ABSTRACT
Allergen immunotherapy has been used for more than 100 y, but only recently underlying immunological mechanisms have started to be understood. New Allergy vaccines are now considered to be full pharmaceutical products, that should comply with general as well as specific pharmaceutical legal framework. GRAZAX® is the first global allergy vaccine developed in compliance with the new legal environment and is thus a reference for developing new allergy vaccines. Here, we provide a rationale description of GRAZAX®, providing a sequential description of its pharmaceutical and clinical development. With more than 25 clinical trials, involving more than 8000 patients, including as well three 5-y prospective clinical trials, GRAZAX® is a key product to understand the unique position of allergen-specific immunotherapy as a disease-modifying intervention.
Introduction
Allergen-specific immunotherapy has been used for more than 100 y but only recently allergy vaccination has been included within the pharmaceutical regulatory framework. Until late 80s, only some general guidelines covering mainly particular quality aspects were issued by different regulatory agencies. After the inclusion of allergen immunotherapy (AIT) products within the European Directive of Medicinal Products, the progressive development of specific European Medicines Agency (EMA) guidelines, and European Directorate for the Quality of Medicines (EDQM), European pharmacopoeia monograph, new projects aiming to develop industrially produced AIT products must follow this demanding regulatory frameworkCitation1. This is the case of GRAZAX®. Moreover, when considering a global product as GRAZAX®, the position of non-European regulatory agencies, for example Centre for Biologics Evaluation and Research (CBER)-FDA, had to be taken into account.Citation2
During the 1990s, different clinical research groups in Italy started to develop a new way of administering AIT by sublingual route. Several small and investigator-driven studies suggested this new route was effective, with a clear improvement in the safety profile compared to subcutaneous vaccines. The publication of meta-analysis on accumulated evidence on sublingual studies, although with a high heterogeneity, concluded that this route was effective. Sublingual/swallowed administration was recommended and, subsequently, an open debate on the optimal dose was initiated.
Origin and research basis for the design of the product
Despite the sublingual/swallow recommendation, studies performed addressing gastric route alone failed to prove clinical effect. Based on this, the idea came up that a better pharmaceutical formulation, aiming to increase bioavailability of the allergen in the oral mucosa, might improve the effect. The Zydis technology from RP Scherer (currently Catalent) consisted of a freeze-dried tablet pharmaceutical presentation that was successfully used in different drug formulations. This formulation presents a unique bioavailability profile that allows an immediate release of the allergen in the oral mucosa with a maximum local concentration.Citation3 The incorporation of a Phleum pratense allergenic extract onto Zydis platform is the basis of GRAZAX®.
Preclinical and toxicology studies
As previously mentioned, GRAZAX® was based on accumulated experience of sublingual IT in human studies that were basically based on glycerinated aqueous formulations. Besides those studies, the toxicological profile of GRAZAX® was investigated in several in vitro and in vivo studies, revealing no concerns for its use in human beings. However, there is currently no clinical experience of the use of GRAZAX® in pregnant and lactating women.
Animal models were developed to investigate sublingual mechanism and specifically to prove that fast-dissolving orodispersible tablet for sublingual administration was able to reduce allergic symptoms in a time- and dose-dependent manner.Citation4
Pharmacokinetics
The allergens in GRAZAX® mainly comprise polypeptides and proteins that are thought to break down to amino acids and small polypeptides in the gastrointestinal tract and in tissues. These allergens are not expected to be absorbed into the vascular system to any significant extent. Consequently, no pharmacokinetic studies are needed to be conducted in animals or in the clinical setting.
Safety testing
GRAZAX® is the first AIT preparation that has been developed prospectively in accordance with Pharmaceutical regulations.
In spite of using an active pharmaceutical ingredient (API) that had been extensively used in other vaccines formulation, in agreement with regulatory bodies of UE and USA, different safety tests were performed following International Council for Harmonisation (ICH) guidelines. Preclinical safety studies included acute and repeated dose toxicity testing, as well as mutagenicity potential (AMES test). No major safety-related observations were found.
Production
Since 1989, directive 89/342 EEC extended the scope of pharmaceutical directive to allergen products. Besides, a specific European Pharmacopoeia monograph on allergen products set the legal basis for allergen product commercialization. Specific note for guidance has been progressively introduced, setting source material as well as final product quality requirements.
Manufacturing layout must follow European Pharmacopeia. An API must be defined and standardized according to regulations. Total biological potency, major allergen content, and appropriate identity test must be implemented. Despite the efforts carried out in the last decades,Citation5 there is no international reference preparation for Phleum individual allergens. To date, only a reference on major allergen for Bet v 1 is implemented.Citation6 Therefore, a careful internal reference system was implemented. Basic standardization and quality check are performed at the API level. As quality consistency is directly dependent on the quality of source material use for API manufacture, vertical control of all the process is guaranteed, and a specific ALK Group Company, dedicated to source material production of allergens, supply the pollen used for API manufacturing.
