ABSTRACT
This study was planned to evaluate whether a 3-month treatment with Lactobacillus rhamnosus GG (LGG) can modify immune system functions in children and adolescents with type 1 diabetes (T1D), leading to an increased immune response to an injectable quadrivalent inactivated influenza vaccine (QIV). A total of 87 pediatric patients with T1D were screened, although 34 patients in the Probiotic group and 30 in the Control group accepted to be vaccinated with QIV and completed the study. Vaccine immunogenicity and safety and the inflammatory cytokine response were studied. Results showed that QIV was immunogenic and safe in T1D pediatric patients and pre-administration of LGG for three months did not substantially modify the QIV humoral immunity. The combination of QIV and LGG reduced inflammatory responses (i.e., IFN-γ, IL17A, IL-17F, IL-6, and TNF-α) from activated PBMCs of pediatric patients with T1D, without dampening the production of seroprotective antibodies. In conclusion, QIV is associated with an adequate immunogenicity in children and adolescents with T1D in presence of a good safety profile. Although a systematic administration of LGG did not result in an improvement of humoral responses to an influenza vaccine, the probiotic did induce important anti-inflammatory effects.
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease characterized by a selective destruction of pancreatic β-cells, leading to insulin deficiency. T1D is associated with an increased risk of infection-related complications, including those associated with influenza. Epidemiological evaluations carried out during seasonal epidemics and the recent 2009 pandemic have shown that patients with T1D, infected by influenza viruses, can face a severe and complicated disease, be hospitalized, and die more frequently than healthy subjects.Citation1 To reduce the total burden of influenza in T1D patients, annual influenza vaccination is recommended for individuals with this disease by all the health authorities.Citation2–Citation5
However, whether influenza vaccines will confer true protection to T1D patients has not precisely defined yet.Citation6 Data collected in T1D patients are scarce. Moreover, results of studies carried out in T1D patients are conflicting.Citation7–Citation9 Seroconversion and satisfactory immune responses to all influenza vaccine antigens have been reported by studies in which adjuvanted vaccines have been used.Citation7,Citation8 On the contrary, in a study performed with the traditional inactivated trivalent influenza vaccine, it was shown that the incidence of non-responders to two vaccine components was significantly increased in T1D patients than in healthy controls.Citation9 Moreover, in the same study, delayed type hypersensitivity reaction to influenza antigens was found to be decreased in patients with elevated glycosylated haemoglobin (HbA1c) concentrations.Citation9 This finding, besides highlighting the existence of a relationship between current glycemic control and protection evoked by immunization, indicated that some T1D patients cannot be protected by influenza vaccines.Citation7–Citation9 Therefore, as a whole, previous studies put into question the efficacy of influenza vaccines in T1D patients.
To improve immune responsiveness to influenza vaccines, several measures have been suggested, including the probiotic use. Probiotics are defined as live microorganisms that, when administered in adequate amounts, will confer a health benefit on the host without any clinical adverse effect.Citation10,Citation11 Among the beneficial effects, probiotics affect the gut microbiome,Citation12 which might be useful to potentiate immune response to vaccines, including those against the influenza virus. Studies in this regard seem to suggest that immunogenicity of both traditional trivalent inactivated influenza vaccine and live attenuated vaccine is significantly increased by the administration of probiotics in healthy adults.Citation13,Citation14 In addition, the use of probiotics can reduce or eliminate gut dysbiosis and consequent cytokine-dependent inflammation, particularly that mediated by interleukin (IL)-17− producing CD4+ T helper (Th17) cells, which stimulate autoimmune responses.Citation15,Citation16 However, which probiotic can lead to the highest immune stimulation and by which schedule (i.e., dose and duration of treatment) it should be administered have not been defined yet. Moreover, studies in younger patients with T1D are currently lacking. This study was planned to evaluate whether a previous 3-month period of Lactobacillus rhamnosus GG (LGG) can modify immune system functions in children and adolescents with T1D in order to lead to an increased immune response to an injectable quadrivalent inactivated influenza vaccine (QIV).
2. Patients and methods
2.1 Study design and population
A prospective, randomised, and single-blind study was carried out at the Pediatric Clinic of University of Perugia, Perugia, Italy, between August 1, 2017 and March 31, 2018. The protocol was approved by the Ethics Committee of Umbria Region and the study was conducted in accordance with the standards of Good Clinical Practice for trials of medicinal products in humans. Written informed consent was obtained from the parents/legal guardian of each enrolled child and from every enrolled subjects aged ≥8 years.
