ABSTRACT
Pneumococcal conjugate vaccines (PCV) have been widely used in high-income countries for more than a decade, resulting in a dramatic reduction in pneumococcal disease caused by vaccine serotypes. PCV has been included in Turkey’s National Immunization Programme since 2009 with PCV7 and continued with PCV13 from 2011. We presented a three-month-old infant who developed mastoiditis secondary to S. pneumoniae serotype 19A after acute otitis media.
To the editors
In the first few years of life, infants and young children experience very frequent upper respiratory infections. Among these, acute otitis media (AOM) is one of the most prominent.Citation1 The etiology of AOM varies with age, the most frequently implicated agents being viruses and bacteria such as non-typable Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. S. pneumoniae is the common cause of AOM in children; mastoiditis remains an important complication of AOM.Citation2,Citation3
Pneumococcal conjugate vaccines (PCV) have been widely used in high-income countries for more than a decade, resulting in a dramatic reduction in pneumococcal disease caused by vaccine serotypes.Citation4 Turkey introduced 7-valent pneumococcal conjugate vaccine (PCV7) in November 2008 followed by 13-valent PCV (PCV13) in April 2011 into the national routine infant immunization schedule. The recommended vaccination scheme for infants with PCV13 is primary doses at months 2, 4, 6 and a booster dose at month 12. PCV13 contains serotype 19A. We present here the clinical case of a three-month-old infant with mastoiditis as a complication of acute otitis media. The responsible serotype of S. pneumoniae was identified as 19A, and it was resistant to penicillin and third-generation cephalosporin.
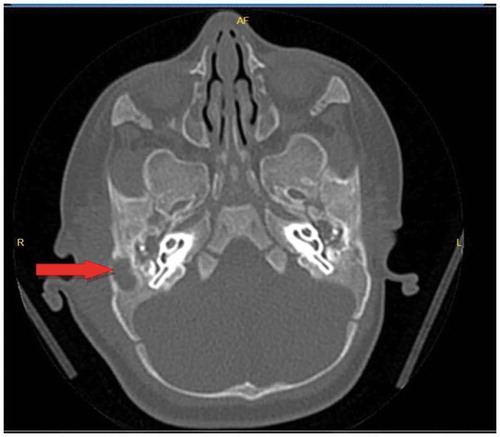
A 3,5 month-old boy was admitted to the hospital with a complaint of swelling behind his right ear. It was learned that he had born at 37 weeks of gestation weighing 3600 gr with normal spontaneus vaginal delivery. At three months of age, he had a case AOM. Amoxicillin clavulanate was given for 10 days, then pus drainage for 4 days occurred and the otitis had apparently resolved. Swelling behind ear developed after 10 days. A physical examination, showed no fever, erythema, inflammatory discharge or pain. There was a protrusion of the right auricle and fluctuation on the lesion. His white blood cell count was 17400 cells/mm3with 46.5% neutrophils, hemoglobin at 10.4 g/dL, platelet count at 604,000/mm3, C-reactive protein (CRP) was 2.3 mg/dL. Temporal computed tomography performed. Bilateral otomastoiditis and destruction of mastoid bone were detected on the right side (). Subperiosteal abscess drainage and myringotomy with placement of a tympanostomy tube to both ears were performed by an otorhinolaryngologist. S. pneumoniae serotype 19A was detected by culture of an abscess sample. This strain was resistant to cefuroxime (MIC >2 mg/L), erythromycin (MIC >0.5 mg/L), penicillin G (MIC ≥4 mg/L) and tetracycline, and sensitive to vancomycin, cefepime, trimethoprim–sulfamethoxazole gentamycin in antibiotic susceptibility test. Cefepime was given at 50 mg/kg per dose every 8 hours for 10 days because of the recent antibiotic treatment history. Mastoiditis was resolved without complication.
