ABSTRACT
Botulinum neurotoxins (BoNTs) are among the most toxic proteins. Vaccination is an effective strategy to prevent botulism. To generate a vaccine suitable for human use, a recombinant non-His-tagged isoform of the Hc domain of botulinum neurotoxin serotype E (rEHc) was expressed in Escherichia coli and purified by sequential chromatography. The immunogenicity of rEHc was evaluated in mice and dose- and time-dependent immune responses were observed in both antibody titers and protective potency. Then, the pilot-scale expression and purification of rEHc were performed, and its immunological activity was characterized. Our results showed rEHc has good immunogenicity and can elicit strong protective potency against botulinum neurotoxin serotype E (BoNT/E) in mice, indicating that rEHc is an effective botulism vaccine candidate. Further, we developed a novel antitoxin against BoNT/E by purifying F(abʹ)2 from pepsin-digested serum IgG of rEHc-inoculated horses. The protective effect of the F(abʹ)2 antitoxin was determined in vitro and in vivo. The results showed that our F(abʹ)2 antitoxin can prevent botulism in BoNT/E-challenged mice and effectively alleviate the progression of paralysis caused by BoNT/E to achieve therapeutic effects. Therefore, our results provide valuable experimental data for the production of a novel antitoxin, which is a promising candidate for the treatment of BoNT/E-induced botulism.
Introduction
Botulism is a neuroparalytic disease caused by botulinum neurotoxins, which are produced primarily by the Gram-positive, spore-forming bacterium Clostridium botulinum.Citation1–Citation3 Naturally occurring botulism in human is caused by serotypes A, B, E and F, while illness in birds, cattle, and horses are caused by type C, D, and G. However, all seven serotypes are lethal to humans. Botulism neurotoxins (BoNTs) are currently known to be the most toxic proteins to humans.Citation4–Citation7
Botulinum neurotoxins of various serotypes are structurally and functionally similar. Botulinum toxin is initially synthesized as a 150-kDa protein. It is then cleaved into a 50-kDa light chain and a 100-kDa heavy chain joined by a disulfide bond. Each BoNT consists three functional domains: the N-terminal catalytic domain (light chain, L domain, 50 kDa), the internal translocation domain (N-terminal of heavy chain, Hn domain, 50-kDa), and the C-terminal receptor-binding domain (C-terminal of heavy chain, Hc domain, 50-kDa).Citation8–Citation10 The intoxication process is divided into three steps. First, the Hc of BoNT binds to the neuronal membrane on the presynaptic side of the peripheral synapse. Then, the receptor-bound toxin is internalized by receptor-mediated endocytosis. The light chain of the toxin passes through the endocytic vesicle membrane and enters the cytoplasm. Once inside the cell, the light chain, which is identified as a zinc endopeptidase, cleaves polypeptides that are essential for neurotransmitter release. Subsequently, the extracellular release of neurotransmitters into the neuromuscular junction is blocked, resulting in a flaccid muscular paralysis that can ultimately lead to death.Citation4,Citation7,Citation11–Citation13
BoNTs are one of the most toxic proteins and classified as category A biological warfare agents.Citation4,Citation14 Thus, there is an urgent need to develop effective vaccines and neutralizing antibodies for the prevention and treatment of botulism. The most commonly used human botulism vaccine is formalin-inactivated pentavalent vaccine (PBT) which prevents serotypes A, B, C, D, and E.Citation3,Citation15–Citation17 However, the availability of this vaccine is limited because it is expensive, difficult to produce and involves a dangerous detoxification process. Post-exposure vaccination is useless with the rapid onset of this disease. Therefore, antitoxins are required for post-exposure treatment. Equine BoNT antitoxins are being widely used to treat adult botulism.Citation2,Citation7,Citation18,Citation19
Based on the information from the development of structurally similar tetanus toxin (TeNT), a novel vaccine-design strategy using BoNT recombinant protein as antigen was developed.Citation2,Citation7,Citation20 The nontoxic protein of the Hc fragment expressed in E.coli was less hazardous than traditional toxin-inactivated vaccine.Citation3,Citation21,Citation22 In previous studies, the non-His-tagged Hc domains of botulinum neurotoxin serotype A, B, and F were successfully expressed and purified. Further evaluation proved that these recombinant proteins were sufficient to elicit complete protective immunity against active botulinum neurotoxins.Citation23–Citation26
In this study, a candidate subunit vaccine and a novel antitoxin against BoNT/E were developed and evaluated. The non-His-tagged Hc domain of BoNT/E (rEHc) was expressed and purified in E. coli. The purified rEHc was formulated with an aluminum adjuvant to develop a subunit vaccine. Preclinical evaluation of this subunit vaccine was performed. In addition, validated rEHc was used as an immunogen to prepare antitoxins. The protective efficacy of the novel antitoxin against BoNT/E was determined in vitro and in vivo.
