vaccine, but fewer annual cases occurred in Years 2 through 4 resulting in decreased cumulative influenza cases as early as Year 2 ().
The impact of using universal vaccine among 18 to 49-year-olds was also measured for two other age groups who are most susceptible to the effects of influenza infection, i.e., children (0–17 years) and older adults (≥50 years). The reduction in annual and cumulative cases in children and adults was comparable in absolute numbers to that among 18 to 49-year-olds. The year-to-year pattern for annual and cumulative cases was also similar for all age groups.
To adjust for the relative sizes of the three age groups, the number of averted influenza cases per 100,000 population was calculated for scenarios when half and all of 18 to 49-year-old vaccinees received universal influenza vaccine. shows these rates for 50% total population influenza vaccine uptake during Year 1. In that year, the number of averted cases was lower (more flu cases) for all age groups, but in Years 2–4, the numbers of averted cases when universal vaccine had been used were greater than the numbers when seasonal vaccine only was used. Moreover, larger proportions of averted cases were observed in Years 2–4 among the groups not receiving the universal vaccine (children and older adults), indicating an indirect protective effect. In any scenario, the use of more universal vaccine in Year 1 resulted in greater proportions of averted cases.
Table 2. Key parameters in the model
Table 1. Vaccination coverage and averted influenza cases when total population coverage in Year 1 = 50%
In the sensitivity analysis simulation, where VE remained the same but total population vaccine coverage was 40% in Year 1, similar effects were observed ( and ). It should be noted that when only 40% of the total population is vaccinated, the number of influenza cases is considerably higher than when 50% is vaccinated, regardless of the type of vaccine received.
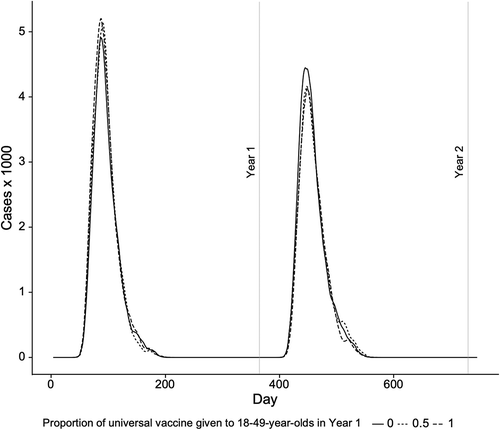
shows the epidemic curves for the effect on the entire population of 50% total vaccine coverage and having none, half and all of 18 to 49-year-old vaccines receive universal vaccine in Year 1. During Year 1, use of all seasonal vaccine and no universal vaccine resulted in fewer influenza cases than scenarios using either half or all universal vaccine among 18 to 49-year-olds. In Year 2, the reverse is true with fewer overall influenza cases resulting from both scenarios using universal vaccine (half or all) among 18 to 49-year-old vaccinees in Year 1.
Discussion
Universal influenza vaccine, with its long-lasting immunity and ability to prevent infection by genetically drifted strains of influenza, offers an opportunity to dramatically reduce morbidity and mortality. A single vaccination given two or three times in a lifetime would likely be preferred by those who are not vaccinated annually because they forget to be vaccinated (no need to remember every year), find vaccination to be inconvenient (vaccination could occur year-round, not just during influenza season), or do not trust the vaccine because the formulation is frequently changed (the universal vaccine binds a less variable portion of the virus). However, in any given season, a broadly protective influenza vaccine might be less effective than a seasonal vaccine that has been carefully matched to one or more of the current circulating strains. To be acceptable from a public policy perspective, the balance between delayed increases in vaccine uptake and potential lower effectiveness of the universal vaccine must be weighed. Indeed, early universal vaccines might not be designed well for influenza B,Citation10 leading to a modest overall effectiveness when both influenza types A and B are considered.
This analysis found that in the short term of a single year, the modestly effective universal vaccine does not decrease the burden of influenza as measured by estimated cases overall, or in any of the three population subgroups – children, adults and older adults. Importantly, by the second year without additional universal vaccine recipients, the number of influenza infections declines, relative to the baseline condition without universal vaccine, at any level of universal vaccine tested. That is, the additional cases averted in the second year (and all subsequent years) are sufficient to fully compensate for the increased number of cases in the initial year of universal vaccine usage. Such findings provide invaluable insights into the potential public health policies and effective vaccination strategies.