The study evaluated the role of a previous 3-month administration of LGG on immunogenicity and safety of a standard intramuscular dose of a split-virion quadrivalent influenza vaccine (Fluarix tetra, GSK, Italy) in 3–18 years old subjects with T1D. summarizes study design and population. Patients were selected on the basis of a computer-generated randomization list from those regularly followed by the Regional Center for T1D of Umbria Region among those undergone at least one previous influenza vaccination with the traditional trivalent inactivated vaccine (TIV) in previous years. Patients with any serious chronic disease, including those considered complication of T1D (i.e., signs of cardiac or renal failure, severe malnutrition, or obesity), Down syndrome or other known cytogenetic disorder, or a known or suspected disease of the immune system were excluded. Patients who had received immunosuppressive therapy, including systemic corticosteroids for more than 14 days, and those with a documented history of hypersensitivity to egg, egg protein, or any other component of the vaccine were also excluded.
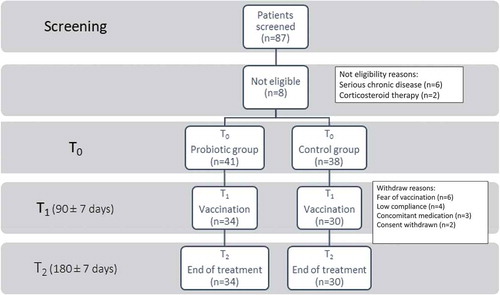
Figure 1. Flow-chart of the study population.
A total of 87 pediatric patients with T1D were screened, although 8 (10.1%) were not eligible. Among the remaining 79 patients, 41 were randomized to receive probiotics (Probiotic group) and 38 were assigned to the Control group. However, 7 patients in the Probiotic group and 8 in the Control group were not vaccinated and withdrew from the study for various reasons. A total of 34 patients in the Probiotic group and 30 in the Control group were vaccinated with QIV and all of them completed the study.
Starting from August 1, 2017, selected patients were randomised 1:1 to receive LGG (Dicoflor 60 Immuno D3, Dicofarm, Italy), containing 1 billion LGG/drop, or placebo (with similar formulation but not containing the probiotic) by a data manager not involved in the clinical follow-up. Patients assigned to the experimental arm received five drops of probiotic twice per day for three months before and after vaccination, whereas control subjects were given five drops of placebo twice per day for the same periods of time. The study was conducted in blinded fashion by using identically labelled packaging for probiotic and placebo. Randomization codes were revealed to the staff at the data monitoring center, who had no contact with the patients; similarly, physicians involved in clinical monitoring (SE, SB) were blinded to the treatment assignment. Six numbered bottles were provided to the enrolled subjects, each bottle being suitable for a 15− day treatment. In order to monitor the compliance of the study regimen, bottles had to be returned within seven days after the end of the 3-month treatment.
Upon returning to the center, enrolled subjects were vaccinated against influenza by means of an injection in the deltoid region of the left arm. Vaccine components for the 2017–2018 Northern hemisphere influenza season included an A/Michigan/45/2015 (H1N1) pdm09-like virus; an/Hong Kong/4801/2014 (H3N2)-like virus; a B/Brisbane/60/2008-like virus (lineage B/Victoria); and a B/Phuket/3073/2013-like virus (lineage Yamagata). On the day of vaccination, enrolled subjects were provided with further six bottles containing probiotic or placebo, thus ensuring the 3-month post-vaccination treatment.
Blood samples for monitoring T1D (i.e., insulin levels and HbA1c percentage) were collected at the time of enrollment, when patients started the probiotic or placebo pre-treatment (time 0), 90 ± 7 days later (time 1; T1) at the time of vaccine administration, and after 180 ± 7 days (time 2; T2), when patients had completed the probiotic or placebo post-treatment. Blood samples harvested at T1 and T2 were also used to evaluate the inflammatory cytokine response.
Safety of the probiotic was monitored within the administration period by registering every clinical symptom that the enrolled subject could associate with the treatment in a dedicated card. Safety of influenza vaccine was measured by collecting data concerning any local and systemic reactions (by the investigators in the first hour after administration, and by the children’s parents/legal guardian for the 14 days after vaccination using a specific diary card). Patients were evaluated for the occurrence of local adverse events (AEs: erythema, swelling/induration, and pain) and questioned about systemic AEs (body temperature ≥ 38°C, rhinitis, irritability, sleepiness, changed eating habits, vomiting, and diarrhoea). Families of each child were also asked to use the previously prepared diary card to assess the type and number of medically-diagnosed upper respiratory tract infections (URTIs: acute pharyngitis, acute otitis media, croup), lower respiratory tract infections (LRTIs: acute bronchitis, wheezing, pneumonia), other infections, antibiotic courses, and lost school days during the study period.
