ABSTRACT
Rabies is a fatal disease that mandates proper prophylaxis after a rabies virus exposure to prevent death. This study evaluated adherence to Centers of Disease Control and Prevention (CDC) recommendations for rabies immune globulin (IG) patient selection, dosing, timing of administration, and anatomical site of administration for rabies postexposure prophylaxis. This retrospective, cross-sectional study included patients who received at least one dose of rabies IG or rabies vaccine at a multi-hospital health system from January 2015 through June 2018. This study included 246 patients, and all of them received at least one dose of rabies vaccine. Two patients had a history of rabies vaccination, did not have an indication for rabies IG, and appropriately did not receive additional rabies IG. Rabies IG was administered to 91% (223 of 244) of patients with an indication. Of 223 patients who received rabies IG, 219 (98%) received doses within 10% of 20 IU/kg of body weight, and all 223 (100%) received rabies IG within 7 days of the first rabies vaccine administration. Only 56% (96 of 170) of patients with a wound that could be infiltrated with rabies IG actually received rabies IG via infiltration into and around the wound. This multi-hospital health system study demonstrated high adherence to guideline recommendations for rabies IG patient selection (91%), dosing (98%), and timing (100%). However, only 56% of eligible patients received rabies IG infiltration at wound sites as recommended by guidelines.
Introduction
Background
Rabies viral infection is a fatal disease that mandates proper prophylaxis after an exposure in order to prevent death. Rabies virus is nearly always transmitted through the bite of an infected animal – wherein contamination of the wound through saliva occurs.Citation1–Citation4 Other routes of viral transmission include animal scratches, mucous membrane contamination, and occult exposures, as can occur with bats. In the United States, human rabies is rare, with on average 1 to 3 cases reported per year. However, approximately 30,000 to 60,000 people come in to contact with potentially rabid animals each year and receive life-saving rabies post exposure prophylaxis.Citation5
Importance
Rabies infection can occur in humans when key elements of the rabies postexposure prophylaxis regimens are omitted or incorrectly administered. The Centers of Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) recommends that humans who are exposed to the rabies virus should receive rabies postexposure prophylaxis, consisting of prompt and thorough wound cleansing followed by vaccination with cell culture-derived rabies vaccine (rabies vaccine) and administration of human rabies immune globulin (rabies IG).Citation6
A series of four rabies vaccine administrations on days 0, 3, 7, and 14 is recommended for rabies postexposure prophylaxis in patients who were not previously vaccinated, and a fifth dose should be administered on day 28 for immunosuppressed patients. If previously vaccinated, patients should receive two booster doses of rabies vaccine; one given on day 0, and one given on day 3.Citation6 Rabies vaccine should be administered in the deltoid muscle in adults and anterolateral thigh in pediatric patients. Administration of rabies vaccine into the gluteal muscle should be avoided due to possible injection into the fat tissue, attenuation of immunogenicity, and risk of sciatic nerve damage.Citation6–Citation15
Among patients who were not previously vaccinated, one dose of rabies IG (20 IU/kg of actual body weight) is indicated within seven days of the first rabies vaccine administration.Citation6,Citation16,Citation17 If delayed beyond this time, rabies IG should not be given, as it may interfere with the active production of antibodies that usually become detectable in the blood 7 days after rabies vaccine administration.Citation17,Citation18 A low dose of rabies IG (<20 IU/kg) is associated with inadequate prophylaxis, while a high dose of rabies IG (>20 IU/kg) is associated with an increased risk of adverse reactions (e.g., injection site reaction) and may suppress vaccine-induced antibody production.Citation16,Citation19 If anatomically feasible, the full dose of rabies IG should be infiltrated into and around the wound to maximize effectiveness and neutralize any virus before it enters the central nervous system from the site of infection.Citation6,Citation20,Citation21 Any remaining volume should be administered intramuscularly into a large muscle distant from the vaccine administration site, as rabies IG may neutralize the rabies antigens in the vaccine if administered in the same site.Citation6,Citation22
Goals of this investigation
This study evaluated adherence to CDC/ACIP recommendations for rabies IG patient selection, dosing, timing of administration, and site of administration for rabies postexposure prophylaxis at a multi-hospital health system.
Materials and methods
Study design and setting
This retrospective, cross-sectional study included patients who received at least one dose of rabies IG or rabies vaccine at a multi-hospital health system (one academic medical center, seven community hospitals, and eight additional free-standing emergency care centers staffed by board-certified physicians) in Houston, Texas. The study was approved by the health system’s institutional review board with a waiver of informed consent. During the study period, rabies IG and rabies vaccine products used at the health system were human rabies IG HyperRAB® S/D 2 mL and 10 mL vials at 150 IU/mL (Grifols Therapeutics Inc., Clayton, NC, USA) and Imovax® rabies human diploid cell culture vaccine 1 mL vials containing ≥2.5 IU of rabies antigen (Sanofi Pasteur SA, Lyon, France). The funding agency, Grifols© Shared Services North America, Inc., was not involved in the design of the study, collection of the data, analysis of the data, interpretation of the data, nor the development of this manuscript.