API consists of a semi-purified (ultra-filtration) aqueous pollen extract. Extraction conditions are optimized to solubilize proteins under physiological conditions. The extract includes all relevant allergens present in the pollen in native form. No further modification aiming to modify immunological recognition of allergenic component is performed. It must be noticed that immunological recognition of these antigens greatly varies from one patient to another.
API is incorporated into gelatine- and mannitol-containing formulation at the nominal dose deeply frozen and freeze-dried. A detailed description of the GRAZAX® manufacturing and standardization process is described by Römmelmayer et al.Citation7
Assays for releasing and characterizing the final product
Specification and release criteria for GRAZAX® are shown in .
Table 1. Batch release specifications for GRAZAX®
Main test includes physical, chemical, immunochemical, and microbiological criteria.
Indication
GRAZAX therapeutic indication is: Treatment of grass pollen induced rhinitis and conjunctivitis in adult and children (>5-y old) patients with clinically relevant symptoms and diagnosed with a positive skin prick test and/or specific IgE test to grass pollen.
GRAZAX® addresses the underlying allergic condition and builds up an immunological tolerance to grass pollen by inducing immunological tolerance towards grass allergens. According to the summary of product characteristics, as a “Disease-modifying treatment of grass pollen induced rhinitis and conjunctivitis in adults and children (5 y or older), with clinically relevant symptoms and diagnosed with a positive skin prick test and/or specific IgE test to grass pollen”.
GRAZAX®is in allergen extracts, grass pollens pharmacotherapeutic group, with the Anatomical Therapeutic Chemical (ATC) code V01AA02.
Mode of action
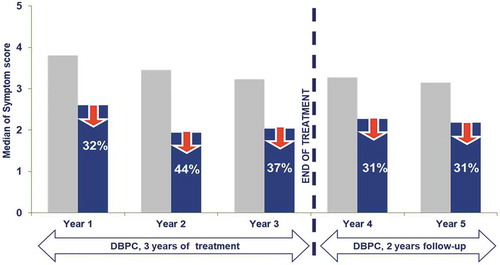
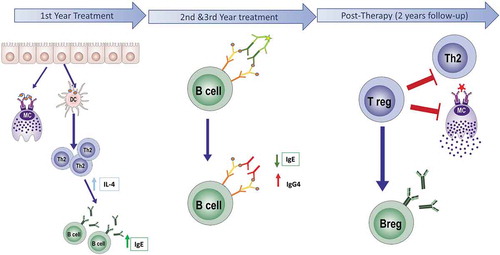
Specific Immunotherapy for allergy treatment has been used for more than 100 y. As previously mentioned, sublingual immunotherapy has only gained attention in the last three decades. There is a consensus that the aim of AIT is to induce allergen-specific peripheral tolerance by the periodic administration of the sensitizing allergen. Mechanisms described in relation to AIT effect include desensitization, T and B regulatory response generation, rebalance of Th1/Th2 responses, and interference of blocking antibodies (IgG4).Citation8 In a recent publicationCitation9 it is described that oral mucosa undergoes a progressive remodelling associated to allergic inflammation. This impaired barrier function of the oral mucosa in allergic subjects constitutes an immunological access point for sublingual immunotherapy products. The sequence of treatment-induced changes in the later immunological events is analyzed in a prospective immunological mechanism study.Citation10,Citation11 This study, carried-out for 5 y (3 of active therapy and 2 y follow-up), demonstrated that long-lasting benefit associated to the intervention is linked to the acquisition of an activated T cell memory regulatory phenotype. This response is generated progressively during the intervention and consolidated after 3 y of continuous treatment. Interestingly, recent studies have demonstrated that if GRAZAX® (or high-dose subcutaneous AIT) is administered only 2 y, this sustained benefit is lost.Citation12 Short-term effect (1–2 months) is generated by desensitization of effector cells, a mechanism that is not fully understood, antigen specific, and quickly lost after therapy discontinuation. Change in antibodies profile, linked to a progressive increase of specific IgG4, is seen after several months of therapy and reaches a peak during the first 2 y of treatment, with a high patient variability. In summary, these data support the need of continuous administration over a 3-year period, as stated in the marketing authorization of the product. In are summarized the immunological mechanisms involved in GRAZAX® effect.
Figure 1. Scheme of the immunological response to allergen-specific immunotherapy (AIT). AIT during the early phase of the treatment (1–4 months), induces both mast cell desensitization and upregulation of Th2 response, mediated by high levels of IgE and IL4. During the active phase (1–3 y), a switch of isotype occurs. The levels of IgG4 increase while IgE significantly decrease. In this period is the maximum effect of IgG4 interference. Later, after 3 y of AIT treatment, in the post-therapy period, the regulatory response is established, and levels of IgE and IL4 are significantly decreased
Clinical development
According to the European regulatory guidelines on the clinical development of products for specific immunotherapy for the treatment of allergic diseases,Citation13 clinical efficacy evidence for seasonal allergies requires randomized double-blind, placebo-controlled (DBPC) long-term clinical trials. The following objectives have to be fulfilled:
Treatment of allergic symptoms as clinical efficacy in first pollen season;
Sustained clinical effect as maintenance of significant and clinically relevant efficacy during 2–3 treatment years;
Long-term efficacy and disease-modifying effect as sustained significant and clinically relevant efficacy in post-treatment years (a minimum of 2 y after stopping immunotherapy);
Curing allergy as sustained absence of allergic symptoms in post-treatment years.
