ABSTRACT
In two pivotal efficacy studies (ZOE-50; ZOE-70), the adjuvanted recombinant zoster vaccine (RZV) demonstrated >90% efficacy against herpes zoster (HZ).
Adults aged ≥50 or ≥70 years (ZOE-50 [NCT01165177]; ZOE-70 [NCT01165229]) were randomized to receive 2 doses of RZV or placebo 2 months apart. Vaccine efficacy and safety were evaluated post-hoc in the pooled (ZOE-50/70) population according to the number and type of selected medical conditions present at enrollment.
At enrollment, 82.3% of RZV and 82.7% of placebo recipients reported ≥1 of the 15 selected medical conditions. Efficacy against HZ ranged from 84.5% (95% Confidence Interval [CI]: 46.4–97.1) in participants with respiratory disorders to 97.0% (95%CI: 82.3–99.9) in those with coronary heart disease. Moreover, efficacy remained >90% irrespective of the number of selected medical conditions reported by a participant.
As indicated by the similarity of the point estimates, this post-hoc analysis suggests that RZV efficacy remains high in all selected medical conditions, as well as with increasing number of medical conditions. No safety concern was identified by the type or number of medical conditions present at enrollment.
PLAIN LANGUAGE SUMMARY
What is the context?
Shingles, or herpes zoster (HZ), is a clinical reactivation of the varicella-zoster virus, which causes chickenpox. HZ refers to the resulting skin rash, which is often accompanied by severe, burning or itching, pain. While HZ generally affects adults over 50 years of age, it is also common in people with weakened immune systems, for example patients undergoing chemotherapy, or those with an underlying medical condition such as diabetes, asthma, or other diseases.
What is new?
Clinical trials have demonstrated that the risk of experiencing HZ in adults over 50 years of age is substantially decreased after administration of the adjuvanted recombinant zoster vaccine (RZV, Shingrix). Additional analyses were performed to determine the impact of common medical conditions on the efficacy and safety of Shingrix. These show that underlying medical conditions were common among the trial participants, but Shingrix was still protective against HZ despite their presence. A similar safety profile was reported by participants receiving either placebo or RZV.
What is the impact?
Shingrix is effective in the prevention of HZ in adults over 50 years of age, including those with one or more of the common medical conditions considered. Results may help inform the use of Shingrix in these individuals.
Introduction
Herpes zoster (HZ) results from reactivation of latent varicella-zoster virus (VZV) in sensory ganglia long after primary infection. Worldwide, the incidence of HZ ranges between 3–5 cases per 1,000 person-years in the general population and increases markedly with age, with more than two-thirds of HZ cases occurring in adults over 50 years of age (YOA).Citation1,Citation2
Medical conditions previously identified as increasing the risk of HZ include systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, chronic obstructive pulmonary disease, asthma, chronic kidney disease, renal failure, hypertension, diabetes mellitus (predominantly type I), depression, and spinal disc herniation.Citation3–5 The increase in HZ risk associated with some of these conditions may result from the immunosuppressive therapy prescribed to treat the disease and/or from underlying cell-mediated immunosuppression associated with the disease.Citation4
Vaccination decreases the risk of HZ.Citation6–9 The adjuvanted recombinant zoster vaccine (RZV, Shingrix), containing a truncated form of VZV glycoprotein E and the AS01B adjuvant system, demonstrated 97.2% and 91.3% vaccine efficacy (VE) against HZ in adults ≥50 (ZOE-50) and ≥70 YOA (ZOE-70), respectively, over an approximate 4-year follow-up period. Efficacy remained >90% among participants ≥80 YOA.Citation7,Citation8 RZV also demonstrated an acceptable safety profile.Citation7,Citation8,Citation10 It is currently licensed in multiple countries for the prevention of HZ in adults ≥50 YOA.
Although adults with a life expectancy of less than 4 years, with immunosuppressive conditions (e.g., resulting from malignancy or HIV infection) or requiring treatment with immune-modifying drugs (e.g., medications used during cancer chemotherapy or organ transplantation) were excluded from entry into the ZOE-50/70 trials,Citation7,Citation8 the eligibility criteria allowed enrollment of adults with medical conditions that are common in the general older adult population. The study population was therefore considered broadly representative of the general older adult population. The two studies were conducted in parallel at the same sites and in an identical manner, allowing the analysis of pooled data from both trials. Adults ≥70 YOA were randomly assigned to the ZOE-50 or ZOE-70 study before being randomized to a study group.