Data management
Study data were extracted from two electronic health records (EHRs) that were used at the health system during the study period. To validate data collection forms, three investigators piloted data collection using a sample of 10 patients, and data collection forms were optimized and standardized based on this pilot. Final data collection forms were built in Microsoft Access® 2013, and standard operating procedures for data collection were developed. Study data were extracted from the EHRs by two independent investigators. Minor data collection discrepancies between the two independent reviewers were arbitrated by one investigator. Major data collection discrepancies were arbitrated through study team discussion and consensus. Additionally, random audits were conducted to ensure data integrity. Two EHRs were used clinically during the study period; one EHR documented both ethnicity and race while the other EHR only documented race. For the purpose of this analysis, patients listed as Hispanic race were categorized as Caucasian/White, and ethnicity was not reported.
Selection of participants
Patients who received at least one dose of rabies IG or rabies vaccine from January 2015 through June 2018 at any facility within the health system were included in this study. Patients were excluded if their date of the first rabies vaccination was missing as evaluation of appropriateness of rabies IG patient selection and timing would not be possible without this information. Patients who received rabies IG at an external facility prior to presenting to a facility in our health system were excluded.
Characterization of animal exposure
Animal exposures were categorized into one or more of the following categories: bite, scratch, lick, direct contact, or unknown encounter. Exposures where the animal touched the patient with no resulting skin penetration were classified as “direct contact”. Exposures where the animal was in close proximity to the patient without making direct contact or where the patient was not aware of direct contact occurring were classified as “unknown encounter.” An example of an unknown encounter is a patient finding a dead bat in the house after waking up in the morning.
Patient selection for rabies IG administration
Patients were categorized into four categories of adherence to CDC/ACIP recommendations for rabies IG patient selection: (1) correct inclusion – rabies IG was indicated and administered, (2) error of omission – rabies IG was indicated but was not administered, (3) error of commission – rabies IG was not indicated but was administered, and (4) correct exclusion – rabies IG was not indicated and was not administered. Investigators collected information on clinicians’ rationale for not administering rabies IG if rabies IG was indicated per CDC/ACIP guideline recommendations.
Rabies IG dosing
All rabies IG doses administered during the same encounter were summed to calculate total dose of rabies IG. The weight-based dose was calculated by dividing the total dose (IU) by actual body weight (kg). If actual body weight was not documented during that encounter, then actual body weight recorded on a previous medical encounter or on the driver’s license was used. Rabies IG doses within 10% of the recommended 20 IU/kg dose were considered appropriate, as orders for some medications (e.g., immune globulin and high-cost medications) can be rounded to the nearest vial size if the rounded dose is within 10% of the calculated dose.Citation6,Citation23–Citation25 Investigators attempted to identify the cause of inappropriate dosing deviations.
Rabies IG timing of administration
The date of receiving the first administration of rabies vaccine was known for all included patients, and in some cases, the first dose of rabies vaccine was administered at an external facility before the patient presented to a facility in our health system. The difference in days between the first administration of rabies vaccine and administration of rabies IG was calculated for each patient who received rabies IG.
Rabies IG anatomical site of administration
According to CDC/ACIP guidelines, the full dose of rabies IG should be infiltrated around and into the wound, if anatomically feasible.Citation6 In this study, a wound was defined as an anatomical location where rabies IG could be infiltrated. These anatomical locations included clearly evident wounds resulting from a skin penetration visible at the time of rabies IG administration, occult wounds resulting from a skin penetration that was too small to detect or healed at the time of presentation, and previously existing wounds that came into contact with the animal. Animal exposure anatomical location categories were created to include the arm (shoulder through the wrist), hand (hand and fingers), leg (hip/gluteal muscle through the ankle), and foot (foot and toes). If an exposure occurred on both the arm and hand, it was categorized as hand. If an exposure occurred on both the leg and foot, it was categorized as foot. Exposure of animal saliva into a mucous membrane such as the patient’s eye or mouth was not eligible for local infiltration and was not considered a wound. When available, investigators collected information on all rabies IG administration sites and the volume of rabies IG administered at each administration site. These administration sites were categorized as either into and around the wound or as an intramuscular site distant from the wound. This data was used to calculate the volume and proportion of total rabies IG dose that was administered into and around the wound among patients where it was anatomically feasible. Patients were excluded from this analysis if the volume of rabies IG administered at each site was not documented.
Rabies IG should be administered in an anatomical site distant from the rabies vaccine administration site.Citation6,Citation26 Therefore, the administration site was collected for all rabies vaccine administrations given at the same time as rabies IG. The rabies IG and rabies vaccine administration sites were compared to identify administration sites that were too close together. Injections were considered too close together if both were given ipsilaterally in the deltoid muscle, thigh area (including vastus lateralis site, rectus femoris site, anterolateral [thigh], or quadriceps), or gluteal area (including dorsogluteal site, ventrogluteal site, gluteal, buttock, or hip).