2.2 Assessment of vaccine immunogenicity
Titers of hemagglutination-inhibiting (HI) antibodies against each of the four influenza strains contained in the 2017–2018 influenza vaccine formulation were determined in all children before vaccine administration (T1) and 90 ± 7 days later (T2). Serum samples taken from the same subject and frozen at −20°C were tested simultaneously for HI antibodies titers against the egg-grown vaccine antigens. HI titers were determined by a standard microtiter method using 0.5% turkey erythrocytes. All sera were treated with receptor-destroying enzyme and heat-inactivated at 56°C for 30 min to remove non-specific inhibitors. Results were expressed as the reciprocal of the highest dilution inhibiting agglutination. In order to calculate geometric mean titers (GMTs), a titer of 1:5 was arbitrarily assigned to non-responders. Immunogenicity end-points were based on the hemagglutination inhibition licensure criteria established by the guideline of the European Medicines Agency (EMA), according to standard methods.Citation17,Citation18 As there are no EMA-defined criteria for children, as previously reported,Citation19,Citation20 immunogenicity was evaluated using the criteria for adults aged 18–60 years, which require at least one of the following for each strain: (1) seroconversion, a ≥ 4− fold increase in HI antibody titer with a titer of ≥ 1:40 being reached in > 40% of the subjects; (2) seroprotection, an HI antibody titer of ≥ 1:40 in > 70% of the subjects; and (3) GMT, a > 2.5− fold increase in the GMT of HI antibodies.
2.3 Determination of the cytokine profile in activated peripheral blood mononuclear cells (pbmcs)
Peripheral blood mononuclear cells (PBMCs) were isolated on a Ficoll-Hypaque gradient from enrolled subjects at both times, T1 and T2. PBMCs (1 × 106 cells/mL) were cultured in vitro in RPMI medium supplemented with 10% FCS, 1 mM glutamine and penicillin/streptomycin (Gibco, Invitrogen CA, USA). Cells were exposed to 1 μg/mL of phytohemagglutinin (PHA; Sigma-Aldrich, MO, USA) or medium alone for 48 hours at 37°C in a humidified 7% CO2 incubator, before harvesting culture supernatants for assessment of cytokine production.
The cytokine profile was analyzed in supernatants from PBMCs either unstimulated or stimulated with PHA by using a 15-plex immunoassay (Bio-Plex Pro Human Th17 cytokine panel, Cat N. 171aa001m; Bio-Rad, CA, USA) and a MAGPIX system (Luminex Corporation). Cytokine levels were represented as the mean ± standard deviation (SD) of the fold changes (i.e., the ratio between cytokine concentrations in PHA-stimulated and unstimulated PBMCs) or as the mean ± SD of the concentrations (pg/mL).
2.4 Statistical analyses
For the primary objective, i.e., comparing immunogenicity of the influenza vaccine in T1D patients that had been pre-treated with LGG or placebo, the null hypothesis was that the treatment with probiotics would increase the seroconversion rate relative to those of the control group. Sample size was determined on the assumption of a seroconversion rate in the control group of 50% and a seroconversion rate for subjects in the probiotic group of 85%. To be able to reject the null hypothesis that the failure rates for experimental and control subjects are equal with a power of 0.8, each group had to enroll 32 patients. All inferential statistical comparisons were based on two-sided tests with alpha = 0.05.
Categorical data were analyzed using contingency tables and the chi-square or the Fisher’s exact test, as appropriate. Pre- and post-vaccination serum antibody titers were used to calculate GMTs, geometric mean fold increase (GMFIs) in serum antibody titers, and seroconversion rates. GMFI was calculated as the geometric mean of post-vaccination serum HI fold-rise from pre-vaccination (ratio of post-vaccination/pre-vaccination) of each subject. Pooled GMTs, GMFRs, and serum responsiveness rates were presented by vaccine type/subtype/lineage (A/H1N1, A/H3N2, B/Brisbane and B/Phuket), participant age, and baseline serostatus. Standard descriptive statistics was used to describe baseline patient characteristics. Central tendency and variability were expressed as mean (±SD) or median (min-max) for continuous variables and number (percent) for categorical variables as appropriate. All statistical analyses were performed using STATA version 14 (STATA Corp., Texas, USA). Categorical data were analyzed using contingency tables and the chi-square or the Fisher’s exact test as appropriate. All analyses were two-tailed, and p values of ≤ 0.05 were considered significant.
3. Results
3.1 Demographic and disease features of the study population
We screened 87 pediatric patients with T1D, although 8 (9.2%) were not eligible. Among the remaining 79 patients, 41 were randomized to receive probiotics (Probiotic group) and 38 were assigned to the Control group. However, 7 patients in the Probiotic group and 8 in the Control group were not vaccinated and withdrew from the study for the following reasons: 6 for fear of vaccination, 4 for low compliance with assigned treatment, 3 for concurrent illness, and 2 for withdrawn consent (). There were no statistically significant differences between the patients who were not randomized and those who were randomized in terms of age, gender, corporeal measures, race, and parental education.