The objective of this post-hoc pooled analysis is to evaluate RZV efficacy against the first or only episode of HZ and to examine RZV safety in participants with selected medical conditions at enrollment.
Methods
The ZOE-50/70 studies were phase III, randomized, observer-blind, controlled trials conducted in parallel in 18 countries in Europe, North America, Latin America, Asia and Australia in adults ≥50 YOA (NCT01165177) and ≥70 YOA (NCT01165229). Participants were randomized 1:1 to receive 2 doses of either RZV or saline placebo 2 months (M) apart.Citation7,Citation8 Protocol summaries are available for both studies at http://www.gsk-clinicalstudyregister.com (studies 110390 and 113077). Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Persons with a confirmed or suspected immunosuppressive or immunodeficient conditions resulting from disease (e.g., malignancy, human immunodeficiency virus infection) or immunosuppressive/cytotoxic therapy (e.g., medications used during cancer therapy, organ transplantation or to treat autoimmune disorders) were excluded. Persons who had received immunosuppressants or immune-modifying drugs for >15 consecutive days within 6 months prior to the first vaccine dose, were also excluded (prednisone <20 mg/day, or equivalent, and inhaled/topical steroids were allowed). Full inclusion and exclusion criteria have previously been published.Citation7,Citation8
Medical conditions of the approximately 30,000 participants were recorded at enrollment in the ZOE-50/70 trials, and those most frequently reported in the ZOE-70 trial were selected and applied for analysis utilizing data from both trials, since the prevalence of most underlying medical conditions increases with age. Efficacy and safety analyses were performed post-hoc in the pooled ZOE-50/70 population (i) according to medical conditions reported by at least 500 participants from either arm of the ZOE-70 study at enrollment (considered to provide an adequate sample size for the purpose of a descriptive analysis), and according to additional medical conditions reported by less than 500 participants in the ZOE-70 study at enrollment but associated with an increased risk of HZ (asthma, depression, respiratory disorders, and renal disorders),Citation3–5 and (ii) according to the number of selected medical conditions reported by study participants at enrollment. For the purpose of the analysis, medical conditions were defined as individual Medical Dictionary for Regulatory Authorities preferred terms (PTs) or grouped PTs representing conditions of similar pathophysiological origin. Details on PT grouping are provided in the Supplementary Table 1. Participants reporting more than one of the PTs within the 15 selected medical conditions were only counted once for the respective analyses.
For the analysis of VE, a suspected HZ case was defined as new unilateral rash with pain that had no alternative diagnosis. Participants were followed for at least 90 days after the onset of HZ or until the rash resolved and the participant was pain-free for 4 weeks. Suspected HZ cases were evaluated and confirmed as described previously by polymerase-chain-reaction or an adjudication committee.Citation7,Citation8 Efficacy was assessed similar to the primary analysis on the pooled modified total vaccinated cohort (mTVC).Citation7,Citation8 This included all participants from the pooled TVC who had received both doses of vaccine/placebo and who had not developed a confirmed HZ case prior to 30 days post-dose 2. VE was defined as 1 minus the ratio of HZ incidence in the RZV group to that in the placebo group.
For assessment of safety, serious adverse events (SAEs) were recorded for all participants for up to 12 months post-dose 2; any events resulting in death, and potentially immune-mediated diseases (pIMDs) were evaluated in all participants over the entire study period. Safety was evaluated in the pooled TVC from the ZOE-50/70 studies, which included all participants administered with at least one dose of RZV.
Results
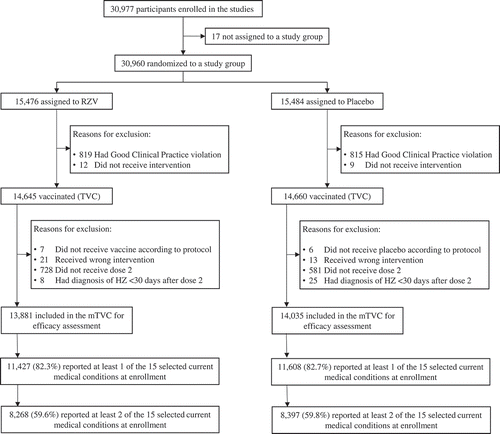
Of the 30,977 participants enrolled in the ZOE-50/70 studies, 13,881 RZV and 14,035 placebo recipients were included in the mTVC. Of these, 82.3% of RZV recipients and 82.7% of placebo recipients had at least 1 of the 15 selected medical conditions at enrollment. A majority (RZV: 59.6%, Placebo: 59.8%) had 2 or more (). In the pooled mTVC, demographic characteristics, including proportions of participants reporting each of the selected medical conditions, were balanced between study groups ().
