ABSTRACT
Background: This exploratory study aimed to assess the immunogenicity and safety of 1 and 2 doses of meningococcal serogroups A and C tetanus toxoid-conjugate vaccine (MenAC-TT) in toddles.
Methods: Healthy participants aged 12–23 months were randomized into two groups to receive 1 or 2 doses of the tested vaccine. The interval was 28 days between two doses. Blood samples were collected at day 0 before the immunization and day 28 post-each dose. Safety observation was conducted during 28 days after each vaccination. Serious adverse event (SAE) was conducted throughout 6 month observation period.
Results: Overall 301 toddles were vaccinated. Twenty-eight days post full-course vaccination, ≥97.20% toddles had titers ≥1:8 and ≥81.48% had titers ≥1:128 for MenA and MenC in the two schedules groups. There were no significant differences between the two schedule groups for each titer thresholds and serogroups. Up to month 12 post the first dose, titers ≥1:8 and 1:128 were declined to 71.32–80.83% and 26.67–57.85% for each meningococcal serogroups. Most adverse reactions (ARs) were mild or moderate, and the incidence of grade 3 ARs was below 3.33%. The incidence of redness was significantly higher in the two doses group than that in the one dose group, in terms of grade 1 and grade 2 were higher. No SAEs were considered causally related to vaccination.
Conclusion: The MenAC-TT showed similarly safety and immunogenicity profile in toddles with two schedules. It will be more important to provide the data for formulating appropriate immunization strategies in different age groups in China.
Introduction
Invasive meningococcal disease (IMD), such as meningitis and meningococcemia, is caused by Neisseria meningitidis and has their highest incidence in infants. The annual number of cases is estimated to be at least 1.2 million with 135,000 deaths over the worldwide. Approximately 10–20% of patients suffer from significant clinical sequelae such as limb loss, deafness, seizures or psychomotor retardation.Citation1–Citation5 The most important disease-causing serogroups of Neisseria meningitidis are MenA, MenB, MenC, MenW and MenY. Their prevalence varies geographically, MenA and MenC are more prominent in Asia, such as China.Citation6 To combat IMD, an increasing number of countries have included vaccines against N. meningitidis in their routine immunization programs.
The specific vaccine use in each country depends on the factors mainly include predominant serogroups, cost and availability. Polysaccharide vaccines were used and in routine immunization in China.Citation7 Polysaccharide meningococcal vaccines have been used widely in high-risk individuals and for the control of outbreaks. These vaccines elicit a largely T-cell-independent response and are poorly immunogenic in young children, and cannot confer long-lasting immunity,Citation8 as well as repeated administration of these vaccines may result in hyporesponsiveness.Citation9 Recommendations for vaccination in the Asia-Pacific region are highly variable.Citation6,Citation10 The vaccination policy of conjugate vaccine in China is currently under review, despite the availability of conjugate vaccines.Citation11 In China, The routine IMD immunization schedule for children aged 6–18 months is two doses of the MenA polysaccharide vaccine at an interval of 3 months, and subsequently a single dose of the MenA plus MenC polysaccharide vaccine at ages 3 and 6 years.Citation11 Study showed that routine use of meningococcal conjugate vaccine in toddlers could expand protection against IMD.Citation12− The introduction of conjugate vaccinations into the expanded program on immunization (EPI) schedule is being considered to increase the proportion of the population protected against MD, and new vaccination strategies may be required including the use of conjugate vaccines.Citation11 This clinical trial is planning to evaluate the immunogenicity and safety of bivalent meningococcal serogroups A and C tetanus toxoid conjugate vaccine in Chinese healthy toddles aged 12–23 months with two different immunization schedules.