GRAZAX® possesses the most complete and comprehensive series of clinical trials ever performed in specific AIT. With a total of 16 DBPC phase I–IIIb trials, in pediatric, adolescents, and adults, involving more than 7000 patients (>1700 aged 5–17-y old, >4400 adults), overall, GRAZAX® has set the clinical reference for future developments in AIT.
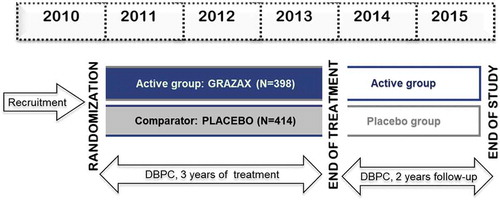
An example of design of 5-y trial is shown in .
Figure 2. Example of a 5-y prospective clinical trial design
Phase I/II trials
Phase I/II trials incorporated 2400 patients and involved 124 clinical groups. The first study was performed in 52 subjects. Doses up to 75,000 Standardized Quality Units Tablets (SQ-T) were administered. In a second study, that involved 84 allergic patients 14, the tested dose was raised until 1,000,000 SQ-T. Cardiovascular system safety pharmacology was an integrated part of chronic repeat-dose toxicology study by electrocardiogram (ECG) and blood pressure measurements. No test-article-related effects on blood pressures or ECG patterns were detected. A further safety study was performed in 43 asthmatic patients. Fifteen of them received the maximum dose of 500,000 SQ-T. Phase I development was completed with two studies performed in children.Citation14
Overall, the product had an adequate safety/efficacy profile. A dose-/time-dependent serological antigen-specific response was reported. Most of the adverse drug reactions were local and mild and appeared during the first days of administration and faded in most of the patients after a week of treatment. No life-threatening reactions were observed. The adequate safety profile of GRAZAX® shown in these three studies allowed advance to further clinical research.
A pivotal Phase II trial in adults, with three administered doses, allowed the identification of the optimum therapeutic dose of 75,000 SQ-T.Citation15–Citation17
An additional safety and efficacy study on patients (n = 114) with mild-to-moderate asthma and rhinoconjunctivitis was performed. Safety in a severe patient subgroup was closely monitored.Citation18 The treatment was well tolerated and had a clear clinical benefit of symptoms/medication scores.
All the above-mentioned studies confirmed that the 75,000 SQ-T (GRAZAX®) dose provided the best risk-benefit profile.
Phase III trials
Pivotal Phase III trial: Pivotal trial was a multicenter, randomized, DBPC, parallel-group study, which aimed to confirm the efficacy of GRAZAX® in patients with seasonal rhinoconjunctivitis with or without mild-to-moderate asthma. A total of 634 grass-allergic patients were included in the study.
Treatment was initiated at least 16 weeks prior to the grass pollen season (GPS) and continued all year round, until the end of the GPS. The study was prolonged for two additional treatment years in about 50% of the subjects, and the effect was also assessed in a DBPC manner two additional years after treatment cessation.
Patients with clinical history of grass pollen–induced allergic rhinoconjunctivitis having received symptomatic treatment during the previous GPS, positive skin prick test against P pratense (Soluprick SQ; ALK), wheal diameter >3 mm; specific IgE against P pratense, IgE class 2; no clinical history of chronic sinusitis or perennial or seasonal allergic rhinitis and/or asthma because of another allergen during – or potentially overlapping – the GPS; no clinical history of severe asthma (Global Initiative for Asthma 2002 step 4 and FEV1 <80% of expected value after treatment with inhaled corticosteroids and short-acting b2-agonists); and no previous treatment by allergen-specific immunotherapy within the previous 5 y. Pregnancy was also an exclusion criterion.
Clinical efficacy was based in allergy symptom and medication scores. They were calculated as the sum of the individual daily scores for each subject during the GPS 2007 divided by the number of subject diary recordings of that score during the same period.
Each day subjects rated their rhinoconjunctivitis and asthma symptoms in the electronic diaries on a scale from 0 to 3 (0 = no, 1 = mild, 2 = moderate, 3 = severe symptoms). A total of 10 types of symptoms were rated. The six rhinoconjunctivitis symptoms were: runny nose, blocked nose, sneezing, itchy nose, gritty feeling or red/itchy eyes, and watery eyes. The four asthma symptoms were cough, dyspnea, wheezing, and exercise-induced asthma. In case of allergic symptoms, subjects had access to symptomatic relief medication (loratadine tablets, levocabastine eye drops, budesonide nasal spray, salbutamol spray, fluticasone inhaler, and prednisolone tablets) provided in a stepwise fashion depending on the persistence and severity of the symptoms. Use of relief medication was recorded in the daily diary and scored according to predetermined criteria.