Table 1. Demographic characteristics (pooled ZOE-50/70 modified Total Vaccinated Cohort)
Figure 1. Participant flow (pooled ZOE-50/70 studies) RZV = participants receiving the adjuvanted recombinant zoster vaccine; Placebo = participants receiving placebo; ZOE-50/70 = RZV efficacy studies in adults ≥50 YOA (NCT01165177) and ≥70 YOA (NCT01165229), respectively; (m)TVC = (modified) total vaccinated cohort; HZ, herpes zoster
Medical conditions in order of decreasing frequency included hypertension, osteoarthritis and vertebral disorders, and dyslipidemia. The most frequently reported conditions known to increase HZ risk included in this analysis were depression and asthma ().
Efficacy against HZ ranged from 84.5% (95% Confidence Interval [CI]: 46.4–97.1) in participants with respiratory disorders to 97.0% (95% CI: 82.3–99.9) in those with coronary heart disease at enrollment. Efficacy was 88.8% (95% CI: 63.6–97.8) in participants with asthma, and 91.2% (95% CI: 81.1–96.6) in those with diabetes. The only medical condition that RZV efficacy did not achieve statistical significance was renal disorders (VE: 86.6% [95% CI: −4.5–99.7]). The low number of participants with this condition limited the statistical power to assess VE. Overall, RZV efficacy was >90% irrespective of the number of the selected medical conditions reported at enrollment by a participant ().
Table 2. Vaccine efficacy against first or only episode of herpes zoster in ZOE-50/70 participants reporting at least 1 of the 15 selected medical conditions at enrollment (pooled modified Total Vaccinated Cohort)
In RZV or placebo recipients reporting at least 1 of the 15 selected medical conditions at enrollment, the proportion of participants experiencing SAEs or deaths was highest among those with chronic conditions such as renal disorders, respiratory disorders, or coronary heart disease. The numbers of SAEs, deaths, or pIMDs were similar in the vaccine and placebo groups for each of the medical conditions (). The frequency of SAEs and deaths increased with the number of medical conditions present at enrollment; however, the frequency of pIMDs did not. Frequencies of SAEs, deaths, and pIMDs were balanced between RZV and placebo recipients reporting any number of these conditions ().
Table 3. Safety of RZV in ZOE-50/70 participants reporting at least 1 of the 15 selected medical conditions at enrollment (pooled Total Vaccinated Cohort)
Discussion
Older adults enrolled in the ZOE-50/70 studies reported underlying medical conditions at a frequency expected in these age groups.Citation11 The observed efficacy across the 15 medical conditions, including the ones associated with an increased HZ risk, was consistent with the efficacy in the overall pooled ZOE-50/70 population ≥70 YOA.Citation8 This is in line with the fact that >80% of the overall ZOE-50 and ZOE-70 study population reported at least 1 of the specified conditions. Other studies have shown that RZV also confers strong protection against HZ in immunocompromised populations who are at highest HZ risk, such as hematopoietic stem cell transplant recipients and patients with hematological malignancies.Citation12,Citation13
The high proportion of participants reporting at least 1 of the selected medical conditions can be explained by the relatively permissive inclusion and exclusion criteria for the ZOE-50/70 studies and the age of study participants. Previous studies have shown that frailty correlates with the number, rather than the nature of accumulated biopsychosocial deficits, which are mostly medical conditions.Citation14,Citation15 Our analysis was consistent with this finding in that the frequency of SAEs and deaths increased with the number of conditions reported at enrollment. Although the point estimates for efficacy were <90% for 5 of the 15 selected medical conditions, the observed trend suggest that a high VE was maintained in participants having up to 6 or more of those conditions. This contrasts with the observed decline in influenza vaccination effectiveness as the level of frailty increases in elderly people.Citation16 This further underscores the ability of RZV to induce robust protection against HZ in older adults with multiple medical conditions.