Results
Purification and analysis of rEHc
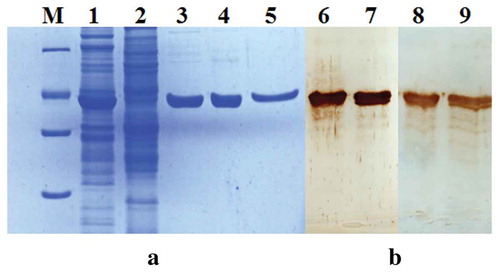
The high-level expression of rEHc in E.coli (approximately 15% of the cell protein) was confirmed by SDS-PAGE (, lane 1–2). Purification of rEHc was carried out using sequential chromatography with ion-exchange (SP and Q) and hydrophobic interaction (HIC) resins (, lane3, 4 and 5). SDS-PAGE analysis of the rEHc product showed approximately 95% purity after three steps. Final product yields ranged from 10 to 15 mg of purified protein per liter of culture. The product was identified by Western blot analysis with anti-serum from post-immunized horse and mouse (, lane 6–7, lane 8–9). The result confirmed that the rEHc preparation has good specificity and purity.
Figure 1. Analysis of purified rEHc protein by SDS-PAGE (a) and western blot (b). The soluble fraction and purified rEHc were subjected to 12% SDS-PAGE and Western blot analysis using hyperimmune anti-BoNT/E serum. Lane 1, pTIG-Trx-EHc transformed BL21 cell lysates; Lane 2, pTIG-Trx vector transformed cell lysates; Lane 3, SP column purified products; Lane 4, Q column purified products; Lane 5, HIC column purified products; Lane 6–7, Western blot analysis using hyperimmune anti-BoNT/E mouse serum; Lane 8–9, Western blot analysis using hyperimmune anti-BoNT/E horse serum; M, protein markers 116, 66.2, 45, 35, 25. 18.4 and 14.4 kDa (from top to bottom).
Immunogenicity of rEHc
To evaluate the immunogenicity of rEHc, mice were vaccinated with rEHc formulated with an aluminum hydroxide adjuvant. As shown in , a dose-dependent immune response was observed and anti-rEHc ELISA antibody titer correlated well with protective potency against BoNT/E. Mice immunized with one dose of rEHc failed to survive the 102 LD50 BoNT/E challenge. However, after the second immunization, mice were completely protected against 102 LD50 BoNT/E but partly protected against 103 LD50 of BoNT/E with relatively low (4.31–5.06) geometric mean titers (GMT). After the final booster immunization, the group GMT (5.27–5.91) was significantly increased and all mice survived the 103 LD50 BoNT/E challenge. The results indicate that the rEHc protein expressed in E.coli has good immunogenicity.
Table 1. Survival, group antibody ELISA titers, and serum neutralization titers of mice after vaccination with rEHc antigen.
Pilot-scale purification and of rEHc
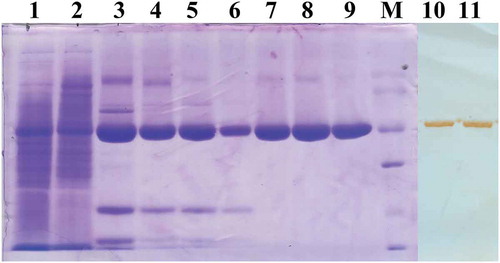
Pilot-scale purification of rEHc was performed using sequential chromatography. SDS-PAGE analysis of each purification step was shown in . SP cation-exchange chromatography efficiently captured the rEHc fragment and the SP product contained approximately 80% of the rEHc fragment (, lane 3). The Q column and Source 30S column purification step successfully removed numerous high molecular weight or low molecular weight contaminants, respectively (, lane 5 and lane 7). After the final gel filtration chromatography column (Superdex 200, GE Healthcare) purification step, a rEHc product with more than 95% purity (, lane 9) was obtained. The specificity and purity of rEHc were further identified by Western blot analysis (, lane 10, 11) with anti-BoNT/E horse serum.