Results
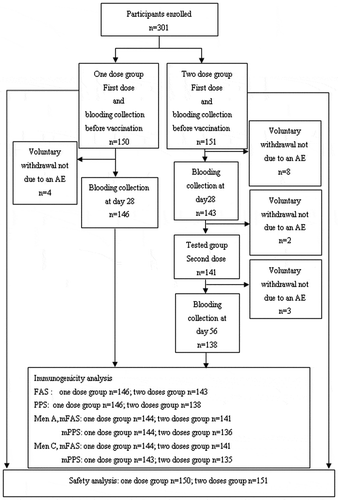
A total of 301 participants were enrolled and randomized (150 in the one dose group and 151 in the two doses group). All the participants were analyzed for safety. Seventeen participants (4 in one dose group and 13 in two doses group) did not complete the study because of voluntary withdrawal not due to an adverse reaction (AR). () For MenA, the modified per-protocol set (mPPS) included 144 in the one dose group and 136 in the two doses group. For MenC, the mPPS included 143 in the one dose group and 135 in the two doses group (). The demographic characteristics (age, sex and body mass index [BMI]) were similar without significant difference between the two study groups. (Showed in , all P> .05.)
Table 1. Baseline characteristics of participants (total vaccinated cohort)
Figure 1. Flow chart for the recruitment of volunteers in the study. Titers for antibody to MenA≥1:8 pre-vaccination were two participants in the one and two doses group, respectively. Titers for antibody to MenC ≥1:8 pre-vaccination were three participants in the one and two doses group, respectively. They were removed from the analysis set (mPPS) for immunogenicity
Immunogenicity
The antibodies to MenA and MenC of participants in mPPS at baseline had titers <1:8, with the geometric mean titer (GMT) of 1.0 in both two schedule groups. At day 28 post completion of the single dose in one dose group and day 28 post completion of the second dose in two doses group, a higher proportion of participants had detectable serum bactericidal activity against MenA and MenC in each group. In the one dose group, seroconversion rates (titers ≥1:8), seroprotection rates (titers ≥1:128) and GMT were 98.61%, 90.97% and 325.7 for MenA, and 97.2%, 84.62% and 227.9 for MenC. Those indicators in the two doses group were 100.00%, 86.76% and 269.4 for MenA, and 98.52%, 81.48% and 177.6 for MenC. The seroconversion and seroprotection rates and GMTs showed no significant differences between the two schedule groups for each titer thresholds and serogroups. (See in .)
Table 2. Percentage of participants with rSBA titers≥1:8 and ≥1:128 and geometric mean titers against the serogroups MenA and MenC (mPPS cohort for immunogenicity)
To the month 12 post the first dose, the seroconversion rate, seroprotection rate and GMT were declined to 71.32%, 57.36% and 53.9 for MenA, and 78.13%, 29.69% and 29.2 for MenC in the dose one group. Those indicators in the two doses group were 79.34%, 57.85% and 67.4 for MenA, and 80.83%, 26.67% and 26.1 for MenC. The immunogenicity variables were no significant difference between the two schedule groups.
Safety
From the first dose to day 28 post completing full-course vaccination, two schedule groups showed good safety profiles. The intensities of most AR were mild or moderate (≤grade 2), the AR of grade 3 were at a low reported rate below 3.33% in both two study groups. The total incidence of solicited AR was 58.28% in the two doses schedule group, which was significantly higher than that found (44.00%) in the one dose group (P = .0132). The incidence of moderate AR (grade 2) was significantly higher in the two doses group than that in the one dose group (P = .0016). The most common symptoms of local solicited ARs were redness and swelling with the incidence of 10.67% and 10.00% in the one dose group compared to 22.52% and 11.92% in the two doses group. The most common symptoms of systemic solicited ARs were fever with the incidence 29.33% vs. 39.07% in the one and two dose groups. The incidences of other ARs were lower below 5.00%. Almost all the symptoms were similar in the two groups, excepting redness was reported significantly in the two doses group (P = .0057) (see ). Only one participant in the two doses group occurred bronchopneumonia at 7 days later post the first dose. This SAE was assessed without relation to vaccination (data were not shown).