Overall, frequencies of SAEs, deaths, and pIMDs were balanced between RZV and placebo recipients within each of the analyzed sub-groups. No vaccine-related safety concerns were identified in participants with any type or number of the selected medical conditions.
This post-hoc analysis has some limitations that should be taken into account when interpreting its results. The ZOE-50/70 studies were not statistically powered to evaluate RZV efficacy and safety by the number and type of participants’ medical conditions. In addition, the number of participants in some sub-groups was limited. This includes medical conditions reported by less than 500 participants in the ZOE-70 study at enrollment but associated with an increased risk of HZ (asthma, depression, respiratory disorders, and renal disorders) and participants with ≥6 of the selected conditions. The present analysis was not adjusted for multiplicity, and being exploratory, its significance level was not controlled. The efficacy and safety analyses by medical condition category did not detect any additional underlying medical condition at enrollment that might have an additive or synergistic effect on the risk of HZ. As the study groups were comparable as a result of randomization, this should have a limited effect on the analyses by type or number of conditions. In addition, study participants were not fully representative of the overall older adult population. Individuals with a life expectancy of less than 4 years at the time of study entry were to be excluded. Persons with diseases requiring treatment with immune-modifying drugs, as well as persons with diseases that are immunosuppressive by nature, were also excluded, limiting the conditions for which we could provide a VE estimate. Therefore, owing to the inclusion/exclusion criteria, persons with some of the conditions associated with the highest HZ risk were not enrolled in the study. Some of these conditions were studied as part of the parallel clinical program with RZV in immune compromised adults (e.g. hematopoietic stem cell transplant recipients and patients with hematological malignancies). No standard definitions were used in the diagnosis; therefore, each selected medical condition could vary with respect to severity, stage, treatment, progression, or type (e.g., diabetes mellitus type). The database did not capture this level of detail. Medical conditions with onset after enrollment were not considered for this analysis. Older adults with severe frailty (e.g., bedridden elderly persons) were also unlikely to participate. Despite their broad geographic diversity, study participants were mostly Caucasian. Nonetheless, the most common diseases in the targeted age group were well represented.
In summary, this study showed that >80% of participants had at least 1 of the 15 selected medical conditions present at enrollment. As indicated by the similarity of the point estimates, this post-hoc analysis suggests that RZV efficacy remains high in all selected medical conditions, as well as with increasing number of medical conditions. Point estimates for efficacy ranged between 84.5–97.0% according to the type of the selected medical conditions (with overlapping 95% confidence intervals) and were >90% even among participants reporting at least 6 of these at enrollment. No safety concern was identified in adults ≥50 YOA presenting these medical conditions.
A plain language summary contextualizing the results and potential clinical research relevance and impact is displayed in the Focus on Patient Section (Supplementary Figure 1).
Authors’ contributions
Detailed authors’ contributions are provided in the supplementary materials.
Disclosure of potential conflicts of interest
TCH, HL, and LO are former employees, and AFD, CH, TM, PVdS, TZ and TM spouse are employees, of the GSK group of companies (GSK). LO, TCH, AFD, HL, TM, PVdS, TZ and TM spouse hold shares or/and stock options from GSK as per their former or current employee remuneration. LO is an employee of CureVac AG. LO and TCH are inventors on a patent owned by GSK and relevant to RZV. TCH served as a paid consultant to GSK outside the submitted work. RWJ received honoraria for consultancy and funding for a meeting he organized from Merck, funding from Sanofi Pasteur MSD to organize a scientific meeting, and honoraria as a consultant for GSK. MJL received fees for serving on advisory boards from Merck and GSK and grant support from Merck and GSK. JEM reports receiving honoraria and fees paid to her institution from GSK, Sanofi Pasteur, Merck and Pfizer, as well as travel support from GSK, Sanofi Pasteur, Merck and Pfizer outside the submitted work. RC reports receiving lecture fees from Pfizer outside the submitted work. JDD reports personal fees from GSK for serving on an advisory board, as well as grants and personal fees from Sanofi Pasteur MSD for an epidemiological study on herpes zoster and an advisory board on Zostavax, respectively, outside the submitted work. ISG received lecture fees and grant support from GSK outside the submitted work and served on the Advisory Board for Shingrix in Canada. In addition, ISG has received research grants and lecture fees from several other pharmaceutical companies in work done for Prime Health Clinical Research outside the submitted work. HI received grants and personal fees from GSK and grants from Japan Vaccine during the conduct of the study, as well as grants and personal fees from Daiichi-Sankyo and grants from Sanofi Pasteur and personal fees from Shionogi outside the submitted work. GK is a freelance statistician at Keyrus Biopharma, serving as a paid consultant for GSK. HL is a current employee of Pfizer and receives stock as part of his employee remuneration. SAM reports research grant from Pfizer, personal fees for continuing professional development talks on adult immunization from Pfizer and Merck, and consulting fees from Pfizer and Merck outside the submitted work, as well as grant from GSK outside the submitted work. JS reports personal fees from GSK and Sanofi Pasteur outside the submitted work. DW reports grants and personal fees from GSK, consulting fees from Maruho and Japan Vaccines, lecture fees from Maruho and Mochida, and grant support from Maruho. LYW received grant from GSK during the conduct of the study, as well as fees for serving on advisory boards from Novartis, GSK, AbbVie and Wyeth. ALC received honoraria paid to his institution from GSK, Merck, and BioCSL/Sequirus outside the submitted work.