This was the first prospective, 5-y clinical trial ever performed in AIT, and demonstrated that clinical benefit was established in the first treatment year, maintained during the two subsequent treatment years and persisted 2 y after discontinuation.Citation19,Citation20 The effect size was comparable over the 5 y ().
Other phase III trials
Pediatric indication was obtained in a DBPC study performed over a sample of 253 grass-allergic children (5–16-y old).Citation21 The results confirmed same clinical benefit and immunological response as shown in adult studies.
Registration in North-America was gained with three DBPC studies, two of those Phase III trials performed in children, adolescents, and adults (5–65-y old), suffering from grass-allergic rhinitis with/without conjunctivitis and/or asthma, carried on in USA and Canada. A total of 1886 patients randomized 1:1 were included. Out of them, 85% were polysensitized and 25% had asthma. The results confirmed previous findings showing that GRAZAX® was effective in polysensitized grass-allergic North American children and adults with allergic rhinitis.Citation22,Citation23
As a summary, according to EMA guidelines on clinical development of products for specific immunotherapy, GRAZAX® has demonstrated clinical efficacy from the first GPS symptoms (as long as treatment is started 4 months before), sustained clinical effect during 3 y administration, and long-term efficacy (at least 2 y after discontinuation), being therefore a disease-modifying treatment.
Post-marketing authorization studies
Further clinical research as well as scientific evidence continue aftermarket authorization. More than 8700 patients have been exposed in 24 post-authorization phase IV and post-marketing surveillance studies. These studies have confirmed the safety profile of GRAZAX®.
In pharmacovigilance studies, safety-related variables were studied. Post hoc analysis from 13 GRAZAX® trials n = 2497; placebo, n = 2139 regarding epinephrine administrations in response to sublingual immunotherapy (SLIT)-tablet-related reactions were analyzed. The authors referred that epinephrine was used 13 times (grass SLIT-tablet, n = 10; placebo, n = 3). Eight administrations were associated with SLIT-tablet-related adverse events: four for systemic allergic reactions and four for local mouth and/or throat swelling. They concluded that epinephrine use is uncommon, typically occurs within the first week of treatment, and is rarely self-administered. All SLIT-tablet-related events treated with epinephrine were nonserious.Citation24
The combined use of different AIT tablets was also investigated post-marketing. This is relevant because many patients are allergic to more than one allergen. In this trial, patients were allergic to both grass and ragweed, and dual administration of grass and ragweed SLIT tablets may be indicated for some of these patients. This multicenter open study including 102 patients found that after tolerability with single SLIT tablet administration has been established (4-week sequential tablet dosing schedule), dual treatment with GRAZAX® and ragweed SLIT tablets was well tolerated and could be followed by simultaneous tablet administration at home.Citation25
Relevant scientific evidence like starting GRAZAX® treatment at any time of the year or even safety profile of intra-seasonal start of GRAZAX® in real-life setting show same tolerability as previously described in DBPC studies. Authors concluded that tolerability data for an intra-seasonal start of grass AIT during routine treatment confirmed the safety profile already known. The good tolerability was associated with high satisfaction and compliance.Citation26–Citation28
Attempts to improve compliance had been also reported post-marketing. Alesina et al. investigated if compliance can be increased by providing the patients an Compliance electronic device (CED) (Memozax; a tablet container with a programmable daily acoustic alarm). They showed that compliance to the treatment with AIT administered for 12 consecutive months is in general good (mean 83%). The use of CED was not associated with a greater compliance. AIT treatment was associated with a significant clinical improvement in >80% of patients with a good tolerability and safety profile.Citation29
Other examples of research aftermarket authorization primarily focuses on clinical benefit more than tolerability. As an example, the influence of the duration of pre-seasonal treatment on clinical efficacy obtained within the GPS was investigated. Data from three multicenter randomized DBPC trials with different pre-seasonal treatment periods were analyzed. Authors concluded that GRAZAX® must be initiated at least 8 weeks prior to the GPS to provide significant clinical efficacy on the first GPS. Longer pre-seasonal treatment period (>8 weeks) improves the clinical efficacy (relative to placebo) during the GPS.Citation30
Other important efficacy issue in clinical practice is related with the polysensitized condition of most pollen allergic patients. Thus, a post hoc analysis of pooled data from six randomized, DBPC trials (n = 1871) comparing the efficacy and safety of GRAZAX® in mono‐ and poly-sensitized subjects concluded that no difference in efficacy and safety of single‐allergen grass AIT was observed between them.Citation31
GAP (GRAZAX® Asthma Prevention) Study
The GAP trial represents the first 5 y prospective multi-national, multicenter study performed in a randomized DBPC design aiming to investigate asthma prevention potential of AIT. It was performed in a pediatric sample of 812 allergic children suffering from rhinitis mediated by grass pollen, without asthma symptoms.