Rabies IG should not be administered into the buttock to avoid possible sciatic nerve damage.Citation11,Citation13–Citation15,Citation27,Citation28 Rabies IG administration into the buttock – including dorsogluteal, gluteus medius, or gluteal sites not otherwise specified – were considered inappropriate in this regard, unless the animal exposure occurred in that area (e.g., dog bite in the gluteal area). Rabies IG administration into the ventrogluteal site or hip was considered appropriate.
Analysis
Patient demographics and baseline characteristics were summarized as means with standard deviations for continuous variables and frequencies with percentages for categorical variables. Associations between covariates and the outcome rabies IG wound infiltration were analyzed using Pearson Chi–squared test or Fisher’s exact test if any cell frequency was ≤5. A two-sided p-value of 0.05 was used to identify statistical significance. Statistical analyses and data management were conducted using STATA version 15 (StataCorp LP, College Station, Texas).
Results
Characteristics of study subjects
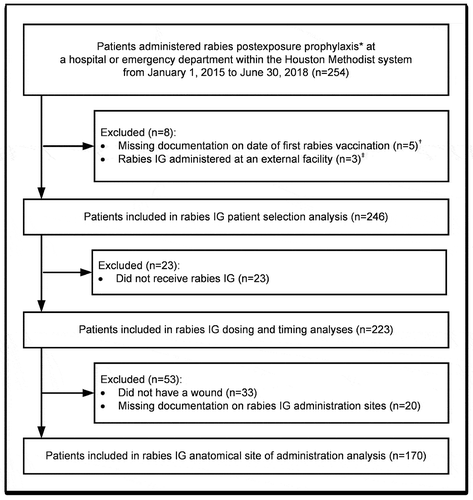
Of 254 patients who received rabies postexposure prophylaxis, five patients were excluded because the date of the first dose of rabies vaccine was unknown, and three patients were excluded because rabies IG was administered at an external facility (). This study included 246 patients who had a mean age of 39 years ± 21 years and mean actual body weight of 73 kg ± 26 kg (). Six patients (2%) were immunosuppressed. Two patients (1%) had a history of rabies vaccination prior to the new rabies exposure. Seventy-four percent of patients presented to a facility that could provide rabies postexposure prophylaxis within one day of animal exposure.
Table 1. Patient demographics.
Figure 1. Patient inclusion flowchart.
IG: immune globulin * Patients who received one or more doses of rabies IG or rabies vaccine were included. † Rabies IG is only indicated during the first 7 days of postexposure prophylaxis among patients who were not previously vaccinated prior to animal exposure. Therefore, patients were excluded if the date of administration of the first rabies vaccine was unknown. ‡ Rabies IG is only indicated for one dose among patients who were not previously vaccinated prior to animal exposure. Therefore, patients who received rabies IG at an external medical facility prior to receiving care at the Houston Methodist system were excluded from this analysis.
Characteristics of animal exposures
The predominant animal exposure type was bite (75%, N = 185). The most common wound locations were upper extremities (45%, N = 111) and lower extremities (27%, N = 67). Among 246 study patients, 48% (N = 118) were exposed to dogs, 25% (N = 61) were exposed to bats, and 14% (N = 34) were exposed to cats (). Among 118 dog exposures, 48% (N = 57) were stray, 28% (N = 33) were domesticated, and 24% (N = 28) had an unknown status. Among 34 cat exposures, 82% (N = 28) were stray, and 9% (N = 3) were domesticated, and 9% (N = 3) had an unknown status.
Patient selection for rabies IG administration
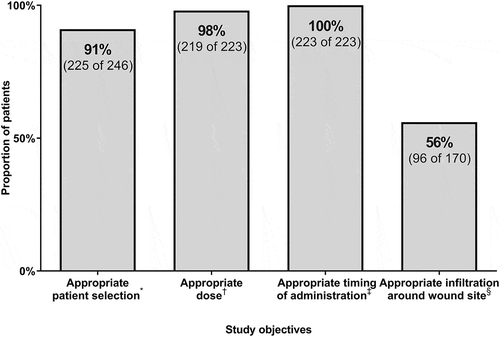
Patient selection for administration of rabies IG was adherent to CDC/ACIP guideline recommendations for 91% (225 of 246) of patients (, , Supplemental Table 1, Supplemental Table 2). Rabies IG was not administered to 100% (2 of 2) of patients who did not have an indication for prophylaxis. Rabies IG was administered to 91% (223 of 244) of patients who had an indication for prophylaxis. Therefore, 21 (9%) patients who had an indication for rabies IG did not receive it. The rationale for not administering rabies IG when indicated was patient refusal (N = 4), providers’ clinical judgment (N = 5), or not documented (N = 12). Physicians (medical doctor or doctor of osteopathic medicine, 56%, 125 of 223) and advanced practice practitioners (nurse practitioner or physician assistant, 44%, 98 of 223) ordered rabies IG. Although advanced practice practitioners (nurse practitioner or physician assistant) ordered 44% of rabies IG doses, they consult with an attending physician during routine patient care in the emergency department at our health system.