Vaccinating adults with universal vaccine also had a protective effect on other age groups who were assumed to be ineligible to receive a universal vaccine, that is, it created herd immunity that reduced infection in older adults and children, both of whom are more vulnerable to influenza infection. In previous research, we found the importance of adult vaccination using seasonal vaccine for reducing infection in children.Citation18–Citation20 Other research has demonstrated that universal influenza vaccine can be cost-effective when cost is ≤$100/dose and VE at both 5 and 10 years is ≥75%, or VE is 50% for 10 years,Citation21 VE values far higher than those used in these analyses. This initial analysis did not consider cost-effectiveness, but potential improvements in public health due to universal vaccine suggest that use of universal vaccine may be cost-effective at lower VE and those analyses should be the focus of further research.
The current definition of a universal vaccine has a proposed VE of 75%.Citation7 This analysis used a conservative VE value of 30%, less than half of the proposed acceptable VE. This scenario, using universal vaccine in only one year with a highly conservative VE estimate, would bias against universal vaccine. Yet we found that it was effective for reducing influenza cases in the group using universal vaccine, as well as other age groups who received only seasonal vaccine. These results indicate that a more effective universal vaccine would have significantly greater public health benefits.
Limitations
FRED is a well-known single-season ABM framework that has been modified to PHIL to allow for multi-year influenza modeling; indeed, one of the major purposes of this paper was to move the public health computer science framework to handle multiple years of infection. Given the number of computations involved in simulating over a million persons with multiple infection opportunities and multiple vaccination opportunities, a sizable supercomputing cluster was needed. An important limitation is its complexity. We have attempted to address this complexity by making certain simplifying assumptions. We strictly controlled for differences in seasonal epidemiology by influenza seeding, transmission dynamics and defined immunity parameters. These parameters may not represent all influenza seasons nor account for other unknown factors. Furthermore, the assumption that seasonal vaccine produces permanent immunity may be questioned. A change in this assumption would increase the complexity of the analysis. These initial experiments were limited in scope and perhaps differ from the way in which universal vaccine would ultimately be employed. However, the simplicity was designed to test the feasibility of the model and to identify important lessons. The value of using agent-based simulation models lies in its ability to account for heterogeneities in population structure that may not be captured as well in other models such as equation-based models (EBM). Conversely, an age-structured EBM may have adequately accounted for those heterogeneities. However, we chose the ABM because this model with its simplifying assumptions can form the basis of subsequent, more complex scenarios in which for instance, universal vaccine is given in several years, is gradually introduced, is given to additional age groups, or other parameter changes such as, universal vaccination takes place year-round, or seasonal vaccination is spread out over several months. Future studies might also explore a variety of higher VE estimates for universal vaccine.
Conclusions
Using a new, multi-year PHIL, this study found that universal vaccination in adults 18–49 years old reduces cases of influenza in Years 2–4 but not in Year 1 among the age group receiving the universal vaccine. The use of universal vaccine also resulted in indirect protective effects among younger and older age groups. Should the VE of universal vaccines that become licensed exceed that assumed in these models, a reduction in influenza burden may be observed as early as the first year of their use. These findings should assist vaccine policy officials and planners as they contemplate future influenza vaccine recommendations.
Methods
Agent-based model of Allegheny County, PA synthetic population
Using a modified version of the FRED ABM framework,Citation17 which enables fine-grained control of age-specific vaccine coverage (PHIL), simulations were conducted using a synthetic population of Allegheny County Pennsylvania based on the 2010 US Census.Citation22 That is, the model contained approximately 1.3 million virtual people (i.e., “agents”) who were assigned to households that represented the geospatial population density and demographics of Allegheny County at the census tract level.
Each agent was assigned to a household and to age-appropriate outside locations; school-aged children to schools and working adults to a workplace. On each weekday, agents start in their households, coming into contact with other household members; then children leave for school and adults for work, coming in contact with others at those locations; and finally, they mix in their neighborhoods and their community in the evening. On weekends, mixing occurs primarily in households and neighborhoods, with only 20% of adults working.Citation22
Influenza transmission
On a given day, each agent may be in one of four influenza disease states: susceptible, exposed, infectious, or removed (i.e., not susceptible due to past exposure or vaccination). The influenza epidemic simulation was initiated by randomly selecting 100 agents and assigning them to the infectious state, a process referred to as “seeding.” Each year, an immunologically distinct influenza strain is introduced (seeded) during a short period at the beginning of each season. Infection induces complete immunity to the infecting strain that lasts for the remainder of the simulation (does not wane). Infection does not induce immunity to other strains.