Both trial design and outcome are described in the first paper published of this trial.Citation32
Trial design follows EMEA guidelines.Citation13 Other international treatment guidelines were also consulted on the different aspects of the study.Citation33–Citation36 The primary end-point of the study, defined as the “difference in time to onset of asthma defined by pre-specified asthma criteria relying on documented reversible impairment of lung function,” was not met.
However, there were multiple secondary end-points that supported the prevention potential of AIT. GRAZAX treatment significantly reduced the risk of experiencing asthma symptoms or using asthma medication at the end of trial (odds ratio = 0.66, p < .036), during the 2-y post-treatment follow-up and during the entire 5-y trial period. Additionally, it had a positive, long-term clinical effect on grass-allergic rhinoconjunctivitis symptoms that were 22–30% reduced (p < .005 for all 5 y). At the end of the trial, the use of allergic rhinoconjunctivitis pharmacotherapy was significantly lower in active treated patients (27% relative difference to 19% placebo, p < .001)Citation37
An interesting finding was the significant decrease of winter asthma symptoms, a measure of bronchial hyperreactivity, in treated patients, in the third winter of treatment, which persisted and increased in effect 2 y after discontinuation. This kinetic is in agreement with the acquisition of a memory T cell regulatory phenotypeCitation11 and supports the systemic benefit of early intervention in allergy.
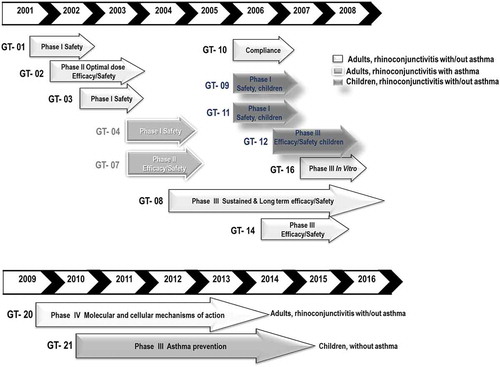
We would like to finish the summary of clinical development of GRAZAX® showing the clinical implications stated in the GAP paper: “The data presented in this article demonstrate that treatment with the SQ grass SLIT tablet modifies the grass pollen allergic disease. The disease modification is expressed by preventing progression from allergic rhinoconjunctivitis symptoms to development of asthma symptoms and reducing rhinoconjunctivitis symptoms and medication use during and after treatment termination”. In are summarized in chronological order the clinical trials performed in Europe.
Figure 4. Summary of clinical trials performed in Europe
Main clinical trials of GRAZAX® clinical development programme are summarized in .
Table 2. Summary of main Clinical trials performed with GRAZAX®
Regulatory issues
GRAZAX® has been the first global AIT product registered following Pharmaceutical parameters. It has set the standard for future AIT product development.
Public-health
GRAZAX® has proved its pharmacoeconomic value. In an evaluation of its value,Citation43,Citation44 it was concluded that GRAZAX® generated an incremental cost per quality-adjusted life year (QALY) of 12,168 GBP, and thus was a cost-effective option for the treatment of allergic rhinoconjunctivitis in a UK pediatric population. If we consider its preventive potential, this value will be further enhanced. In fact, AIT in general, and GRAZAX®, when administered following recommended schedule, has a unique therapeutic profile, as can modify the natural course of allergy.
Product availability
GRAZAX® is currently available in EU countries, Norway, Switzerland, US, Canada, Turkey, Russia, and Australia.
Advantages/disadvantages relative to other products
The only AIT product for grass allergy with a similar product design is ORALAIR®Citation45 Apart from the formulation, that has a clear influence in the product availability,Citation3,Citation46 the main difference is that Oralair is recommended for pre-co-seasonal administration. As previously mentioned in the mode of action section, this administration schedule addresses mainly desensitization mechanisms, but might slow the generation of a regulatory response that is pivotal for sustained benefit.Citation11 In fact, in a prospective 5-y trial, ORALAIR® showed a decline in the effect in the second follow-up study. Other difference between both products is based on the allergenic composition of GRAZAX® (one grass species) versus ORALAIR® (five grass species). Election of a single grass species was made in base to the high cross-reactivity observed in serological responses to different grass species.Citation47,Citation48 In fact, in general, grass allergens present multiple isoforms, and thus a single species will incorporate different molecular variants of each allergen. Moreover, there is clinical evidence that a grass vaccine can be further simplified even to single isoform of relevant grass allergens.Citation49 Recently, it has been shown that some patients, mostly resident in the south of Europe, could have a higher sIgE recognition to a five species vaccine.Citation50 Currently, there is no evidence of clinical superiority in base to the number of grass species included in the vaccine composition.