A total of 34 patients in the Probiotic group and 30 in the Control group were vaccinated with QIV and all of them completed the study. T1D was diagnosed at a mean age of 7.97 years (range, 1–16 yrs) in the Probiotic group and at 7.91 years (range, 1–15 yrs) in the Control group. shows the demographic baseline characteristics of the study population who received QIV and completed the study. There were no statistically significant differences between the two groups in terms of age, gender, corporeal measures, race, and parental education. None had received an immunosuppressive therapy in the previous six months.
Table 1. Baseline characteristics of children and adolescents with type 1 diabetes (T1D) vaccinated with inactivated quadrivalent influenza vaccine (QIV) according to assumption (Probiotic group) or no assumption (Control group) of Lactobacillus rhamnosus GG.
T1D specific features, like HbA1c blood levels, were similar in the two groups (). Approximately 70% of patients in both groups showed an HbA1c level lower than 8.5%. The T0 HbA1c level appeared significantly higher in the Probiotic group (8.45 vs 7.7, p value 0.01), a difference that persisted also at T2 (p value 0.03). No relationship between the effect of probiotic administration and HbA1c level was observed. Few patients in both groups presented diabetes complications and concomitant diseases.
Table 2. Parameters associated with clinical and laboratory complications in children and adolescents with type 1 diabetes (T1D) vaccinated with inactivated quadrivalent influenza vaccine (QIV) according to assumption (Probiotic group) or no assumption (Control group) of Lactobacillus rhamnosus GG.
3.2 Effects of probiotic administration on the humoral response of influenza vaccination in pediatric patients with T1D
shows the antigen-specific, humoral responses of the two groups after the vaccine administration. Approximately 60% of subjects in the Probiotic group had baseline-specific antibody titers ≥ 40 upon HI assay against the A/H1N1 and B/Brisbane influenza viruses, more than 90% for A/H3N2, and less than 30% for B/Phuket. In the Control group, approximately 50% of the subjects had baseline-specific antibody titers ≥ 40 upon HI assay against the A/H1N1 and B/Phuket influenza viruses, whereas about 70% had a seroprotection for B/Brisbane and more than 90% for A/HongKong.
Table 3. Geometric Mean Titer (GMT) and Haemagglutination inhibition (HI) antibody responses (seroprotection and seroconversion) against seasonal influenza strains in children and adolescents with type 1 diabetes (T1D) vaccinated with inactivated quadrivalent influenza vaccine (QIV) according to assumption (Probiotic group) or not (Control group) of Lactobacillus rhamnosus GG.
Three months after vaccine administration, almost all patients were seroprotected for both A strains () and no statistically significant differences were found between the two groups. Almost 90% of subjects of both groups reached a seroprotection for B/Brisbane. For B/Phuket, in the Probiotic group, the reached level of seroprotection (about 80%) was less high, although the difference with Control group was not statistically significant (29.4% at T1 to 76.5% at T2 in the Probiotic group vs 40.0% at T1 to 83.3% at T2 in the Control group). The Probiotic and Control group showed a seroconversion rate of 79.4% and 66.7% against A/H1N1, 44.1% and 46.7% against A/H3N2, respectively, with no significant difference between groups. There was a similar seroconversion rate against the B strain in the Probiotic group as compared to the Control group (67.6% vs 56.7% for the B/Brisbane and 55.9% vs 46.7% for the B/Phuket, respectively), although without a statistically significant difference.
GMTs and fold increase against A and B strains were not different between the groups.
3.3 Effects of probiotic administration on safety of influenza vaccination in pediatric patients with T1D
Solicited and unsolicited local and systemic AEs were similar between the two groups. During the probiotic treatment, fever (i.e., axillary temperature) was reported only in one child belonging to the Probiotic group, and fever for less than 24 hours was recorded in two control subjects. Two patients in the Probiotic group experienced abdominal pain, whereas none was reported in the Control group. After vaccination, three patients reported rhinitis (two in the Probiotic group and one in the Control group). Three subjects needed one antipyretic dose (two in the Probiotic group and one in Control group). One patient in the Control group, and none in the Probiotic group, reported diarrhea for one day. No antibiotic assumption was recorded.
During the study period, URTIs and LRTIs were reported by three and one patients in the Probiotic group respectively (2 pharyngitis, 1 acute otitis media, 1 acute bronchitis) and by 4 and 2 patients in the Control group respectively (2 pharyngitis, 2 acute otitis media, 1 acute bronchitis, 1 wheezing).