Figure 2. Analysis of pilot-scale purified rEHc protein by SDS-PAGE and western blot. The soluble fraction and pilot-scale purified rEHc were subjected to 12% SDS-PAGE and Western blot analysis using hyperimmune anti-BoNT/E serum. Lane 1, pTIG-Trx-EHc transformed BL21 cell lysates; Lane 2, Flow-through fraction of SP column; Lane 3, SP column purified products; Lane 4, Loading sample of Q column; Lane 5, Q column purified products; Lane 6, Loading sample of Source 30S column; Lane 7, Source 30S column purified products; Lane 8, Loading sample of Superdex 200 column; Lane 9, Superdex 200 column purified products; Lane 10–11, Western blot analysis using hyperimmune anti-BoNT/E horse serum; M, protein markers 97.4, 66.2, 42.7, 31, 20.1 and 14.4 kDa (from top to bottom).
Furthermore, the molecular character of the pilot-scale rEHc was determined by N-terminal sequencing, mass spectrum, spectral scanning, and other methods. The rEHc solution was verified by ELISA, DNA dot blot, Culture method and Gel limulus reagent method (Supplementary material Table S1). The results showed that rEHc has correct sequence, molecular weight and conformation. Besides, no side effects were observed in abnormal toxicity study, such as loss of weight. Validated antigen batches were used for further experiments.
Immunological activity of pilot-scale rEHc
Efficacy study was carried out in Balb/c mice. Mice were vaccinated with 1 μg or 10 μg of pilot-scale rEHc followed with 103 or 104 LD50 of biologically active BoNT/E challenge. As shown in Table S2, a dose-dependent strong immune response was observed and the anti-rEHc ELISA antibody titer correlated well with protective potency. Mice vaccinated with two doses of rEHc were completely protected against 103 LD50 of BoNT/E. Only 40% of mice with two doses of 10 μg antigen survived the 104 LD50 BoNT/E challenge, but after the third immunization, all mice survived the 104 LD50 BoNT/E challenge.
To further evaluate the immunogenicity of rEHc and determine the ED50 of the pilot-scale rEHc. Mice were i.m. injected with two doses of 0.0039, 0.0156, 0.0625, 0.25, 1 or 4 μg of rEHc and challenged with 103 LD50 of BoNT/E. As shown in , a dose-dependent protective potency against BoNT/E was observed. Mice injected with two doses of ≥1 μg rEHc were completely protected against 103 LD50 of BoNT/E. The calculated ED50 for this potency assay was 0.256 μg with two doses. These results showed that rEHc elicited a strong immune response and provided potent protection against BoNT/E in Balb/c mice, indicating that this antigen has a functionally active conformation and can be used as a subunit candidate vaccine against BoNT/E.
Table 2. Survival, group antibody ELISA titers, and serum neutralization titers of mice after vaccination with rEHc vaccine.
Horse serum antibody titers and neutralization potency assay
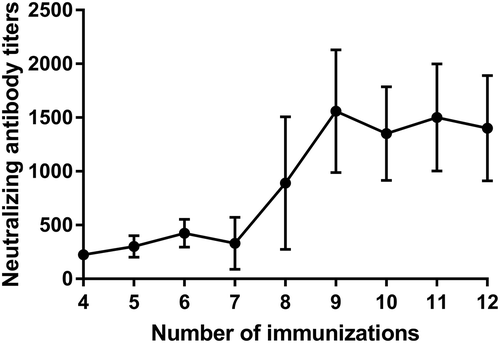
Antibody titers and neutralization potency of sera from immunized horses were analyzed by ELISA and BoNT/E neutralization assay, respectively. As shown in and , the first six immunizations elicited a strong immune response with an average of 425 IU/ml neutralizing antibody against BoNT/E. After two boost immunizations, an increase in total IgG and neutralizing antibody titers was observed. From the ninth immunization, the neutralizing antibody titer increased to more than 103 IU/ml, which reached our standard for producing antitoxins. These results indicated that rEHc is effective in inducing high levels of neutralizing antibodies in horses.
Figure 3. Anti-rEHc antibody titers of horse sera. Horses were immunized with rEHc and sera samples were collected 7 days after each immunization, and anti-rEHC antibody titer was measured by ELISA. Serum sample from individual horse was assayed, and the geometric mean titers (GMT) were calculated.