Future product development needs
Allergen-specific Immunotherapy, when correctly used, is the only available pharmacotherapy with disease-modifying potential in allergic pathologies. Nearly 70% of the patients develop a sustained memory regulatory T cell response.Citation11 The problem is that there are no adequate biomarkers to monitor and predict successful AIT intervention. There is an urgent need to develop new biomarkers strategies.Citation51,Citation52 New biomarkers, more connected to the disease, should allow not only monitor individual patient responses but also to compare different pharmacological interventions and prove the value of AIT intervention.
Grazax has set a clinical standard in AIT development. Better specific intervention strategies should be focused in obtaining long-term benefit after discontinuation with shorter treatment regimes, to increase patient compliance. This might be achieved for example by using better formulations or incorporating adjuvants in the formulation.
New prevention studies, with a focus on secondary prevention (asthma, poly-sensitization), should be developed with the lessons learned from the GAP trial.Citation32
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Bonertz A, Roberts GC, Hoefnagel M, Timon M, Slater JE, Rabin RL, Bridgewater J, Pini C, Pfaar O, Akdis C, Goldstein J. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: a global perspective on the regulation of allergen products. Allergy. 2018;73:64–76. doi:10.1111/all.13266.
- Bonertz A, Roberts G, Slater JE, Bridgewater J, Rabin RL, Hoefnagel M, Timon M, Pini C, Pfaar O, Sheikh A, Ryan D. Allergen manufacturing and quality aspects for allergen immunotherapy in Europe and the United States: an analysis from the EAACI AIT guidelines project. Allergy. 2018;73:816–26. doi:10.1111/all.13357.
- Ohashi-Doi K, Kito H, Du W, Nakazawa H, Ipsen H, Gudmann P. Bioavailability of house dust mite allergens in sublingual allergy tablets is highly dependent on the formulation. Int Arch Allergy Immunol. 2017;174:26–34. doi:10.1159/000479693.
- Brimnes J, Kildsgaard J, Jacobi H, Lund K. Sublingual immunotherapy reduces allergic symptoms in a mouse model of rhinitis. Clin Exp Allergy. 2007;37:488–97. doi:10.1111/j.1365-2222.2006.02624.x.
- van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, et al. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63:310–26. doi:10.1111/j.1398-9995.2007.01612.x.
- Kaul S, Zimmer J, Dehus O, Constanzo A, Daas A, Buchheit KH, et al. Validation of ELISA methods for quantification of the major birch allergen Bet v 1 (BSP090). Pharmeur Bio Sci Notes. 2017;2017:69–87.
- Römmelmayer ALL, Larsen JN. GRAZAX®: an oromucosal vaccine for treating grass pollen allergy with immunotherapy. In: Jorgensen L, Nielsen HM, editors. Delivery technologies for biopharmaceuticals: peptides, proteins, nucleic acids and vaccines; 2009.
- Berings M, Karaaslan C, Altunbulakli C, Gevaert P, Akdis M, Bachert C, Akdis CA. Advances and highlights in allergen immunotherapy: on the way to sustained clinical and immunologic tolerance. J Allergy Clin Immunol. 2017;140:1250–67. doi:10.1016/j.jaci.2017.08.025.
- Rosace D, Gomez-Casado C, Fernandez P, Perez-Gordo M, Dominguez MDC, Vega A, Belver MT, Ramos T, Vega F, Marco G, et al. Profilin-mediated food-induced allergic reactions are associated with oral epithelial remodeling. J Allergy Clin Immunol. 2019;143:681–90 e1. doi:10.1016/j.jaci.2018.03.013.
- Suarez-Fueyo A, Ramos T, Galan A, Jimeno L, Wurtzen PA, Marin A. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J Allergy Clin Immunol. 2014;133(130–8):e1–e2. doi:10.1016/j.jaci.2013.09.043.
- Varona R, Ramos T, Escribese MM, Jimeno L, Galan A, Wurtzen PA, Vega F, Marín A, Martín S, Carrera AC, Blanco C. Persistent regulatory T-cell response 2 years after 3 years of grass tablet SLIT: links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy. 2019;74:349–60. doi:10.1111/all.13553.
- Scadding GW, Calderon MA, Shamji MH, Eifan AO, Penagos M, Dumitru F, et al. Effect of 2 years of treatment with sublingual grass pollen immunotherapy on nasal response to allergen challenge at 3 years among patients with moderate to severe seasonal allergic rhinitis: the grass randomized clinical trial. Jama. 2017;317:615–25. doi:10.1001/jama.2016.21040.
- Guideline on the clinical development on products for specific immunotherapy for the treatment of allergic diseases. Doc. Ref. CHMP/EWP/18504/2006. © European Medicines Agency, 2008. 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK.
- Ibanez MD, Kaiser F, Knecht R, Armentia A, Schopfer H, Tholstrup B, Bufe A. Safety of specific sublingual immunotherapy with SQ standardized grass allergen tablets in children. Pediatr Allergy Immunol. 2007;18:516–22. doi:10.1111/j.1399-3038.2007.00556.x.
- Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:802–09. doi:10.1016/j.jaci.2005.12.1358.