Infectious agents encounter susceptible agents as they visit their households, schools and workplaces. When an agent in the infectious state comes in contact with agents in the susceptible state, influenza may be transmitted, in which case, any newly infected agent moves to the exposed state. The function that is used to calculate the probability of successful transmission is dependent on both the age of the individuals coming into contact, as well as the type of place (i.e., school, work, home, community). The details of this transmission function remain unchanged and are described in detail in Grefenstette, 2013.Citation17 Upon successful transmission, an agent moves from the susceptible state to the exposed state for a latent period drawn from a truncated Weibull distribution with a mean of 1.9 days (). At the conclusion of the latent period, the agent moves to the infectious state for an infectious period drawn from a truncated Weibull distribution with a mean of 4.1 days (). It is assumed that one third (33%) of the agents who enter the infectious state are asymptomatic and are 50% less infectious than a symptomatic agent.Citation23,Citation24 Half (50%) of all symptomatic agents were randomly assigned to stay home from school or work, thus limiting contact with others who may be in the susceptible state. These parameters and assumptions are consistent with previously published ABM studiesCitation25–Citation31 and are summarized in . Influenza transmission in the model was calibrated to match age-specific attack rates in the presence of vaccination. Transmissibility matched as closely as possible the age-specific attack ratesCitation32 and translated to an effective reproduction number of 1.27.
Vaccination
Three vaccination strategies were tested in the base case in which total population coverage was 50% and universal vaccine replaced a) none; b) half; and c) all seasonal vaccine for vaccinated 18 to 49-year-olds. In sensitivity analyses, total population coverage was reduced to 40%. In both levels of influenza vaccine coverage, seasonal VE was assumed to be 60% and universal VE was assumed to be 30%. Vaccine coverage distribution was proportional to the age-specific coverage published in Molinari et al..Citation31 For the sake of simplicity in modeling, all vaccination was assumed to take place in the first week. Without this simplification, the number of simulations required to achieve stability in the model would be dramatically higher and decrease the ability to interpret the effect of introducing a broadly protective, long-lasting influenza vaccine. Seeding occurred after vaccination was complete. The seasonal influenza vaccine used in this model was assumed to produce permanent immunity to the particular target strain assigned to the vaccine. Furthermore, there was no cross-protection conferred by the seasonal vaccine. The universal vaccine given only to adults 18–49 years old was assumed to confer immunity to all influenza strains throughout the simulation. For both seasonal and universal vaccines, effectiveness was assumed not to vary based on the age of the person vaccinated. A relatively low VE for universal vaccine and relatively high VE for seasonal vaccine were selected to create conservative estimates that would be upheld in scenarios with more effective universal vaccine and/or less effective seasonal vaccine.
Simplifying assumptions
Because PHIL used a multi-year modeling of seasonal influenza in the presence of vaccination, this model made other simplifying assumptions. Firstly, the model did not account for population aging over the 4-year time horizon of the study; that is, the population does not age from Year 1 to Year 4. This assumption reduced the difficulties of older teenagers aging into the adult 18 to 49-year-old group, thereby changing the percentage of this age group that was protected by universal vaccine. Secondly, the universal vaccine was assumed to be given only once during Year 1, thus keeping the number of individuals who have received universal vaccine constant over the 4-year time horizon. Thirdly, vaccination did not change agents’ day-to-day behaviors. Finally, the total number of doses given each year including the universal vaccine given in Year 1 was held constant. Thus, in Years 2–4, the number of individuals receiving vaccine stayed the same as in Year 1, while the number of individuals protected was equal to the number vaccinated with seasonal vaccine plus the number vaccinated with universal vaccine in Year 1 (adjusted by the stated vaccine effectiveness values).
Calibration
To produce an agent-based model suitable for multi-year seasonal influenza epidemics, the calibration used age-specific attack rates.Citation32 The age-specific contact rates were adjusted using a modification of a multi-objective, parallel genetic algorithmCitation33 until the resulting age-specific attack rates matched those of Molinari.Citation31 The baseline calibration was carried out using 60% VE for seasonal vaccine and age-specific coverage listed in . After calibration, the PHIL ABM was used to test the effect of universal vaccine given to adults aged 18–49 years, then the indirect effect of those vaccination strategies on children and older adults was similarly tested. In total, 6,000 simulations were conducted. Vaccination strategies were tested at two levels of total population coverage (40% and 50%), in which universal vaccine replaced a) none; b) half; and c) all seasonal vaccine for all vaccinated 18 to 49-year-olds. For each of these six vaccination strategies, 1,000 individual stochastic simulations were averaged, resulting in age-specific numbers of influenza cases. All simulations were run on the Olympus High Performance Computing Cluster at the Pittsburgh Supercomputing Center.