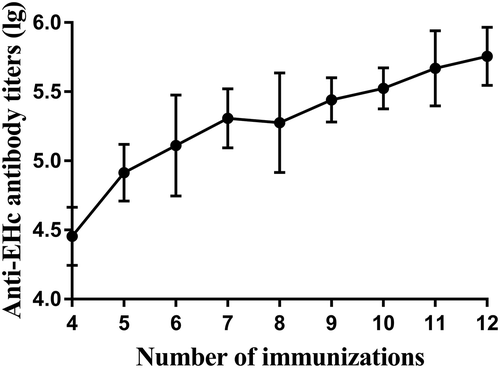
Figure 4. Neutralizing antibody titers of horse sera. Horses were immunized with rEHc and sera samples were collected 7 days after each immunization and anti-BoNT/E and neutralizing antibody titer assay was performed according to Pharmacopoeia of the People’s Republic of China (PPRC, Appendix XII H: the potency assay of botulinum antitoxin).
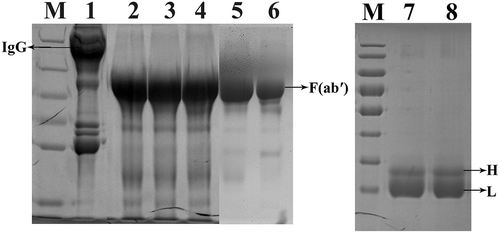
Preparation of equine IgG and F(abʹ)2 fragments
The multi-step purification of the F(abʹ)2 products was performed. SDS-PAGE analysis of each step and the final products was shown in . The IgGs were completely digested after the first step (, lane2). The purified proteins migrated as a single 100 kDa major band under non-reducing conditions (, lane5), whereas under reducing conditions (, lane 7–8) they appeared as bands of 25–31 kDa. The results showed that no albumin and detergent-resistant high molecular weight aggregates were detected under non-reducing conditions, and no undigested heavy chains were observed under reducing conditions. The purity of the F(abʹ)2 fraction reached about 85.6%, and the protein content was 32.9 mg/ml. In addition, the neutralizing potency of the F(abʹ)2 fragments against BoNT/E was 2000 IU/ml. These results indicated that the F(abʹ)2 antitoxin has the desired purity and potency.
Figure 5. Analysis of purified F(abʹ)2 fragments during various purification steps by SDS-PAGE under reducing (Lane 1–6) and non-reducing (Lane 7–8) conditions. Lane 1, Hyperimmune horse sera diluted with pyrogen-free distilled water (1:2 v/v); Lane 2, Pepsin digested horse sera; Lane 3, Ammonium sulfate precipitate sample; Lane 4, Alum adsorbed sample; Lane 5, Centrifugal filtrated F(abʹ)2 fragments; Lane 6, A standard horse antitoxin for BoNT/A. Lane 7–8, Centrifugal filtrated F(abʹ)2 fragments under non-reducing conditions; M, protein markers 180, 130, 95, 72, 55, 43, 34, and 26 KDa (from top to bottom).
In vivo potency assay of the antitoxin against Bont/E in the mouse model
The in vivo protective efficacy of the antitoxin against BoNT/E was determined in mice. The results are shown in . The paralysis status of the intoxicated mice was alleviated or delayed, and significant protection was observed in F(abʹ)2 antitoxin pretreated mice. Mice pretreated with 10 μL antitoxin survived completely with 20 LD50 BoNT/E challenge within 3 days or 5 LD50 BoNT/E challenge within 7 days. For low-dose F(abʹ)2 antitoxin (2.5 μL) treatment, prevention of BoNT/E intoxication was effective for 5 LD50 BoNT/E within 3 days or 100 LD50 BoNT/E within 1 day. In conclusion, the use of the novel F(ab’)2 antitoxin to protect mice against homologous neurotoxin exposure is effective, and the protection is time- and dose-dependent.
Table 3. Preventive effect of the new antitoxin against BoNT/E in mice.
The therapeutic role and dose-dependency of the new F(abʹ)2 antitoxin against BoNT/E were investigated in a mouse model. The results () showed that the F(abʹ)2 antitoxin can delay the onset of paralysis in the intoxicated mice and protect the mice from the lethal effects of the toxin. For the high dose of BoNT/E intoxication (20 LD50), treatment was effective within 1 h after exposure but not within 3 h, even with 25 μL of F(abʹ)2 antitoxin. For the low dose of BoNT/E intoxication (1 LD50), treatment was still effective with 10 μL F(abʹ)2 antitoxin 6 h after exposure. Notably, mice that survived within 1 h of challenge with 1LD50 BoNT/E by treating with F(ab’)2 antitoxin showed a significant improvement in survival after treatment with another dose of antitoxin. These results indicate that the novel F(ab’)2 antitoxin is effective in treating homologous BoNT/E intoxication in a mouse model with a time- and dose-dependent therapeutic effect.