Table 3. Common symptoms analysis of solicited local and systemic ARs occurring after vaccination
Discussion
In this study, the conjugate MenAC-TT vaccine showed the profile of sturdy immunogenicity at day 28 post primary schedule in healthy toddlers aged 12–23 months in China. The immune response at the terms of seroconversion rates (rSBA titres ≥1:8) for MenA and MenC, was over 97% with one or two doses immune schedules. Seroprotection rates of titers ≥1:128 was high and approximate 80–90%. It was worth noting that GMT at day 28 post the vaccination in the one dose group was little higher than that at day 28 post the second dose in two dose group, even no significant difference was shown. Compared with the first dose in the two doses group, the second dose scarcely increased the immunogenicity which seemed was elicited mostly at the first dose (see in the ). Cutland et al. showed in 2017, 2 month interval of two dose MenACWY-TT just a little bit increased at month 3 compared to month 1.Citation13 In addition, study showed that the immunological hyporesponsiveness may was induced to the participant who had history of polysaccharide vaccine vaccination, especially to younger children.Citation14,Citation15 According to the national immunization program in China, the infants should complete two dose meningococcal polysaccharide vaccine before age of 12 months, the participants in the group aged 12–23 months all had history of two dose of meningococcal polysaccharide A vaccine. It showed the hyporesponsiveness in term of lower GMT and or seroconversion rates after two dose vaccination compared to the first dose, even compared to other studies with one dose of meningococcal polysaccharide diphtheria toxoid conjugate vaccine (MenACYW–D) and MenACWY-TT.Citation8,Citation13 Nevertheless, it speculated that the vaccines may be given too closely together at 4 weeks. The intervals in other studies were about 3–6 months with an increased immunogenicity post dose 2.Citation12,Citation16
Studies of conjugated meningococcal vaccine in toddlers aged 12–23 months showed that one dose of vaccination elicited highly immunogenic. A large phase III study in Finland, in the terms of proportions of toddlers with titers of rSBA ≥1:8 against MenA and MenC were 99.7% in the MenACWY-TT group on the day 42 after the one dose, and the titers of MenC-rSBA ≥1:8 was 97.5% in the MenC group.Citation17 Result from another phase III study demonstrated that a single dose of MenACWY-TT in toddlers was highly immunogenic, and the proportion of rSBA titres ≥1:8 for all serogroups were ≥97.3%.Citation18 Furthermore, one phase III study showed that one or two doses MenACWY-TT lead to similar immune response in toddlers aged 12–14 months, as assessed by the rSBA assay with proportions of rSBA titres ≥1:8 about 92.8–97.8%.Citation19 Although the interpretation of historical comparisons is difficult because of changes in population exposure and laboratory differences in SBA procedures, our results in this study showed similar immunogenicity with those studies at day 28 post the primary vaccination.
In 2006, Auckland et al. found that comparing to unvaccinated ones, subjects with vaccine failure response to the capsular polysaccharide of MenC by eliciting higher SBA titers in convalescent serum samples and IgG avidity in acute serum samples, but remain occurred MenC cases and not confer protection.Citation20 The booster response is not sufficiently rapid to prevent the invasion which occurred within a few days of colonization. Although evidence showed that meningococcal serogroup C conjugate vaccine (MCC)-induced immune memory, the high level of direct protection decline subsequently.Citation21,Citation22 Immunological memory alone was insufficient to provide long-term disease protection. Persistence of antibodies may be a more appropriate correlate of protection, rather than the booster response which could not be relied upon to provide protection from meningococcal disease.Citation20 Few available studies evaluate antibody persistence after primary vaccination with Men-ACWY conjugate vaccines in toddlers. Vesikari et al. phase 2 follow-up study in 2012, the results showed that 98.8% and 90.8% of original Men-ACWY-TT-recipients aged 12–23 months had rSBA titers ≥1:8 against MenA and MenC up to 3 years after vaccination.Citation23 The result of Vesikari et al. phase 3 study in 2015 showed that 59.9–74.1% and 35.9–40.4% of toddlers retained rSBA titers ≥1:8 against MenA and MenC at year 3 and 4 after priming.Citation24 Although no direct evidence of efficacy of MCC vaccine, a comprehensive surveillance program was initiated in the UK in terms of herd immunity. The surveillance showed that a reduction of 67% in attack rate of those unvaccinated occurred from 2001 to 2002, compared with cases reported in 1998–1999.Citation25 Up to month 12 in this study, 71.32% and 79.34% of the participants with the rSBA titers ≥1:8 against MenA, 78.13% and 80.83% of those against MenC. The seroprotective antibody levels declined markedly in both two schedule groups, especially those against MenC. This situation had been seen in the UK in the subjects between 12 and 15 months who had a boost of MCC.Citation26 The percentages of subjects with rSBA titers ≥1:8 was declined from 92% to 54% at month 12 after boost. Those data suggest that the immunization strategy (2 + 1 schedule) had not any substantial impact on sustaining seroprotecion. This supports a need for older childhood or adolescent booster vaccination to enhance population immunity.Citation27 In 2003, the UK had changed immunization schedule to one dose at 3 month of age and one dose at 12 month, meanwhile introduced an adolescent booster at age 13–14 years and a booster dose under the age of 25 years.Citation28 It will be more important to provide the data for formulating appropriate immunization strategies in different age groups in China.