Table 2. Adherence to CDC/ACIP recommendations in rabies postexposure prophylaxis.
Figure 2. Proportion of patients who achieved adherence to guideline recommendations for human rabies immune globulin patient selection, dosing, timing, and anatomical site of administration in rabies postexposure prophylaxis.
* Proportion of patients who were treated according to guideline recommendations on patient selection for rabies immune globulin administration † Proportion of patients who received a rabies immune globulin dose that was within 10% of the Food and Drug Administration-approved dose of 20 IU/kg ‡ Proportion of patients who received rabies immune globulin within 7 days of the first dose of rabies vaccine § Proportion of patients who received rabies immune globulin infiltration into and around the wound among patients who had a wound and documented rabies immune globulin administration sites.
Six immunocompromised patients were included. All six received rabies IG and their first dose of rabies vaccine during the ED visit. One patient was admitted to the hospital and received a second dose of rabies vaccine during the admission. At discharge, immunocompromised patients were referred to primary care doctors (N = 4), a geriatric medicine clinic (N = 1), and a family medicine clinic (N = 1) for follow-up to complete the rabies vaccination series.
Rabies IG dosing
Rabies IG doses ranged from 9.8 IU/kg to 22.0 IU/kg, and 98% (219 of 223) of patients appropriately received a dose that was within 10% of the Food and Drug Administration-approved dose of 20 IU/kg (18 IU/kg to 22 IU/kg) (). A transcription error by a pharmacist resulted in one dose of 10 IU/kg. Administration errors by nurses resulted in one dose of 9.8 IU/kg and one dose of 22 IU/kg. An order entry error by a provider resulted in one dose of 17.6 IU/kg. The rabies IG dose was rounded to the nearest vial size for 13% (30 of 223) of doses. Of the 30 doses where the total rabies IG dose-matched a vial size increment (multiple of 300 IU), one was a nurse administration error, one was a 20 IU/kg dose for a 60 kg patient that matched a vial size, 24 were rounded down to the nearest vial size, and four were rounded up to the nearest vial size.
Rabies IG timing of administration
Of 223 patients who received rabies IG, 223 patients (100%) received rabies IG within the first 7 days of the first dose of rabies vaccine as recommended by the CDC/ACIP guidelines (, ).
Rabies IG infiltration into and around the wound
Of 223 patients who received rabies IG, 33 patients did not have a wound, and 20 patients had missing documentation on rabies IG administration sites. Of the 170 patients included in this anatomical site of administration analysis, only 96 (56%) of eligible patients received infiltration of rabies IG into and around the wound (). Patient age, sex, medical facility type, provider type, animal type, wound location, and exposure type as bite, scratch, lick, or direct contact were not associated with infiltration of rabies IG into and around the wound among 170 eligible patients (). When wound location was collapsed to a binary variable to identify wounds on extremities (including arms and legs, but not including hands and feet), a wound location of extremity was associated with being more likely to have received infiltration of rabies IG into and around the wound (67% [53 of 79] for extremity versus 47% [43 of 91] for other wound locations, p = .01). As an exploratory analysis, a logistic regression model was developed to adjust for age category (pediatric, adult, or geriatric), provider type, medical facility, sex, animal, presence of a bite, and presence of a scratch. In this model, a wound location of extremity (arm or leg) was associated with infiltration of rabies IG into and around a wound (adjusted odds ratio of 2.1, 95% CI 1.1 to 4.2, P = .03).
Table 3. Association of covariates with rabies IG local wound infiltration.
For 33 patients who received rabies IG but who did not have a wound, at least some of the rabies IG dose was administered into the hip for 13 patients, deltoid for 10 patients, buttock for 6 patients, and thigh for 6 patients. The administration location was not documented for three patients.
Volume of rabies IG infiltration into and around the wound
Of 170 patients with wounds that could be infiltrated with rabies IG and clear documentation of anatomical sites where rabies IG was administered, only 143 patients had clear documentation of the exact volumes of rabies IG administered at each anatomical site and were included in this volume analysis. The mean total volume of rabies IG administered was 10.0 mL ± 3.6 mL. The mean volume infiltrated into and around the wound was 3.2 mL ± 4.3 mL, which accounted for 33% ± 43% of the total rabies IG dose administered. The full rabies IG dose was infiltrated into and around the wound for 26% (37 of 143) of patients, and none of the rabies IG dose was infiltrated into and around the wound for 52% (74 of 143) of patients. Among the 32 patients who received a partial rabies IG dose infiltrated into the wound, the mean total volume of rabies IG administered was 10.8 mL ± 3.8 mL, and the mean volume infiltrated into and around the wound was 3.4 mL ± 2.5 mL, which accounted for 32% ± 21% of the total dose.