SJH and KP declare no conflict of interest.
ZOE-50/70 study group
Anitta Ahonen, Eugene Athan, Johan Berglund, Won Suk Choi, Ferdinandus J. de Looze, Maria Giuseppina Desole, Meral Esen, Brecht Geeraerts, Wayne Ghesquiere, Lars Rombo, Antonio Volpi.
Trademark statement
Shingrix is a trademark owned by or licensed to the GSK group of companies.
Supplemental Material
Download MS Word (102 KB)Acknowledgments
The authors would like to thank all study participants, the clinical investigators and study nurses involved in the ZOE-50 and ZOE-70 trials. The authors are also grateful to Anne Schuind (GSK) for draft reviewing and scientific input, and to Modis c/o GSK for editorial assistance and manuscript coordination. Medical writing services were provided by Alpár Pöllnitz and editorial assistance and publication coordination were provided by Quentin Deraedt.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2019.1627818
Additional information
Funding
References
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81:928–30. doi:10.1212/WNL.0b013e3182a3516e.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi:10.1136/bmjopen-2014-004833.
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clinic Proc. 2017;92:1806–21. doi:10.1016/j.mayocp.2017.10.009.
- Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case-control study. Bmj. 2014;348:g2911. doi:10.1136/bmj.g2911.
- Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39:537–44. doi:10.1007/s15010-011-0162-0.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi:10.1056/NEJMoa051016.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–32. doi:10.1056/NEJMoa1603800.
- Schmader KE, Levin MJ, Gnann JW Jr., McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su S-C, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54:922–28. doi:10.1093/cid/cir970.
- Lecrenier N, Beukelaers P, Colindres R, Curran D, De Kesel C, De Saegher J-P, Didierlaurent AM, Ledent EY, Mols JF, Mrkvan T, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines. 2018;17:619–34. doi:10.1080/14760584.2018.1495565.
- Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–62. doi:10.1016/S0140-6736(14)61347-7.
- de la Serna J, Campora L, Chandrasekar P, El Idrissi M, Gaidano G, López Fauqued M, Oostvogels L, Schwartz S, Sullivan K, Szer J, Bastidas A. Efficacy and safety of an adjuvanted herpes zoster subunit vaccine in autologous hematopoietic stem cell transplant recipients 18 years of age or older: first results of the phase 3 randomized, placebo-controlled ZOE-HSCT clinical trial. BMT Tandem Meetings, Salt Lake City, Utah. 2018: abstract LBA2.
- Dagnew AF, Ilhan O, Lee W-S, Woszczyk D, Kwak J-Y, Bowcock S, Sohn SK, Rodriguez Macías G, Chiou T-J, Quiel D, et al. Immunogenicity, safety and efficacy assessment of the adjuvanted recombinant zoster vaccine in adults with hematologic malignancies: a phase 3, randomized clinical trial. ID Week. 2018: abstract 140.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi:10.1186/1471-2318-8-24.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–36. doi:10.1100/tsw.2001.58.
- Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, Dos Santos G, McElhaney JE, Ambrose A, Boivin G, Bowie W, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216:405–14. doi:10.1093/infdis/jix282.