3.4 Effects of probiotic administration and influenza vaccination on cytokine production by pbmcs from pediatric patients with T1D
To evaluate possible effects of the influenza vaccine (in the presence or absence of probiotic administration) on cellular immunity, the production of multiple cytokines was measured in culture supernatants of purified PBMCs stimulated with the PHA mitogen. Secretion of soluble CD40 ligand (sCD40L), a costimulatory molecule often associated with inflammatory/autoimmune diseases was also monitored.Citation21–Citation23 Results showed that PHA failed to significantly increase the production of IL-10, IL-17A, IL-6, and tumor necrosis factor α (TNF-α) in PBMCs from patients having received the probiotic treatment alone, i.e., prior vaccination (T1), but not the control treatment. Moreover, although still significant, the increase in interferon γ (IFN-γ) and IL-17F was less in PBMCs from patients having received the probiotic as compared to controls (). At three months after vaccination (T2), no difference in cytokine profile could be observed in the two groups (i.e., receiving or not receiving the probiotic), with IL-17F being the only cytokine significantly upregulated in PBMCs from both groups after PHA-activation (). However, when comparing the overall cytokine profile between T1 and T2 conditions in each group (i.e., prior and after vaccination; ), a general reduction in the PHA activation of PBMCs from the Control group (i.e., receiving the vaccine alone) could be observed. Interestingly, the probiotic administration significantly contained the production of IL-17F prior (T1) and after (T2) vaccination in PHA-activated PBMCs, as compared to controls, while IFN-γ was significantly restrained by the probiotic treatment only at the T1 ().
Figure 2. Cytokine profile in T1D PBMCs after activation with PHA.
Cytokine levels were determined in culture supernatants of T1D PBMCs after stimulation with PHA, from control and probiotic groups before (T1, panel A) and after (T2, panel B) vaccination. Cytokine levels are represented as the mean ± SD of the fold changes (i.e., the ratio between cytokine concentrations in PHA-stimulated and unstimulated PBMCs). Cytokine concentrations are analyzed by two-tailed unpaired Student’s t test (PHA-stimulated versus unstimulated). * P < .05; ** P < .01.
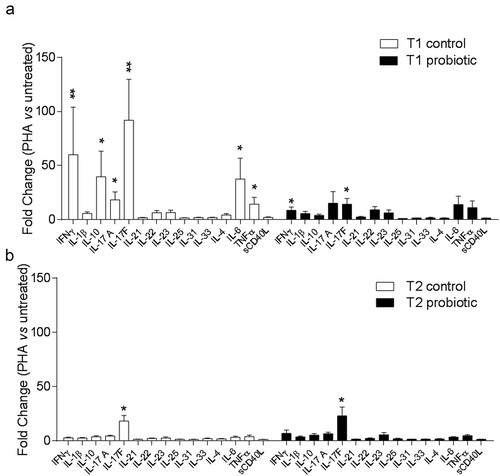
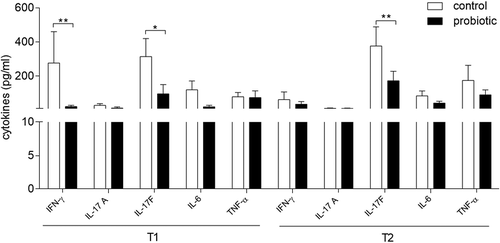
Figure 3. Levels of selected proinflammatory cytokines in PHA-activated PBMCs.
Cytokine levels were determined in culture supernatants of T1D PBMCs after stimulation with PHA, from control and probiotic groups before (T1) and after (T2) vaccination. Cytokine levels are represented as the mean ± SD of the concentrations (pg/ml) and are analyzed by two-tailed unpaired Student’s t test (probiotic versus control). * P < .05; ** P < .01.
4. Discussion
This study aimed at evaluating efficacy, safety, and tolerability of the QIV in combination with LGG probiotic treatment in children and adolescents with T1D. Results showed that (i) QIV is immunogenic and safe; (ii) pre-administration of LGG for three months does not substantially modify the QIV humoral immunity; (iii) both QIV and LGG reduce inflammatory responses of blood immune cells.