Rabies IG administration near a rabies vaccine administration site
Both rabies IG and rabies vaccine were administered during the same medical encounter for 218 patients. Of these, the administration site for either rabies IG or rabies vaccination was not clearly documented for 32 patients. Among the 186 patients with clear documentation, administration of rabies IG and rabies vaccine were documented at the same administration site for 10% (N = 19) of patients, which may neutralize the effectiveness of rabies vaccine.Citation6,Citation22 The locations of co-administration of both rabies IG and rabies vaccine included the deltoid muscle (58%, 11 of 19), ventrogluteal injection site (32%, 6 of 19), and vastus lateralis injection site (11%, 2 of 19).
Rabies IG administration into the buttock
Rabies IG was injected into the buttock for 17% (37 of 223) of patients, and these administration sites were considered inappropriate. Patient age ranged from 5 to 84 years old. None of the 37 patients with rabies IG administration in the buttock were newborns, infants, or toddlers. A focused chart review of these 37 patients was unable to identify any documentation of sciatic nerve injury following rabies IG gluteal intramuscular injection during any of encounters at the health system for up to 21 days following rabies IG administration.
Discussion
In 2015, two patients in the United States and one patient in Puerto Rico died from rabies confirmed by virology testing after not receiving rabies postexposure prophylaxis.Citation29 Although the incidence of human rabies in the United States is low, it is almost always fatal after patients become clinically ill. Thus, appropriate postexposure prophylaxis is critical to prevent central nervous system infection and rapid death.Citation5,Citation20 This study evaluated adherence to the CDC/ACIP guideline on rabies IG patient selection, dosing, timing, and anatomical site of administration at a multi-hospital health system. Although the animals most commonly reported in this study were dog, bat, and cat, bats are the animals which are most commonly found to be infected with rabies in the United States and in Texas.Citation30 Nine percent (N = 21) of patients with an indication for rabies IG did not receive rabies IG. Although four patients refused rabies IG, the other 17 errors of omission may have been avoidable through additional provider education and EHR clinical decision support. All 223 doses of rabies IG were appropriately started within 7 days of the first dose of rabies vaccine, and 98% of rabies IG doses were given on the same day as the first dose of rabies vaccine. Although 98% of rabies IG doses were the correct dose of within 10% of 20 IU/kg, four dosing errors were identified. These types of dosing errors can be prevented with EHR safety enhancements related to dose calculation and communication between providers and nurses regarding prescribed rabies IG administration volume at each administration site.
Failure to infiltrate rabies IG into and around eligible wounds was the largest area of non-adherence to guideline recommendations observed in this study. Although 170 patients who received rabies IG were eligible to receive rabies IG into and around the wound, only 56% (N = 96) of patients actually received rabies IG infiltration into and around the wound. Administration of rabies IG into and around the wound is critical to maximize effectiveness, and treatment failures have been previously reported when rabies IG was not appropriately infiltrated into wounds.Citation31–Citation34 A study of 192 patients who received 40 IU/kg of equine rabies IG with confirmed infiltration into and around the wound reported that 39% (N = 75) of patients received full infiltration at the wound site, and 38% (N = 72) of patients received 50% or less of the rabies IG dose at the wound site.Citation35 Another study reported that a less than adequate volume of rabies IG was infiltrated into and around the wounds of 42% of patients who received postexposure prophylaxis in the emergency department in another region of the United States.Citation36 A new World Health Organization guideline published in April 2018 recommends infiltration of rabies IG into and around the wounds exclusively and no longer recommends injecting the remaining dose at distant muscles. This new recommendation underscores the perceived importance of infiltrating rabies IG into and around the wound.Citation37
Among 143 patients with wounds and documented volumes of rabies IG administration at each administration site, the proportion of rabies IG dose that was infiltrated into and around the wound was full (100%) for 37 patients, partial (>0% and <100%) for 32 patients, and none (0%) for 74 patients. Possible explanations for no infiltration into and around the wound include patient preference, anatomical complexity, lack of provider awareness of administration recommendations, or inaccurate EHR documentation. Patients receiving partial infiltration of rabies IG into and around the wound represent a situation where the provider demonstrated intention to perform appropriate infiltration but was unable to adequately infiltrate the full dose. This may have been due to concerns related to injection volume constraints, risk of compartment syndrome, or risk of injection site reactions.