The two study schedules showed the similar immunogenicity, even similar incidences of AR post the first dose (data were not shown) the incidences of common local and systemic symptoms included redness, swelling were higher in the two doses group post the second dose, related to vaccination times. The incidence of ARs in this study was approximate to other studies, and the safety can be acceptable.Citation29,Citation30
One limitation in this study was no control group. A Control is important both for reasons of safety comparator as well as to lend reassurance that there was non-specific rSBA titers were not generated.The aim of this exploratory study just to tentatively provided data for the schedule of experimental vaccine in toddles. More rigorous experimental design and larger sample size is need in the future research to supplied data for immunization strategies.
In conclusion, this study confirmed that two schedules of MenAC-TT induced the similar immune response with a high seroconversion rates of serogroups MenA and MenC over 97% at day 28 post full course vaccination, and declined up to month 12, especially for MenC. MenAC-TT had a good safety profile with little higher incidence of ARs in terms of the symptom of redness and severity of grade 2 in the two doses group caused by more vaccination times. It will be more important to provide the data for formulating appropriate immunization strategies in different age groups in China.
Methods
Study design
This exploratory study was one part of a randomized phase 3 clinical trial (ClinicalTrials.gov: NCT03714737), which was conducted between March, 2016 and October, 2017 in Jiangsu province of China. Protocol was designed by the Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) and OLYMVAX biopharmaceutical Co., LTD (the study sponsor and manufacture of the vaccine). It was approved by the institutional ethics committee of JSCDC. Parents or legal representatives provided written informed consent before enrollment. Healthy participants aged 12–23 months were randomized in a 1:1 ratio to receive one or two doses the tested vaccine. The interval in two doses group was 28 days. Blood samples were collected for immunogenicity assessment on day 0 and day 28 post-each dose. Safety date was collected from day 0 to day 28 post-each dose and serious adverse event was observed extend to 6 months after vaccination.
Participants
Eligible participants were healthy children aged 12–23 months and never received meningococcal conjugate vaccine or meningococcal polysaccharide vaccine over 3 months. Key exclusion criteria included a history (confirmed clinically, serologically or microbiologically) of meningococcal disease, acute disease/infection according to the investigator or fever (axillary temperature > 37.0°C on the day of vaccination), history of allergy or allergic to any ingredient of vaccine, bleeding disorders or history of known thrombocytopenia, immunodeficiency conditions, receipt of subunit or inactivated vaccine, or immunoglobulin in past 7 days, receipt of attenuated live vaccine in past 14 days, had acute illness or acute onset of chronic disease in last 3 days before vaccination.
Sample size
In the initial randomized phase 3 clinical trial, the expected seroconversion of bactericidal antibodies against group A and group C of meningococcal bacteria was over 80% in the control vaccine group after the primary vaccination,Citation31 and the rates in the experimental vaccine group was assumed no lower than those in the control vaccine group at the same time with the non-inferiority margin value −0.1. The minimum number of subjects in the experimental group and the control group was calculated to be 252, with about 15% shedding rate. The sample size of each group was adjusted to be 300. That is, 300 people in each age group, the experimental group and the control group. The age group 6–23 months in initial phase 3 trial was divided into two sub-age group with a sample size of 150 in each. So the sample size of each groups in exploratory study was 150.