LGG, together with some other strains of the same species, is among the most investigated probiotic bacteria with established human health efficacy data.Citation24–Citation26 Starting from these premises, administration of LGG could be expected to act as an immunoadjuvant for vaccines,Citation26,Citation27 including those against influenza infection. In addition, a better immune response to vaccines has been evidenced in subjects that were fed with milk supplemented with LGG. Addition of 10 billions per day of LGG for five weeks to a chemically acidified clotted milk increased neutralizing antibody titers against poliovirus serotype 1 and affected the formation of specific IgA and IgG in serum of individuals given the trivalent oral polio vaccine during the second week of the study.Citation28 A better immune response to hepatitis B vaccine given in the first months of life was evidenced in children fed with a formula supplemented with probiotics including Lactobacillus rhamnosus LRB at the dose of 280 millions/day from birth to the end of the 6th month.Citation29
However, contrarily to what has been demonstrated for other probiotics,Citation30 there is limited evidence on the impact of LGG on response to influenza vaccines. In a double-blind, placebo controlled, study carried out in adults who had received live attenuated influenza vaccine followed by LGG for a month, it was shown that greater seroprotection rates in comparison to controls were achieved only for the H3N2 vaccine strain, whereas no differences were evidenced for H1N1 and B strains.Citation31 Differences among studies are difficult to explain, as several factors may have conditioned the immune response to the various vaccines. Antibody production depends on the antigen and host characteristics, on type of adjuvant as well as dose and duration of vaccine administration. Dose, in particular, seems very critical. Evaluation of the effects of Lactobacillus rhamnosus Lcr35 on human monocyte-derived immature dendritic cells (DCs) led to the conclusion that upregulation of several genes involved in immune responses could occur only at high doses. Maturation of DCs, secretion of cytokines (TNFα, IL-1β, IL-12p70, IL-12p40, and IL-23) and a strong pro-inflammatory effect were dose-related.Citation32 In our study, 10 billions/day of LGG were given. This and even lower doses have been found effective in the prevention and treatment of acute diarrhea,Citation33,Citation34 in other gastrointestinal diseases,Citation35 and in the prevention of allergic diseases.Citation36 In our study, three months after vaccine injection, seroconversion and seroprotection rates and GMT against A/H1N1 and A/H3N2 vaccine antigens were high and quite similar in treated patients and in controls given placebo. Antibodies against B influenza virus antigens were slightly higher in patients receiving LGG, but differences did not reach statistical significance. Therefore, we cannot exclude that the dose administered to our T1D patients was too low to obtain a significant stimulation of the immune system such to increase humoral responses to the administered influenza vaccine. This means that further studies are needed to evaluate whether, how and when LGG can be administered to increase humoral immunity of T1D patients against influenza vaccine antigens.
However, the analysis of the cytokine profile in whole PBMCs activated by PHA indicated that the used dose of LGG is not absolutely ineffective. In fact, patients’ pretreatment with the probiotic alone (T1) significantly reduced the activation of PBMCs by PHA in terms of upregulation of proinflammatory cytokines, such as IFN-γ, IL17A, IL-17F, IL-6, and TNF-α, as compared to control patients. Moreover, the IL-17F production by PHA-activated PBMCs was contained by the probiotic treatment prior (T1) and after vaccination (T2). Therefore, on one hand, we might hypothesize that the used dose of LGG did not potentiate QIV immunogenicity because of its intrinsic anti-inflammatory effects, similarly to what observed in allergic diseases.Citation36 On the other, the observed LGG effects could be beneficial for patients affected by an autoimmune disease such as T1D. Although we cannot exclude the occurrence of changes in the proportion of distinct T cells subsets (i.e., including Th17 cells), the negative modulation by LGG of the cytokine production by PHA-activated PBMCs may resemble the ‘T-cell exhaustion’ phenomenon, which has been suggested as a potential therapeutic strategy for patients with autoimmune diseases, including T1D.Citation37–Citation39 Moreover, although PHA constitutes a means of activating polyclonal T cells, the observed down-modulated production of IL-6 and TNF-α by the probiotic would indicate that the use of whole PBMCs would allow to detect effects also on other cells, possibly antigen-presenting cells such as dendritic cells and macrophages. Interestingly, recent data provided by this and ananother group indicated that a high IL-6 signaling is pathogenetic in pediatric autoimmune diabetes and maneuvers aimed at blocking the activation of the IL-6 receptor will restore immunoregulatory pathways in PBMCs fromthese patients.Citation40,Citation41
The QIV vaccine itself induced similar immunomodulatory effects on PHA-activated PBMCs (T2, Control group) despite an effective induction of protective anti-influenza antibodies by B cells, which do require the help of T cells in their function. Therefore, although we did not measure the cytokine production by influenza virus-specific T cells, our data may indicate that the use of either QIV or LGG can restrain ongoing autoimmune responses but not the activation of naïve or memory effective T cell clonotypes to influenza antigens. Would this be true, LGG could be even better than QIV because it was more potent in reducing the release of IL-17F, a cytokine produced by pathogenetic Th17 cells in T1D.Citation42,Citation43 As a whole, these data indicated that either probiotic treatment or influenza vaccination reduces the production of several standard inflammatory cytokines (namely, IFN-γ, IL17A, IL-17F, IL-6, and TNF-α) from PHA-activated PBMCs of pediatric patients with T1D, without dampening the production of seroprotective antibodies. Although generally recognized as an immunoregulatory cytokine, IL-10 may also have proinflammatory effects in chronic inflammationCitation23 and therefore its reduction − observed here − may be relevant for the T1D disease.