During the study time frame, providers had access to a rabies IG product with a concentration of 150 IU/mL. In February 2018, a rabies IG product with a concentration of 300 IU/mL became available in the United States.Citation26,Citation38 Among these 32 patients with partial infiltration, only 32% of the volume of the rabies IG dose was infiltrated into and around the wound. This proportion could possibly have been doubled to 64% using the concentrated, 300 IU/mL product, without increasing the physical volume of rabies IG injected. These 32 patients represent 13% of the 246 patients who received rabies postexposure prophylaxis at our health system. Assuming that the study data is generalizable across the United States, up to 3,900 to 7,800 (13% of 30,000 to 60,000) patients who receive rabies postexposure prophylaxis in the United States annually would benefit from having access to 300 IU/mL concentration of rabies IG that may increase the proportion of rabies IG dose infiltrated into and around the wounds.Citation5
According to the CDC/ACIP guideline, any remaining volume of rabies IG that could not be infiltrated locally into a wound should be administered at a large muscle that is distant from the rabies vaccine administration site.Citation6 In this study, 9% of the patients who received rabies IG and the rabies vaccine during the same encounter received both products into the same muscle group, with the deltoid muscle being the most common location of co-administration.
Rabies IG should not be injected into the buttock due to the risk of sciatic nerve injury, and any remaining volume of rabies IG that could not be infiltrated into the wound should be injected in the deltoid muscle or into the lateral thigh muscle.Citation11,Citation13–Citation15,Citation26–Citation28 In this study, rabies IG was inappropriately administered into the buttock in 17% of the patients. This inappropriate administration technique may become less common in the future if providers adopt 2018 World Health Organization recommendations to infiltrate rabies IG into and around the wound exclusively and avoid administration of the remainder of the rabies IG dose at distant muscles.Citation37
During this study period, the health system did not use a structured order set in the EHR to provide clinical decision support for rabies postexposure prophylaxis. Investigators believe that this study’s data can be used to develop clinical decision support in the EHR coupled with a system-wide education campaign for emergency department clinical staff to improve adherence to CDC guideline recommendations. To maximize impact, the clinical decision support would need to first prompt providers to determine the vaccination status of the patient as no prior vaccination (common pathway) or prior vaccination (less common pathway). Among those identified as having no prior vaccination, the clinical decision support should display a bundled set of orders for rabies IG and rabies vaccine. Among those identified as having prior vaccination, only rabies vaccination monotherapy should be displayed. The clinical decision support for rabies vaccine should list the deltoid muscle as the preferred site of administration. The clinical decision support for rabies IG orders should remind providers and nurses to infiltrate as much of the rabies IG dose into and around the wound that is anatomically feasible, avoid the administration of rabies IG into the buttock, and avoid concurrent rabies IG administration in the same site as the rabies vaccine. The EHR should provide structured documentation fields to allow the provider to clearly document the exact volume of rabies IG that should be infiltrated into and around the wound and what volume should be administered at distant muscle sites. Additionally, the clinical decision support could display the appropriate phone number and prompt the provider to engage in a phone consult with a Texas public health authority priority to ordering rabies postexposure prophylaxis. A future study could develop and deploy this proposed intervention and measure its impact on adherence to guideline recommendations for rabies postexposure prophylaxis presented in this study.
Limitations
This study used secondary data that was collected and stored in the EHR for the primary purpose of supporting patient care and is subject to all inherent limitations of using this type of data such as non-standardized documentation by clinical staff and some missing data. Investigators attempted to minimize missing data from structured EHR fields by reviewing unstructured data sources such as typed and handwritten notes by clinical staff. Despite these efforts, 20 patients were excluded from the anatomical site of administration analysis because rabies IG administration sites were not clearly documented. Providers’ rationale for not infiltrating eligible wounds with rabies IG was not reliably documented in the EHR and could not be extracted and reported in this analysis.
Although this study did not provide long term follow-up to detect the development of rabies infection, no cases of human rabies were reported by the state of Texas to the CDC during the 2015 to 2018 study period.Citation5 The initiation of rabies postexposure prophylaxis and exposures to animals suspected of having rabies are not reportable events in Houston, Texas. Therefore, investigators were not able to estimate the overall incidence of rabies postexposure prophylaxis in Houston during the time frame of this study.
This study did not evaluate all animal exposures that were treated at our health system to determine which animal exposures did or did not qualify for rabies postexposure prophylaxis. Rather, this study evaluated utilization of rabies IG among patients who were determined to have a qualifying exposure by their treating provider, as indicated by the providers’ decision to administer rabies IG or rabies vaccine. Therefore, the prevalence of treatment for animal exposure and appropriateness of providers’ determination of which animal exposures qualify for rabies postexposure prophylaxis could not be evaluated by this study design.
Conclusion
In summary, our study evaluated adherence to CDC guideline recommendations for rabies IG patient selection, dosing, timing, and anatomical site of administration in the absence of clinical decision support in the EHR. Providers in the emergency department did not prescribe rabies IG for 9% of patients undergoing rabies postexposure prophylaxis who had an indication for rabies IG. Although rabies IG dosing and timing of dose were mostly adherent to guideline recommendations, many issues of non-adherence were identified with regard to anatomical location of rabies IG administration. Rabies IG was only infiltrated into and around the wound for 56% of patients with eligible wounds, which may limit the effectiveness of rabies IG. Rabies IG was administered into the buttock in 17% of 223 patients who received rabies IG, which may increase the risk for sciatic nerve damage. Among 186 patients with clear documentation of administration sites for both rabies vaccine and rabies IG during the same encounter, 10% of patients received administration of both rabies IG and rabies vaccine into the same muscle group, which may neutralize the effectiveness of rabies vaccine. This study data support the development of clinical decision support in the EHR to improve compliance with guideline recommendations with a special emphasis on guidance for anatomical location of administration for rabies IG.