Vaccine
The study vaccine was a polysaccharide protein conjugate vaccine manufactured by OLYMVAX biopharmaceutical Co., LTD, prepared with serogroups A and C meningococcal polysaccharides, each covalently bound to tetanus toxoid (TT). polysaccharides of MenA and MenC were obtained by fermented, formalin-inactivated, purified. Each dose of MenAC-TT vaccine was 0.5 mL contained 10 μg each of MenA and MenC polysaccharides conjugated to TT (total TT content 23.6–58.3 μg), added 5–10 mg lactose as a stabilizer to freeze dry with 0.5 mL sodium phosphate buffer as the vaccine diluent.
Serologic evaluations
Functional meningococcal antibody activity against MenA and MenC antigens contained in MenAC-TT was measured using a serum bactericidal assay (SBA) with rabbit complement.Citation32 The strains of CMCC29019 (MenA) and CMCC29026 (MenC) for rSBA was afforded by National Center for Medical Culture Collections (CMCC). The lower limit of quantitation of the rSBA was a titer of 2. Assays were performed by the National Institute for Food and Drug Control (NIFDC, Beijing, China). The analysis set of immunogenicity was evaluated in mPPS (the participant who was seronegative pre-vaccination (titers <1:8), met all eligibility criteria, and adhered to the protocol). The primary endpoint was the seroconversion (defined as titers ≥1:8 post vaccination in initially seronegative participants (pre-vaccination titers <1:8)) against MenA and MenC at day 28 post each dose. Secondary endpoints included the rates of seroprotecion (rSBA titers ≥1:128) at day 28 post each dose.Citation8 The more conservative threshold of rSBA titres ≥1:128 was been previously associated with seroprotection against MenC,Citation13,Citation15,Citation18,Citation33 and were applied to MenA in this study.Citation8,Citation29
Safety evaluation
Safety endpoints included: (1) the occurrence and intensity of solicited injection site reactions and systemic reactions occurring from day 0 through day 7 after vaccination, (2) the occurrence of unsolicited adverse events within 28 days after vaccination and (3) the occurrence of SAE occurring at any time during the study.
Adverse reactions were graded according to the scale, derived from the Division of Microbiology and Infectious Diseases pediatric toxicity table.Citation34 Injection site adverse reactions, such as erythema, indurate and swell, were categorized as grade 1 (<10mm), grade 2 (10–25mm), grade 3 (26–50mm) or grade 4 (>50mm). Pain was categorized as grade 1 (touching with a slight reaction), grade 2 (touching with a moderate reaction such as crying), grade 3 (touching with strong reaction such as scream and rejection) or grade 4 (hospitalization or emergency department visit). Itching was categorized as grade 1 (mild itching in injection site), grade 2 (moderate itching in injection site) or grade 3 (itching in the whole body). Systemic adverse reactions were graded for fever, allergic reaction, fatigue, vomiting, loss of appetite, fidgety (irritable, abnormal crying) and diarrhea. Fever was categorized as grade 1 (axillary temperature, 37.1~37.5°C), grade 2 (axillary temperature, 37.6~39.0°C), grade 3 (axillary temperature, >39.0°C). Fatigue was categorized as grade 1 (no interference with activity), grade 2 (some interference with activity), grade 3 (significantly prevent daily activity) or grade 4 (hospitalization or emergency department visit). Loss of appetite was categorized as grade 1 (intake less than normal), grade 2 (miss a meal or two), grade 3 (without eating for 24 h), grade 4 (without eating and administration of intravenous injection). Nausea, vomiting and diarrhea were categorized as grade 1 (once or twice per 24 h, no interference with activity), grade 2 (twice or five times per 24 h with limited activity), grade 3 (over six times in 24 h, administration of intravenous injection) or grade 4 (hypotension, hospitalization with nutritional supplements in other ways). Fidgety (irritable, abnormal crying) was categorized as grade 1 (sleep less than normal), grade 2 (difficult to fall asleep), grade 3 (persistent or prolonged crying).