This study has the limitation that immune response to QIV was evaluated before vaccination and 3 months after in order to avoid, for ethical reasons, blood exams in addition to those required for T1D control. However, although it is possible that the inflammatory response is different at one and three months after a vaccine dose, QIV showed a good immunogenicity in our patients and it is not expected that an analysis performed one month post-vaccination could be associated with differences between the groups. In addition, another limitation is represented by the fact that the number of study patients was quite limited.
5. Conclusions
Our study showed that QIV is associated with an adequate immunogenicity in children and adolescents with T1D in presence of a good safety profile. Although a systematic administration of LGG did not result in an improvement of humoral responses to an influenza vaccine, the probiotic did induce anti-inflammatory effects that might deserve further investigations for the control of autoimmunity in T1D children and adolescents.
Disclosure of potential conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Funding
References
- Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi:10.1136/bmj.f5061.
- World Health Organization. Influenza. Vaccine use. Accessed October 30, 2018. http://www.who.int/influenza/vaccines/use/en/
- Centers for Disease Control and Prevention. Influenza (Flu). Influenza and people with diabetes. Accessed October 30, 2018. https://www.cdc.gov/flu/diabetes/index.htm
- European Center for Disease Prevention and Control. Guidance. Priority risk groups for influenza vaccination. Accessed October 30, 2018. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0808_GUI_Priority_Risk_Groups_for_Influenza_Vaccination.pdf
- Esposito S, Principi N. European Society of Clinical Microbiology Infectious Diseases (ESCMID) Vaccine Study Group (EVASG). Influenza vaccination and prevention of antimicrobial resistance. Expert Rev Vaccines. 2018;17:881–88. doi:10.1080/14760584.2018.1525298.
- Goeijenbier M, van Sloten TT, Slobbe L, Mathieu C, van Genderen P, Beyer WEP, Osterhaus ADME. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35:5095–101. doi:10.1016/j.vaccine.2017.07.095.
- Zuccotti GV, Scaramuzza A, Riboni S, Mameli C, Pariani E, Tanzi E, Zanetti A, Radaelli G. Long-lasting immunogenicity of a virosomal vaccine in older children and young adults with type I diabetes mellitus. Vaccine. 2009;27:5357–62. doi:10.1016/j.vaccine.2009.06.082.
- Zuccotti GV, Pariani E, Scaramuzza A, Santoro L, Giani E, Macedoni M, Gazzarri A, Anselmi G, Amendola A, Zanetti A. Long-lasting immunogenicity and safety of a 2009 pandemic influenza A(H1N1) MF59-adjuvanted vaccine when co-administered with a 2009-2010 seasonal influenza vaccine in young patients with type 1 diabetes mellitus. Diabet Med. 2011;28:1530–36. doi:10.1111/j.1464-5491.2011.03449.x.
- Diepersloot RJ, Bouter KP, Beyer WE, Hoekstra JB, Masurel N. Humoral immune response and delayed type hypersensitivity to influenza vaccine in patients with diabetes mellitus. Diabetologia. 1987;30:397–401.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi:10.1038/nrgastro.2014.66.
- Principi N, Cozzali R, Farinelli E, Brusaferro A, Esposito S. Gut dysbiosis and irritable bowel syndrome: the potential role of probiotics. J Infect. 2018;76:111–20. doi:10.1016/j.jinf.2017.12.013.
- Yousefi B, Eslami M, Ghasemian A, Kokhaei P, Salek Farrokhi A, Darabi N. Probiotics importance and their immunomodulatory properties. J Cell Physiol. 2018. Epub Oct 14 doi:10.1002/jcp.27559
- Lei WT, Shih PC, Liu SJ, Lin CY, Yeh TL. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9:E1175. doi:10.3390/nu9111175.
- Yeh TL, Shih PC, Liu SJ, Lin CH, Liu JM, Lei WT, Lin CY. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:217–30. doi:10.2147/DDDT.S155110.
- Insel R, Knip M. Prospects for primary prevention of type 1 diabetes by restoring a disappearing microbe. Pediatr Diabetes. 2018;19:1400–06. doi:10.1111/pedi.12756.
- Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, Lernmark Å, Hagopian WA, Rewers MJ, She J-X, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589–94. doi:10.1038/s41586-018-0620-2.