Simulations
Base Case Simulation: Total population vaccine coverage = 50% in Year 1, seasonal VE = 60%, universal VE = 30%
In the base case in Year 1, where no universal vaccine was given, 27.1% of all 18 to 49-year-olds were vaccinated with all receiving seasonal vaccine and none receiving universal vaccine. In the scenario where half of 18 to 49-year-old vaccinees received the universal vaccine in Year 1, an additional 13.6% of adults were covered by the universal vaccine, resulting in a combined coverage of 40.7% for adults aged 18–49 years in Years 2–4. Coverage for the other age groups, who all received the seasonal vaccine in all years of the study, was 54.1%, for a total population (all age groups) coverage of 50%. (See , left.) Thus, the same number of doses is given each year, but the number of protected individuals changes from Year 1 to Years 2–4. These vaccine distributions were chosen to preclude additional complexities that might change the epidemiology of the influenza season due to different vaccination coverage levels, allow for direct year-to-year comparisons, and isolate the effect of giving universal vaccine in place of seasonal vaccine in Year 1. Universal vaccine was not assumed to be given again in Years 2–4 for the same reasons.
Sensitivity Simulation: Total population vaccine coverage = 40% in Year 1, seasonal VE = 60% and universal VE = 30%
In Year 1, a total of 21.6% of adults ages 18–49 years received an influenza vaccine, with all being a seasonal vaccine and no universal vaccine received. Coverage for the other age groups, who all received the seasonal vaccine was 52.6%, for a total population (all age groups) coverage of 40%. In the scenario in which one half (10.8%) of 18 to 49-year-old vaccinees received the universal vaccine in Year 1 and a half (10.8%) received seasonal vaccine, 32.4% of 18 to 49-year-olds were vaccinated in Years 2–4. In the scenario in which all (21.6%) of 18 to 49-year-old vaccinees received universal vaccine and none received seasonal vaccine in Year 1, a total of 54.2% were vaccinated in Years 2–4. (See .)
Disclosure of potential conflicts of interest
Dr. Zimmerman has research funding from Pfizer, Inc., Merck & Co., Inc. and Sanofi Pasteur, Inc. Dr. Nowalk has research funding from Merck & Co., Inc. and Pfizer Inc. None of the other authors has conflicts to report.
Clinical Trial Number
Not applicable
Abbreviations
| IIV | = | inactivated influenza vaccine |
| ABM | = | agent-based model |
Additional information
Funding
References
- Scorza FB, Tsvetnitsky V, Donnelly JJ. Universal influenza vaccines: shifting to better vaccines. Vaccine. 2016;34(26):2926–33. doi:10.1016/j.vaccine.2016.03.085.
- Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008;121(7):S28–S35. doi:10.1016/j.amjmed.2008.05.005.
- Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, Sovran J, Stephens D, Katz J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30(32):4807–12. doi:10.1016/j.vaccine.2012.05.011.
- Seasonal flu vaccination: why don’t more Americans get it? [accessed 2018 Feb 15]. https://www.rand.org/content/dam/rand/pubs/research_briefs/2011/RAND_RB9572.pdf.
- Seale H, Heywood AE, McLaws M-L, Ward KF, Lowbridge CP, Van D, MacIntyre CR. Why do I need it? I am not at risk! Public perceptions towards the pandemic (H1N1) 2009 vaccine. BMC Infect Dis. 2010;10(1):99. doi:10.1186/1471-2334-10-99.
- Uscher-Pines L, Maurer J, Kellerman A, Harris KM. Healthy young and middle age adults: what will it take to vaccinate them for influenza? Vaccine. 2010;28(46):7420–22. doi:10.1016/j.vaccine.2010.08.095.
- Status of Vaccine Research and Development of Universal Influenza Vaccine. Prepared for WHO PD-VAC. In: WHO; 2014.
- Krammer F, García-Sastre A, Palese P. Is it possible to develop a “universal” influenza virus vaccine? Toward a universal influenza virus vaccine: potential target antigens and critical aspects for vaccine development. Cold Spring Harb Perspect Biol. 2017;a028845. doi:10.1101/cshperspect.a028845.
- Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, Paules CI, Graham BS, Fauci AS. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis. 2018;218(3):347–54. doi:10.1093/infdis/jiy103.
- Egorov AY. The challenges of creating a universal influenza vaccine. MIR J. 2016;3(1). doi:10.18527/2500-2236-2016-3-1-31-41.
- Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013;64:189–202. doi:10.1146/annurev-med-120611-145115.
- Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, Zimmerman RK, Gaglani M, Lamerato L, Spencer SM, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–27. doi:10.1093/cid/cit736.
- McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk MP, Raviotta JM. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis. 2015;211(10):1529–40.
- Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, Chung JR, Piedra PA, Avadhanula V, Nowalk MP. Influenza vaccine effectiveness against 2009 pandemic influenza A (H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis. 2016;213(10):1546–56. doi:10.1093/infdis/jiv577.
- Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, Monto AS, McLean HQ, Belongia EA, Gaglani M. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63(12):1564–73. doi:10.1093/cid/ciw635.
- Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, et al. Influenza vaccine effectiveness in the United States — 2015/16 season. N Engl J Med. 2017;377:534–43. doi:10.1056/NEJMoa1700153.
- Grefenstette JJ, Brown ST, Rosenfeld R, DePasse J, Stone NT, Cooley PC, Wheaton WD, Fyshe A, Galloway DD, Sriram A, et al. FRED (a Framework for Reconstructing Epidemic Dynamics): an open-source software system for modeling infectious diseases and control strategies using census-based populations. BMC Public Health. 2013;13:940. doi:10.1186/1471-2458-13-940.
- Depasse JV, Nowalk MP, Smith KJ, Raviotta JM, Shim E, Zimmerman RK, Brown ST. Does cost-effectiveness of influenza vaccine choice vary across the U.S.? An agent based modeling study. Vaccine. 2017;35:3974–81. doi:10.1016/j.vaccine.2017.05.093.
- DePasse J, Smith KJ, Raviotta JM, Shim E, Nowalk MP, Zimmerman RK, Brown ST. Does choice of influenza vaccine type change disease burden and cost-effectiveness in the United States? An agent-based modeling study. Am J Epidemiol. 2017;185(9):822–31. doi:10.1093/aje/kww229.
- Raviotta JM, Smith KJ, DePasse J, Brown ST, Shim E, Nowalk MP, Zimmerman RK. Cost‐effectiveness and public health effect of influenza vaccine strategies for US elderly adults. J Am Geriatr Soc. 2016;64(10):2126–31. doi:10.1111/jgs.14323.
- Lee BY, Tai JH, McGlone SM, Bailey RR, Wateska AR, Zimmer SM, Zimmerman RK, Wagner MM. The potential economic value of a ‘universal’(multi‐year) influenza vaccine. Influenza Other Respi Viruses. 2012;6(3):167–75. doi:10.1111/j.1750-2659.2011.00288.x.
- Patterns of Metropolitan and Micropolitan Population Change: 2000 to 2010. [accessed 2018 Feb 15]. https://www.census.gov/library/visualizations/2012/dec/c2010sr-01-pyramid.html
- Longini IM Jr., Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–33. doi:10.1093/aje/kwh092.
- Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–14. doi:10.1038/nature04017.
- Elveback LR, Fox JP, Ackerman E, Langworthy A, Boyd M, Gatewood L. An influenza simulation model for immunization studies. Am J Epidemiol. 1976;103(2):152–65. doi:10.1093/oxfordjournals.aje.a112213.
- Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–52. doi:10.1038/nature04795.
- Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103(15):5935–40. doi:10.1073/pnas.0601266103.
- Halloran ME, Ferguson NM, Eubank S, Longini IM Jr., Cummings DA, Lewis B, Xu S, Fraser C, Vullikanti A, Germann TC, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci U S A. 2008;105(12):4639–44. doi:10.1073/pnas.0706849105.
- Cooley P, Brown S, Cajka J, Chasteen B, Ganapathi L, Grefenstette J, Hollingsworth CR, Lee BY, Levine B, Wheaton WD, et al. The role of subway travel in an influenza epidemic: a New York City simulation. J Urban Health. 2011;88(5):982–95. doi:10.1007/s11524-011-9603-4.
- Cooley P, Lee BY, Brown S, Cajka J, Chasteen B, Ganapathi L, Stark JH, Wheaton WD, Wagener DK, Burke DS. Protecting health care workers: a pandemic simulation based on Allegheny County. Influenza Other Respir Viruses. 2010;4(2):61–72. doi:10.1111/j.1750-2659.2009.00122.x.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. doi:10.1016/j.vaccine.2007.03.046.
- FluVax View. [accessed 2018 Feb 15]. http://www.cdc.gov/flu/fluvaxview/index.htm.
- Biscani F, Izzo D, Martens M: esa/pagmo2: pagmo 2.6 (Version v2.6). 2017.
Appendix
Table A1. Vaccination coverage and averted influenza cases when total population coverage in Year 1 = 40%