Disclosure of potential conflicts of interest
JTS received research funding from Grifols© Shared Services North America that was used to support fellowship training for ER and TI. The authors do not have any additional financial disclosures that are related to this research. GSH, LNB, EIS, and ATT report no conflict of interest.
Author Contributions
* Dr. Hwang and Dr. Rizk are co-primary authors who contributed equally to the manuscript.
JTS and LNB conceived the study design. JTS obtained research funding. GSH, ER, and JTS developed data collection tools. GSH, ER, TI, EIS, and ATT collected data. JTS and ER supervised data collection and managed the data, including quality control. JTS analyzed the data. ER, GSH, and TI drafted the manuscript. All authors revised the manuscript and approved the final version of the manuscript.
Supplemental Material
Download MS Word (30.5 KB)Acknowledgments
The authors acknowledge Daniela Espino PharmD for providing valuable input regarding clinical documentation practices of emergency department nurses and physicians. The authors acknowledge Stephen Scholand, MD and Robert Garrison, DVM, MS for providing expert opinion regarding rabies IG infiltration best practices and for providing thoughtful feedback on the manuscript prior to submission.
Supplementary Materials
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Plotkin SA. Rabies. Clinical Infectious Diseases. 2000;30:4–12. doi:10.1086/313632.
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, et al. Human rabies prevention–United States, 2008: recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2008;57:1–28.
- Brunker K, Mollentze N. Rabies Virus. Trends in Microbiology. 2018;26:886–87. doi:10.1016/j.tim.2018.07.001.
- Centers for Disease Control and Prevention. Rabies-Exposure to the Virus. 2011. [Accessed 2019 January 11]. https://www.cdc.gov/rabies/transmission/exposure.html
- Centers for Disease Control and Prevention. Human Rabies. 2017. [Accessed 2019 Jan 10]. https://www.cdc.gov/rabies/location/usa/surveillance/human_rabies.html
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett SM, Levis R, Meltzer MI, Schaffner W, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR. 2010;59:1–9.
- Centers for Disease Control and Prevention. Human rabies despite treatment with rabies immune globulin and human diploid cell rabies vaccine–thailand. MMWR Morb Mortal Wkly Rep. 1987;36:759–60.
- Shill M, Baynes RD, Miller SD. Fatal rabies encephalitis despite appropriate post-exposure prophylaxis. The New England Journal of Medicine. 1987;316:1257–58. doi:10.1056/NEJM198705143162006.
- Fishbein DB, Sawyer LA, Reid-Sanden FL, Weir EH. Administration of human diploid-cell rabies vaccine in the gluteal area. The New England Journal of Medicine. 1988;318:124–25. doi:10.1056/NEJM198801143180219.
- Shaw FE Jr., Guess HA, Roets JM, Mohr FE, Coleman PJ, Mandel EJ, Roehm RR, Talley WS, Hadler SC. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine. 1989;7:425–30.
- Advisory Committee on Immunization Practices. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1994;43:1–38.
- Bharti OK, Sharma V. Failure of postexposure prophylaxis in a patient given rabies vaccine intramuscularly in the gluteus muscle, Himachal Pradesh, India. Ind J Critical Care Med. 2018;22:189–90. doi:10.4103/ijccm.IJCCM_521_17.
- Gilles FH, French JH. Postinjection sciatic nerve palsies in infants and children. The Journal of Pediatrics. 1961;58:195–204.
- Kline DG, Kim D, Midha R, Harsh C, Tiel R. Management and results of sciatic nerve injuries: a 24-year experience. Journal of Neurosurgery. 1998;89:13–23. doi:10.3171/jns.1998.89.1.0013.
- Jung Kim H, Hyun Park S. Sciatic nerve injection injury. J Inter Med Res. 2014;42:887–97. doi:10.1177/0300060514531924.
- Helmick CG, Johnstone C, Sumner J, Winkler WG, Fager S. A clinical study of Merieux human rabies immune globulin. J Biol Stand. 1982;10:357–67.
- Khawplod P, Wilde H, Chomchey P, Benjavongkulchai M, Yenmuang W, Chaiyabutr N, Sitprija V. What is an acceptable delay in rabies immune globulin administration when vaccine alone had been given previously? Vaccine. 1996;14:389–91.
- Vodopija I, Sureau P, Smerdel S, Lafon M, Baklaic Z, Ljubicic M, Svjetlicic M. Interaction of rabies vaccine with human rabies immunoglobulin and reliability of a 2-1-1 schedule application for postexposure treatment. Vaccine. 1988;6:283–86.