- Rak S, Yang WH, Pedersen MR, Durham SR. Once-daily sublingual allergen-specific immunotherapy improves quality of life in patients with grass pollen-induced allergic rhinoconjunctivitis: a double-blind, randomised study. Qual Life Res. 2007;16:191–201. doi:10.1007/s11136-006-9110-3.
- Aberer W, Hawranek T, Reider N, Schuster C, Sturm G, Kranke B. Immunoglobulin E and G antibody profiles to grass pollen allergens during a short course of sublingual immunotherapy. J Investig Allergol Clin Immunol. 2007;17:131–36.
- Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardized grass allergen tablets in asthmatics with rhinoconjunctivitis. Allergy. 2006;61:185–90. doi:10.1111/j.1398-9995.2005.00949.x.
- Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, Riis B, Grønager PM, Durham SR. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008;121:512–8 e2. doi:10.1016/j.jaci.2007.10.039.
- Durham SR, Emminger W, Kapp A, de Monchy JG, Rak S, Scadding GK, Wurtzen PA, Andersen JS, Tholstrup B, Riis B, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;129:717–25 e5. doi:10.1016/j.jaci.2011.12.973.
- Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, Knecht R, Stephan V, Tholstrup B, Weisshaar C, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173.e7. doi:10.1016/j.jaci.2008.10.044.
- Maloney J, Bernstein DI, Nelson H, Creticos P, Hébert J, Noonan M, Skoner D, Zhou Y, Kaur A, Nolte H. Efficacy and safety of grass sublingual immunotherapy tablet, MK-7243: a large randomized controlled trial. Ann Allergy Asthma Immunol. 2014;112:146–153.e2. doi:10.1016/j.anai.2013.11.018.
- Hebert J, Blaiss M, Waserman S, Kim H, Creticos P, Maloney J, Kaur A, Li Z, Nelson H, Nolte H. The efficacy and safety of the Timothy grass allergy sublingual immunotherapy tablet in Canadian adults and children. Allergy Asthma Clin Immunol. 2014;10:53. doi:10.1186/1710-1492-10-41.
- Nolte H, Casale TB, Lockey RF, Fogh BS, Kaur A, Lu S, Nelson HS. Epinephrine Use in Clinical Trials of Sublingual Immunotherapy Tablets. J Allergy Clin Immunol Pract. 2017;5:84–9 e3. doi:10.1016/j.jaip.2016.08.017.
- Maloney J, Berman G, Gagnon R, Bernstein DI, Nelson HS, Kleine-Tebbe J, Kaur A, Li Q, Nolte H. Sequential treatment initiation with timothy grass and ragweed sublingual immunotherapy tablets followed by simultaneous treatment is well tolerated. J Allergy Clin Immunol Pract. 2016;4:301–9 e2. doi:10.1016/j.jaip.2015.11.004.
- Reich K, Gessner C, Kroker A, Schwab JA, Pohl W, Villesen H, Wüstenberg E, Emminger W. Immunologic effects and tolerability profile of in-season initiation of a standardized-quality grass allergy immunotherapy tablet: a phase III, multicenter, randomized, double-blind, placebo-controlled trial in adults with grass pollen-induced rhinoconjunctivitis. Clin Ther. 2011;33:828–40. doi:10.1016/j.clinthera.2011.06.006.
- Schwab JA, Wolf H, Schnitker J, Wustenberg E. Safety and tolerability of an intra-seasonal initiation of the SQ-standardised grass allergy immunotherapy tablet: a non-interventional observational study investigating the feasibility during routine administration. Clin Drug Investig. 2013;33:719–26. doi:10.1007/s40261-013-0115-8.
- Schwab JA, Wolf H, Schnitker J, Wustenberg E. Intra-seasonal initiation of the SQ-standardised grass allergy immunotherapy tablet routinely applied by allergy specialists and general practitioners with experience in treatment of allergy: a non-interventional observational study. Pulm Ther. 2018;4:45–57. doi:10.1007/s41030-018-0050-1.
- Alesina R, Milani M, Pecora S. A multicenter, randomized, parallel-group trial assessing compliance, tolerability, safety, and efficacy to treatment with grass allergy tablets in 261 patients with grass pollen rhinoconjunctivitis. J Allergy (Cairo). 2012;2012:673502.
- Calderon MA, Birk AO, Andersen JS, Durham SR. Prolonged preseasonal treatment phase with Grazax sublingual immunotherapy increases clinical efficacy. Allergy. 2007;62:958–61. doi:10.1111/j.1398-9995.2007.01416.x.
- Nelson H, Blaiss M, Nolte H, Wurtz SO, Andersen JS, Durham SR. Efficacy and safety of the SQ-standardized grass allergy immunotherapy tablet in mono- and polysensitized subjects. Allergy. 2013;68:252–55. doi:10.1111/all.12074.