Table 4. Therapeutic effect of the new antitoxin against BoNT/E in mice.
Discussion
Botulinum neurotoxins (BoNTs) are one of the most toxic proteins, which are easy to manufacture, deliver, and weaponize. BoNTs have been developed as biological warfare agents by many countries and organizations. Thus, developing suitable drugs for the prevention and treatment of Botulism in order to deal with the potential bio-security threats is very urgent. In previous studies, the colleagues have established a strategy of cloning the Hc gene of botulinum neurotoxin into an expression vector to express and purify recombinant non-His-tagged Hc protein. Purification and characterization of the recombinant Hc proteins of serotypes A, B and F have been completed, demonstrating good safety and efficacy against botulism.Citation23–Citation28
In this study, we presented the production and evaluation of a recombinant non-His-tagged Hc subunit vaccine as a candidate subunit vaccine for human use against BoNT/E. The prokaryotic expression vector pTIG-Trx-EHc was successfully constructed. Recombinant EHc (rEHc) protein was highly expressed in E. coli and purified by sequential chromatography. The potency of rEHc against BoNT/E was assessed in mice. The results showed that rEHc formulated with an aluminum hydroxide adjuvant was efficacious in eliciting protective antibodies against natural BoNT/E. Mice with two immunizations were protected against 102 LD50 BoNT/E but partly protected against 103 LD50 BoNT/E with relatively low (4.31–5.06) geometric mean titers (GMT). After the third immunization, the group GMT (5.27–5.91) increased significantly and all of these mice survived 103 LD50 BoNT/E challenge. These results indicate that rEHc has a functionally active conformation and represents a good subunit candidate vaccine against BoNT/E.
Since rEHc is a suitable candidate vaccine, large quantities of rEHc will eventually be needed. Thus, in this study, pilot-scale production of rEHc was carried out. A pilot-scale rEHc expression and purification protocol were established. After four purification steps, each pilot-scale purification yielded approximately 900 mg rEHc with a purity of up to 95% based on HPLC analysis. Mouse assay results demonstrated that the pilot-scale rEHc has good immunogenicity and can effectively elicit protective antibodies against BoNT/E. Mice with two or three doses of rEHc were completely protected against 103 LD50 or 104 LD50 of BoNT/E challenge, respectively. To further evaluate the immunogenicity of rEHc and determine the ED50 of the pilot-scale rEHc, mice were i.m. injected with a range of 0.0039 μg to 4 μg of four-fold diluted rEHc. Mice injected with two doses of ≥1μg EHc were completely protected against 103 LD50 of BoNT/E. The ED50 was calculated to be 0.256 μg with two doses. In conclusion, the rEHc antigen has a correct sequence, molecular weight and specific immunological activity. Thereafter, serval batches of pilot-scale rEHc were prepared, and the stock solutions were verified by ELISA, DNA dot blot, culture method and Gel Limulus reagent method.