Figure 1. Thickening of the skin in the pre-post auricular region and with arrow bilateral otomastoiditis and destruction of mastoid bone was detected on the right side is seen
Serotype 19A emerged as a predominant serotype in several countries in both children and adults after the introduction of PCV7. In contrast with the experience with PCV7, implementation of PCV13 immunization in the routine infant programs of several countries has resulted in significant declines in the incidence of serotype 19A in all age groups. A case-controlled study found significant PCV13 effectiveness against serotype 19A in children 2–59 months of age.Citation5 One study showed that children presenting with AOM were less likely to carry PCV13 serotypes (19A, 7F, 6C) in the nasopharynx if they had received at least one dose of PCV13 compared with children who had received PCV7 only.Citation6 The current patient was vaccinated with one dose of PCV13 at two months of age, and AOM with serotype 19A developed one month after vaccination. One-dose vaccination was not protective for our patient. Ceyhan et al. reported that reduction in nasopharyngeal carriage of vaccine serotypes also led to a substantial herd effect (indirect protection) in the older children and adults in Turkey.Citation7
After the introduction of PCV7, there was an initial decrease in antimicrobial resistance (AMR), but it was followed by an increase in AMR serotype 19A.Citation8 The same effect was shown after PCV10 vaccination for serotype 19A.Citation9 In a study from New Zealand after PCV10 vaccination, serotype 19A was found to be the most common penicillin-resistant serotype.Citation10 Countries such as France, Israel, and the USA, which have introduced PCV13 into the infant national immunization program, have seen reductions in AMR due to the relevant serotypes, particularly AMR serotype 19A.Citation5,Citation11,Citation12 In contrast with the emergence of AMR serotype 19A after the introduction of PCV7 or PCV10, to date, no single 19A-like AMR pneumococcal serotype has emerged globally in countries using PCV13.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Pichichero ME, Casey JR and Almudevar A. Reducing the frequencyof acute otitis media by individualized care. Pediatr Infect Dis J. 2013;32:473–78. doi:10.1097/INF.0b013e3182862b57.
- Rodgers GL, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27:3802–10. doi:10.1016/j.vaccine.2009.04.021.
- Bluestone CD, Klein JO. Intratemporal complications and sequelae of otitis media. In: Bluestone CD, Stool SE,editors. Pediatric otolaryngology. 3rd ed. Philadelphia: Saunders; 2003. p. 687.
- Fitzwater S, Chandran A, Santosham M, Johnson H. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. The Pediatric Infectious Disease Journal. 2012;31(5):501–08. doi:10.1097/INF.0b013e31824de9f6.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case control study. Lancet Respir Med. 2016;4:399–406. doi:10.1016/S2213-2600(16)00052-7.
- Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012;31:297–301. doi:10.1097/INF.0b013e318247ef84.
- Ceyhan M, Ozsurekci Y, Gurler N, Oksuz L, Aydemir S, Ozkan S, Aydemir S, Ozkan S, Yuksekkaya S, Keser Emiroglu M, et al. Serotype distribution of Streptococcus pneumoniae in children with invasive diseases in Turkey: 2008–2014. Hum Vaccin Immunother. 2016;12(2):308–13. doi:10.1080/21645515.2015.1078952.
- Dagan R, Klugman KP. Impact of conjugate pneumococcal vaccines on antibiotic resistance. Lancet Infect Dis. 2008;8:785–95. doi:10.1016/S1473-3099(08)70281-0.
- Potin M, Fica A, Wilhem J, Cerda J, Contreras L, Escobar C, Moreno G, Muñoz A, Véliz L. Statement of the Advisory Immunization Committee of the Chilean Society of Infectious Diseases on the emergence of serotype 19A pneumococcal infectionand the use of pneumococcal conjugated vaccine in Chilean children. Rev Chilena Infectol. 2016;33:304–06. doi:10.4067/S0716-10182016000300009.
- Institute of Environmental Science and Research Ltd (ESR). Invasive pneumococcal disease in New Zealand. Wellington (NZ): Ministry ofHealth; 2014. [accessed 2017 Aug 4]. https://surv.esr.cri.nz/PDF_surveillance/IPD/2014/2014IPDAnnualReport.pdf.
- Kempf M, Varon E, Lepoutre A, Gravet A, Baraduc R, Brun M, Chardon H, Cremniter J, Croizé J, Dalmay F, et al. Decline in antibiotic resistanceand changes in the serotype distribution of Streptococcus pneumoniaeisolates from children with acute otitis media; a 2001–2011 survey by the French Pneumococcal Network. Clin Microbiol Infect. 2015;21:35–42. doi:10.1016/j.cmi.2014.08.009.
- Rudolph K, Bruce M, Bulkow L, Singleton R, Zulz T, Gounder P, Hurlburt D, Bruden D, Hennessy T, Rudolph K, et al. Clonal diversity of penicilin nonsusceptible invasive pneumococal isolates in Alaska, 2001–2014. Presented at: 10th International Symposium on Pneumococci and Pneumococcal Disease (ISPPD); 2016; Glasgow, Scotland, UK