- WHO Global Influenza Surveillance Network. The WHO Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. Accessed May 21, 2019. http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf
- European Medicines Agency. Guideline on Influenza Vaccine. Accessed May 21, 2019. https://www.ema.europa.eu/en/ema-redirect?redirect_type=document&lang=en&doc_id=WC500211324&doc_ext=pdf
- Esposito S, Giavoli C, Trombetta C, Bianchini S, Montinaro V, Spada A, Montomoli E, Principi N. Immunogenicity, safety and tolerability of inactivated trivalent influenza vaccine in overweight and obese children. Vaccine. 2016;34:56–60. doi:10.1016/j.vaccine.2015.11.019.
- Esposito S, Mastrolia MV, Ghio L, Paglialonga F, Terranova L, Scala A, Edefonti A, Principi N. Influenza immunization in hemodialyzed or kidney transplanted adolescents and young adults. Expert Rev Vaccines. 2014;13:1059–66. doi:10.1586/14760584.2014.935768.
- Filion LG, Matusevicius D, Graziani-Bowering GM, Kumar A, Freedman MS. Monocyte-derived IL12, CD86 (B7-2) and CD40L expression in relapsing and progressive multiple sclerosis. Clin Immunol. 2003;106:127–38.
- Ludwiczek O, Kaser A, Tilg H. Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis. 2003;18:142–47. doi:10.1007/s00384-002-0425-4.
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–77. doi:10.4049/jimmunol.180.9.5771.
- Shah NP. Functional cultures and health benefits. Int Dairy J. 2007;17:1262–77. doi:10.1016/j.idairyj.2007.01.014.
- Brusaferro A, Cavalli E, Farinelli E, Cozzali R, Principi N, Esposito S. Gut dysbiosis and paediatric Crohn’s disease. J Infect. 2019;78:1–7.
- Fang H, Elina T, Heikki A, Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29:47–52. doi:10.1111/j.1574-695X.2000.tb01504.x.
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–12.
- De Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005;44:406–13. doi:10.1007/s00394-004-0541-8.
- Soh SE, Ong DQ, Gerez I, Zhang X, Chollate P, Shek LP, Lee BW, Aw M. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28:2577–79. doi:10.1016/j.vaccine.2010.01.020.
- Zimmermann P, Curtis N. The influence of probiotics on vaccine responses - A systematic review. Vaccine. 2018;36:207–13. doi:10.1016/j.vaccine.2017.08.069.
- Davidson LE, Fiorino AM, Snydman DR, Hibberd PL. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65:501–07. doi:10.1038/ejcn.2010.289.
- Evrard B, Coudeyras S, Dosgilbert A, Charbonnel N, Alamé J, Tridon A, Forestier C, Heimesaat MM. Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PLoS One. 2011;6:e18735. doi:10.1371/journal.pone.0018735.
- Szajewska H, Kołodziej M. Systematic review with meta-analysis: lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment Pharmacol Ther. 2015;42:1149–57. doi:10.1111/apt.13404.
- Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: lactobacillus GG for treating acute gastroenteritis in children–updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38:467–76. doi:10.1111/apt.12403.
- Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302–10. doi:10.1111/j.1365-2036.2011.04665.x.
- Esposito S, Castellazzi L, Garbarino F. Can probiotic administration during pregnancy and the first year of life effectively reduce the risk of infections and allergic diseases in childhood? J Biol Regul Homeost Agents. 2014;28:565–73.
- Long SA, Thorpe J, DeBerg HA, Gersuk V, Eddy J, Harris KM, Kreutzfeldt M, Page N, Zimmer G, Geier F, et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol. 2016;1:eaai7793. doi:10.1126/sciimmunol.aah6817.
- McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523:612–16. doi:10.1038/nature14468.
- Carney EF. T-cell exhaustion limits immune reactivity and is associated with good prognosis in autoimmune disease. Nat Rev Rheumatol. 2015;11:501. doi:10.1038/nrrheum.2015.101.
- Hundhausen C, Roth A, Whalen E, Chen J, Schneider A, Long SA, Wei S, Rawlings R, Kinsman M, Evanko SP, et al. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med. 2016;8:356ra119. doi:10.1126/scitranslmed.aaf0746.
- Orabona C, Mondanelli G, Pallotta MT, Carvalho A, Albini E, Fallarino F, Hill AA, Wang X, Frye SV, Earp HS, et al. Deficiency of immunoregulatory indoleamine 2,3-dioxygenase 1in juvenile diabetes. JCI Insight. 2018;3:96244. doi:10.1172/jci.insight.97941.
- Abdel-Moneim A, Bakery HH, Allam G. The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother. 2018;101:287–92. doi:10.1016/j.biopha.2018.02.103.
- Li Y, Liu Y, Chu CQ. Th17 cells in type 1 diabetes: role in the pathogenesis and regulation by gut microbiome. Mediators Inflamm. 2015;2015:638470. doi:10.1155/2015/125380.