Botulinum antitoxin is the only effective post-exposure therapy and defense method against BoNT. The antitoxin currently available for the treatment of botulism is equine antitoxin, which consists of purified F(abʹ)2 fragments derived from horses immunized with a formaldehyde-inactivated toxin (toxoid). However, aside from the fact that there are many disadvantages in the production of the toxoid, the main problem of this method is that it may induce toxin-binding but non-neutralizing antibodies. The non-neutralizing antibodies reduce the potency of the antitoxin preparations, which can result in the need for high doses and may cause serious side effects. Therefore, there is an urgent need to develop safe and highly potent novel botulinum antitoxins. A potential solution to this problem is to immune animals with nontoxic recombinant BoNT fragment, such as the Hc domain of BoNT. The Hc domain is nontoxic and contains key neutralizing epitopes that are effective in inducing strong neutralizing antibodies against BoNTs. Therefore, the Hc domain may represent good immunogen to replace the toxoid for the production of antitoxins.Citation14,Citation19,Citation29
In this study, a novel antitoxin against BoNT/E was prepared from pepsin-digested serum IgG of horses inoculated with the rEHc antigen. The rEHc antigen was used to immune horses to prepare equine serum with high neutralizing antibody titers. After multiple purification steps, the IgG was successfully digested and F(abʹ)2 fragment with high purity (85.6%), and high protein content (32.9 mg/ml) was obtained. The neutralizing potency of the F(abʹ)2 fragment against BoNT/E was 2000 IU/ml or 60.8 IU/mg. Furthermore, the in vivo potency assay results suggested that the novel antitoxin is effective in the treatment and prevention of BoNT/E intoxication in a mouse model and the therapeutic and protective effects are time- and dose-dependent. Early treatment, especially before obvious paralysis, was most effective in preventing paralysis progression and protecting exposed mice from BoNT/E. Additionally, mice that survived 1 h after intoxication by treating with F(ab’)2 antitoxin showed a significant improvement in survival after treatment with another dose of antitoxin. Therefore, our result indicated that the rEHc antigen is a highly potent immunogen in inducing high titer of neutralizing antibodies against BoNT/E. And the novel F(ab’)2 antitoxin is a potential therapeutic drug for the prevention and treatment of BoNT/E.
In conclusion, we successfully expressed and purified a non-His-tagged rEHc protein in E.coli. The results showed that rEHc is immunologically active and can elicit a strong immune response to protect mice from BoNT/E challenge, indicating that rEHc represents a good potential candidate subunit vaccine for human use. Thereafter, we demonstrated the strategy for the development of a potent novel botulinum antitoxin using rEHc as an immunogen. The F(abʹ)2 antitoxin produced from rEHc-immunized horses is effective in the prevention and treatment of BoNT/E intoxication.
Further manufacture and characterization of the rEHc subunit vaccine and novel rEHc-based antitoxin will be performed for preclinical studies. Preclinical safety of the novel antitoxin will be assessed and valuable experimental data will be provided for potential clinical use. This method is valuable for the development of other F(ab′)2 antitoxins, and the obtained antitoxins can have better specificity and safety than those prepared from toxoids.
Materials and method
Animals
Balb/c mice (6–8 week, female) and KM mice (15–18 g, female) were purchased from Beijing Laboratory Animal Center (Beijing, China) and housed in pathogen-free conditions. Ten 4–6 years old, healthy brown horses (300–350 kg in weight) were purchased from Chifeng Bo En Pharmaceutical Co., LTD (Chifeng, China) and housed in here with standard conditions. All animal experiments were approved by our Institution Animal Care and Use Committee (Beijing Institute of Biotechnology) and performed in accordance with the ethical guidelines of our institution and the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Expression of rEHc in E. coli
To produce recombinant rEHc antigen in E. coli, a prokaryotic expression vector pTIG-Trx-EHc was constructed. In brief, the Hc domain of BoNT/E (rEHc, amino acids 853–1291, ~48 kDa, Genbank under the accession number HM231315) was amplified by PCR with primer pairs 5ʹTAA GGAGG AATTC TAATG AACAA ATTCT TCAAG, 3ʹTCA GTGGT GGTGG TGGTG GTGCT CGAGT CATTA.Citation30–Citation32 The PCR products were digested with EcoR I and Xho I restriction enzyme to excise the rEHc DNA fragment, which was cloned into a pTIG-Trx expression vector cut with the same enzymes. The resulting recombinant plasmid pTIG-Trx-EHc was confirmed by sequencing and subsequently transformed into E. coli strain BL21 (DE3) (TRANSGEN BIOTECH, Beijing, China) and grown in Luria Bertani (LB) medium containing 100 μg/ml ampicillin at 37 ℃ until the optical density at 600 nm reached 0.6–1.0. Isopropyl-β-D-thiogalactopyranoside (IPTG, Promega, Madison, WI, USA) was added to a final concentration of 0.4 mM for another 3 h. The cells were centrifuged at 8000 rpm for 10 min and resuspended in buffer A (20 mM sodium phosphate, pH 7.4), then lysed by ultrasonic homogenizer and centrifuged for the supernatant containing rEHc.