Statistical analyses
Statistical comparisons were made using two-sided tests with an alpha value of 0.05. Geometric mean titers (GMT) were summarized with 95% confidence intervals (CIs) and compared by the Student’s t-test or t’ test, respectively. Comparison of seroconversion rate, rate of titers ≥1:128, baseline characteristics and adverse reactions/events rate were conducted by Chi-square test or Fisher’s exact test. The statistical analyses were performed by an independent statistician using SAS (version 9.1, SAS Institute Inc., Cary, NC, USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
F-C Zhu, H-G Li, Y-M Hu, J-X Li and F-Y Meng contributed to conception and design of the study; K Chu and Q Liang coordinated the clinical aspects of the study and contributed to collecting data and managing participants; Li Luo analyzed the data; J-L Hu wrote the paper; All authors read and approved the final manuscript.
Trial Registration
ClinicalTrials.gov: NCT03714737
Abbreviations
Acknowledgments
The authors would like to thank the volunteers who participated in this study, and also thank all the investigator of Jiangsu Provincial Center for Diseases Control and Prevention and Lianshui Center for Diseases Control and Prevention, who were responsible for collecting data and managing participants.
Additional information
Funding
References
- Abio A, Neal KR, Beck CR. An epidemiological review of changes in meningococcal biology during the last 100 years. Pathog Glob Health. 2013;107:373–80. doi:10.1179/2047773213Y.0000000119.
- Al-Tawfiq JA, Clark TA, Memish ZA. Meningococcal disease: the organism, clinical presentation, and worldwide epidemiology. J Travel Med. 2010;17:3–8. doi:10.1111/j.1708-8305.2010.00448.x.
- Kirsch EA, Barton RP, Kitchen L, Giroir BP. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996;15:967–78.
- Safadi MA, Barros AP. Meningococcal conjugate vaccines: efficacy and new combinations. J Pediatr. 2006;82:S35–44. doi:10.2223/JPED.1495.
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23:S274–9.
- Borrow R, Alarcón P, Carlos J, Caugant DA, Christensen H, Debbag R, et al. The global meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. doi:10.1080/14760584.2017.1258308.
- World Health Organization Western Pacific Region. China, 2014 [accessed 2018 Dec 24] http://www.wpro.who.int/immunization/documents/epi_country_poster_2014_chn.pdf.
- Kim DS, Kim MJ, Cha SH, Kim HM, Kim JH, Kim KN, et al. Safety and immunogenicity of a single dose of a quadrivalent meningococcal conjugate vaccine (MenACYW-D): a multicenter, blind-observer, randomized, phase III clinical trial in the Republic of Korea. Int J Infect Dis. 2016;45:59–64. doi:10.1016/j.ijid.2016.02.010.
- Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev. 2006;19:142–64. doi:10.1128/CMR.19.1.142-164.2006.
- Borrow R, Lee JS, Vázquez JA, Enwere G, Taha MK, Kamiya H, et al. Meningococcal disease in the Asia-Pacific region: findings and recommendations from the global meningococcal initiative. Vaccine. 2016;34:5855–62. doi:10.1016/j.vaccine.2016.10.022.
- Li J, Shao Z, Liu G, Bai X, Borrow R, Chen M, et al. Meningococcal disease and control in China: findings and updates from the global meningococcal initiative (GMI). J Infect. 2018;76:429–37. doi:10.1016/j.jinf.2018.01.007.
- Noya F, McCormack D, Reynolds DL, Neame D, Oster P. Safety and immunogenicity of two doses of quadrivalent meningococcal conjugate vaccine or one dose of meningococcal group C conjugate vaccine, both administered concomitantly with routine immunization to 12- to 18-month-old children. Can J Infect Dis Med Microbiol. 2014;25:211–16. doi:10.1155/2014/237560.
- Cutland CL, Nolan T, Halperin SA, Kurugol Z, Ahmed K, Perrett KP, et al. Immunogenicity and safety of one or two doses of the quadrivalent meningococcal vaccine MenACWY-TT given alone or with the 13-valent pneumococcal conjugate vaccine in toddlers: A phase III, open-label, randomised study. Vaccine. 2018;36:1908–16. doi:10.1016/j.vaccine.2018.02.013.