- Valovirta E, Berstad AK, de Blic J, Bufe A, Eng P, Halken S, Ojeda P, Roberts G, Tommerup L, Varga EM, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ-standardized grass allergy immunotherapy tablet in children with grass pollen-induced allergic rhinoconjunctivitis. Clin Ther. 2011;33:1537–46. doi:10.1016/j.clinthera.2011.09.013.
- Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, Worm M, Wahn U, Bousquet J. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65:1525–30. doi:10.1111/j.1398-9995.2010.02474.x.
- GINA. Global Strategy for Asthma Management and Prevention 2006. Global Initiative for Asthma – GINA. Fontana, WI 53125, USA. https://ginasthma.org/
- Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M, Helms PJ, Hunt J, Liu A, Papadopoulos N, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5–34. doi:10.1111/j.1398-9995.2007.01586.x.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. doi:10.1111/j.1398-9995.2007.01620.x.
- Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sorensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141:529–38 e13. doi:10.1016/j.jaci.2017.06.014.
- Malling HJ, Lund L, Ipsen H, Poulsen L. Safety and immunological changes during sublingual immunotherapy with Standardized Quality grass allergen tablets. J Investig Allergol Clin Immunol. 2006;16:162–68.
- Kleine-Tebbe J, Ribel M, Herold DA. Safety of a SQ-standardised grass allergen tablet for sublingual immunotherapy: a randomized, placebo-controlled trial. Allergy. 2006;61:181–84. doi:10.1111/j.1398-9995.2006.00959.x.
- Calderon M, Essendrop M. Specific immunotherapy with high dose SO standardized grass allergen tablets was safe and well tolerated. J Investig Allergol Clin Immunol. 2006;16:338–44.
- Nelson HS, Nolte H, Creticos P, Maloney J, Wu J, Bernstein DI, Wu J, Bernstein DI. Efficacy and safety of timothy grass allergy immunotherapy tablet treatment in North American adults. J Aller Clin Immunol. 2011;127:72–80. doi:10.1016/j.jaci.2010.11.035.
- Blaiss M, Maloney J, Nolte H, Gawchik S, Yao R, Skoner DP. Efficacy and safety of timothy grass allergy immunotherapy tablets in North American children and adolescents. J Allergy Clin Immunol. 2011;127:64–71. doi:10.1016/j.jaci.2010.11.034.
- Poole CD, Bannister CA, Andreasen JN, Andersen JS, Currie CJ. Estimation of health-related utility (EQ-5D index) in subjects with seasonal allergic rhinoconjunctivitis to evaluate health gain associated with sublingual grass allergen immunotherapy. Health Qual Life Outcomes. 2014;12:99. doi:10.1186/1477-7525-12-99.
- Horn A, Zeuner H, Wolf H, Schnitker J, Wustenberg E. Health-related quality of life during routine treatment with the sq-standardised grass allergy immunotherapy tablet: a non-interventional observational study. Clin Drug Investig. 2016;36:453–62. doi:10.1007/s40261-016-0388-9.
- Blanco C, Bazire R, Argiz L, Hernandez-Pena J. Sublingual allergen immunotherapy for respiratory allergy: a systematic review. Drugs Context. 2018;7:212552. doi:10.7573/dic.212552.
- Lund K, Kito H, Skydtsgaard MB, Nakazawa H, Ohashi-Doi K, Lawton S. The importance of tablet formulation on allergen release kinetics and efficiency: comparison of freeze-dried and compressed grass pollen sublingual allergy immunotherapy tablet formulations. Clin Ther. 2019;15:S0149–S2918.
- Duffort O, Quintana J, Ipsen H, Barber D, Polo F. Antigenic similarity among Group 1 allergens from Grasses and Quantitation ELISA using monoclonal antibodies to Phl p 1. Int Arch of Allergy and Immunol. 2008;145:283–90. doi:10.1159/000110887.
- Johansen N, Weber RW, Ipsen H, Barber D, Broge L, Hejl C. Extensive IgE cross-reactivity towards the Pooideae grasses substantiated for a large number of grass-pollen.sensitized subjects. Int Arch Allergy Immunol. 2009;150:325–34. doi:10.1159/000226233.
- Cromwell O, Niederberger V, Horak F, Fiebig H. Clinical experience with recombinant molecules for allergy vaccination. Curr Top Microbiol Immunol. 2011;352:27–42.
- Batard T, Sanjuan A, Denis L, Nguyen H, Montagut A, Sastre J, Rak S, Cuiné JF. Two grass pollen tablets commercially available for allergy immunotherapy display different IgE epitope repertoires. Clin Transl Allergy. 2019;27:13.
- Eguiluz-Gracia I, Tay TR, Hew M, Escribese MM, Barber D, O’Hehir RE, Torres MJ, Torres MJ. Recent developments and highlights in biomarkers in allergic diseases and asthma. Allergy. 2018;73:2290–305. doi:10.1111/all.13628.
- Barber D, Escribese MM. Predictive biomarkers in allergen specific immunotherapy. Allergol Immunopathol. 2017;45(Suppl 1):12–14. doi:10.1016/j.aller.2017.09.003.