- Cabasso VJ, Loofbourow JC, Roby RE, Anuskiewicz W. Rabies immune globulin of human origin: preparation and dosage determination in non-exposed volunteer subjects. Bull World Health Organ. 1971;45:303–15.
- World Health Organization. Rabies. 2018. [Accessed 2019 January 10]. https://www.who.int/en/news-room/fact-sheets/detail/rabies
- Dean DJ, Baer GM, Thompson WR. Studies on the local treatment of rabies-infected wounds. Bull World Health Organ. 1963;28:477–86.
- Salva EP, Dimaano EM, Villarama JB, Suquilla JT. An evaluation of the safety and potency of equine rabies immunoglobulin through measurements of suppression on vaccine induced antibody production among healthy volunteers. Philip J Inte Med. 2014;52:1–7.
- Bailey AM, Holder MC, Baker SN, Weant KA. Rabies prophylaxis in the emergency department. Adv Emergency Nursing Journal. 2013;35:110–19. doi:10.1097/TME.0b013e31828f0b79.
- National Health Service. Clinical guidelines for immunoglobulin use (second edition update). 2012. [Accessed 2018 December 18]. https://www.nsd.scot.nhs.uk/Documents/clinimmumoMarch12.pdf
- Fahrenbruch R, Kintzel P, Bott AM, Gilmore S, Markham R. Dose Rounding of biologic and cytotoxic anticancer agents: a position statement of the Hematology/Oncology Pharmacy Association. J Oncol Pract. 2018;14:e130-e6.
- HyperRab® [rabies immune globulin (human)] [package insert]. Grifols Therapeutics Inc., Research Triangle Park (NC). 2018.
- HyperRab® S/D (rabies immune globulin [human]) [package insert]. Grifols Therapeutics Inc., Clayton (NC). 2012.
- Bergeson PS, Singer SA, Kaplan AM. Intramuscular injections in children. Pediatrics. 1982;70:944–48.
- Birhane MG, Cleaton JM, Monroe BP, Wadhwa A, Orciari LA, Yager P, Blanton J, Velasco-Villa A, Petersen BW, Wallace RM. Rabies surveillance in the United States during 2015. J Am Vet Med Assoc. 2017;250:1117–30. doi:10.2460/javma.250.10.1117.
- Ma X, Monroe BP, Cleaton JM, Orciari LA, Li Y, Kirby JD, Chipman RB, Petersen BW, Wallace RM, Blanton JD. Rabies surveillance in the United States during 2017. J Am Vet Med Assoc. 2018;253:1555–68. doi:10.2460/javma.253.12.1555.
- Bharti OK, Madhusudana SN, Gaunta PL, Belludi AY. Local infiltration of rabies immunoglobulins without systemic intramuscular administration: an alternative cost effective approach for passive immunization against rabies. Hum Vaccin Immunother. 2016;12:837–42. doi:10.1080/21645515.2015.1085142.
- Bharti OK, Madhusudana SN, Wilde H. Injecting rabies immunoglobulin (RIG) into wounds only: A significant saving of lives and costly RIG. Hum Vaccin Immunother. 2017;13:762–65. doi:10.1080/21645515.2016.1255834.
- Wilde H, Sirikawin S, Sabcharoen A, Kingnate D, Tantawichien T, Harischandra PA, Chaiyabutr N, de Silva DG, Fernando L, Liyanage JB, et al. Failure of postexposure treatment of rabies in children. Clin Infect Dis. 1996;22:228–32. doi:10.1093/clinids/22.2.228.
- Madhusudana SN, Aggarwal P, Tripathi KK. Failure of rabies postexposure treatment with purified chick embryo cell (PCEC) vaccine. Vaccine. 1989;7:478–79.
- Quiambao BP, Dy-Tioco HZ, Dizon RM, Crisostomo ME, Teuwen DE. Rabies post-exposure prophylaxis with purified equine rabies immunoglobulin: one-year follow-up of patients with laboratory-confirmed category III rabies exposure in the Philippines. Vaccine. 2009;27:7162–66. doi:10.1016/j.vaccine.2009.09.036.
- Jerrard DA. The use of rabies immune globulin by emergency physicians. The Journal of Emergency Medicine. 2004;27:15–19. doi:10.1016/j.jemermed.2004.02.005.
- World Health Organization. Weekly epidemiological record, No 16. 2018. [Accessed 2019 April 17]. https://apps.who.int/iris/bitstream/handle/10665/272371/WER9316.pdf?ua=1
- Hanna K, Cruz MC, Mondou E, Corsi E, Vandeberg P. Safety and neutralizing rabies antibody in healthy subjects given a single dose of rabies immune globulin caprylate/chromatography purified. Clinical Pharmacology: Advances and Applications. 2018;10:79–88. doi:10.2147/CPAA.S166454.