- Borrow R, Goldblatt D, Andrews N, Richmond P, Southern J, Miller E. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J Infect Dis. 2001;184:377–80. doi:10.1086/322024.
- Idoko OT, Okolo SN, Plikaytis B, Akinsola A, Viviani S, Borrow R, et al. The impact of pre-existing antibody on subsequent immune responses to meningococcal A-containing vaccines. Vaccine. 2014;32:4220–27. doi:10.1016/j.vaccine.2014.04.052.
- Javadekar B, Ghosh A, Kompithra RZ, Awasthi S, Perminova O, Romanenko V, et al. Safety and immunogenicity of two doses of a quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in indian and russian children aged 9 to 17 months. Indian Pediatr. 2018;55:1050–55.
- Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measlesmumps-rubella-varicella vaccine during the second year of life: an open, randomized controlled trial. Vaccine. 2011;29:4274–84. doi:10.1016/j.vaccine.2011.03.043.
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, Fischbach T, Zinke H, Pankow-Culot H, Papaevangelou V, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine. 2011;29:4264–73. doi:10.1016/j.vaccine.2011.03.009.
- Dhillon S, Pace D. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT; Nimenrix((R))): A review. Drugs. 2017;77:1881–96. doi:10.1007/s40265-017-0828-8.
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, Miller E. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis. 2006;194:1745–52. doi:10.1086/509619.
- Borrow R, Goldblatt D, Andrews N, Southern J, Ashton L, Deane S, Morris R, Cartwright K, Miller E. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United kingdom. J Infect Dis. 2002;186:1353–57. doi:10.1086/344324.
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–67. doi:10.1016/S0140-6736(04)16725-1.
- Vesikari T, Forstén A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–903. doi:10.4161/hv.22166.
- Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup C vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34:e298–307. doi:10.1097/INF.0000000000000897.
- Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Bmj. 2003;326:365–66. doi:10.1136/bmj.326.7385.365.
- Borrow R, Andrews N, Findlow H, Waight P, Southern J, Crowley-Luke A, Stapley L, England A, Findlow J, Miller, et al. MillerKinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccine Immunol. 2010;17:154–59. doi:10.1128/CVI.00384-09.
- Ishola DA, Borrow R, Findlow H, Findlow J, Trotter C, Ramsay ME. Prevalence of serum bactericidal antibody to serogroup C Neisseria meningitidis in England a decade after vaccine introduction. Clin Vaccine Immunol. 2012;19:1126–30. doi:10.1128/CVI.05655-11.
- Findlow H, Borrow R. What would be the best schedule for prevention of meningococcal disease in all ages? The UK experience. Paediatr Drugs. 2016;18:83–87. doi:10.1007/s40272-016-0169-1.
- Bona G, Castiglia P, Zoppi G, de Martino M, Tasciotti A, D’Agostino D, Han L, Smolenov I. Safety and immunogenicity of a CRM or TT conjugated meningococcal vaccine in healthy toddlers. Vaccine. 2016;34:3363–70. doi:10.1016/j.vaccine.2016.05.009.
- European Medicines Agency. Nimenrix: assessment report (EMEA/H/C/002226/II/0049). 2016 [accessed 2016 Nov 10] http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/002226/WC500220577.pdf.
- Wu X, Cai XL, Li MQ, Li YP, Ma B, Yuan L, et al. Study on the safety and immunogenicity of a new home-made vaccine of freeze-dried group A/C meningococcal polysaccharide conjugate. J Appl Prev Med. 2015;21:152–55.
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine. 2005;23:2222–27. doi:10.1016/j.vaccine.2005.01.051.
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001;69:1568–73. doi:10.1128/IAI.69.3.1568-1573.2001.
- Division of Microbiology and Infectious Diseases (DMID). Pediatric toxicitytables November 2007 Draft. 2007 [accessed 2019 Apr 2] https://www.niaid.nih.gov/sites/ default/files/dmidpedtox.pdf.