Purification of rEHc
Purification of recombinant rEHc was carried out using sequential chromatography with ion-exchange (SP and Q) and hydrophobic interaction (HIC) resins. All chromatography steps were performed on an AKTA Explorer (GE Healthcare, Piscataway, NJ, USA). Firstly, a HiTrapTM SP FF (Pharmacia Biotech, Uppsala, Sweden) column was equilibrated with 10 column volumes (CV) of buffer A and then loaded with the filtered lysates. The recombinant protein was eluted using step elution with 6 CV of 200 mM NaCl in equilibration buffer A. The obtained sample was dialyzed into buffer B (20 mM sodium phosphate, pH 8.0). Secondly, a HiTrapTM Q FF (Pharmacia) column was equilibrated with 10 CV of buffer B and then loaded with dialyzed SP product. The column was washed with 6 CV of buffer B and the flow-through fraction containing the recombinant rEHc product was collected. Thirdly, The HiTrapTM Phenyl FF (Pharmacia) column was equilibrated with 10 CV of high-ionic-strength buffer (2.5 M NaCl in 20 mM sodium phosphate, pH 7.4). The Q product was pre-adjusted to 2.5 M NaCl and loaded into the column, which was then washed with 10 CV of equilibration buffer, and the recombinant protein was eluted using six CV of 1.5 M NaCl in the equilibration buffer. The final product was dialyzed to remove residual salt and stored at −20ºC.
The soluble samples and purified rEHc products were verified by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using hyper-immune horse or mouse anti-rEHc antiserum.
Immunization of mice
Balb/c mice (6–8 week) were randomly assigned to different treatment groups (10 mice per group). Mice were vaccinated intramuscularly (i.m.) once, twice or three times with 1 μg or 10 μg of rEHc diluted into PBS (pH 6.0) containing 0.2% (w/w) alhydrogel (Aluminum hydroxide gel, Brenntag Biosector, Denmark). PBS was used as a negative control. The injections were administered at three-week intervals. Three weeks after each vaccination, mice were challenged with an active preparation containing 102 or 103 LD50 (50% lethal doses) of active BoNT/E (Iwanai strain, from National Institutes of Food and Drug Control, Beijing, China) diluted in 20 mM sodium phosphate buffer (pH 6.5) and observed for 7 days to record their survival.
Serum antibody titer measurement and BoNT/E neutralization assay
Sera from mice in different treatment groups were screened for anti-rEHc antibody titers by ELISA as previously described.Citation23 Serum antibody titers were determined for each mouse, and the geometric mean titer (GMT) for each group was determined.
The neutralizing potency of the mouse sera was measured using a BoNT/E neutralization assay as previously described.Citation25,Citation26 Briefly, serially diluted anti-BoNT/E serum was mixed with a standard concentration of BoNT/E (102 LD50/ml) and incubated for 15 min at 37°C. KM mice (15–18 g) were intraperitoneally (i.p.) injected with the mixtures in a total volume of 0.5 ml/mouse (four mice in each group) then monitored for 7 days to record their survival. The neutralizing antibody titers in the sera were calculated as international units per milliliter (IU/ml) according to a WHO (World Health Organization) BoNT/E antitoxin.
Pilot-scale purification and verification of rEHc
Pilot-scale purification of rEHc was performed at the Pilot Production Base of our institute. The correct single clones of pTIG-Trx-EHc transformed E.coli BL21(DE3) were cultured in 30 L Terrific Broth (TB) medium containing 100 μg/ml ampicillin at 37 ℃ in a fermentation cylinder (Sartorius BIOSTAT U50) until the optical density at 600 nm reached 0.6–1.0. Isopropyl-β-D-thiogalactopyranoside (IPTG, Promega, Madison, WI, USA) was added to a final concentration of 0.4 mM for the expression of rEHc at 25ºC for 6 h. The fermentation process was under strict quality control to maintain the stability of the products. The bacterial cells were harvested by centrifugation (8000 rpm, 20 min, 4°C), and the expression products were analyzed by SDS-PAGE. The cells were resuspended in buffer A and lysed by cell homogenizer. The supernatant was collected by centrifugation.
The pilot-scale purification of rEHC was proceeded in four steps. The first two steps are consistent with before. In the third step, a Source 30S column (GE Healthcare) was equilibrated with three CV of 50 mM NaCl in buffer A and then loaded with eluted Q product previously pooled with equal volume of 50 mM NaCl in buffer A. After equilibrium, the column was eluted by linear gradient elution with 0–50% of 1 M NaCl in buffer A to collect the highest concentration of protein at the elution peaks. In the final step, the elutate containing rEHc was loaded into a gel filtration chromatography column (Superdex 200, GE Healthcare) equilibrated with 100 mM NaCl in buffer A. The flow-through fraction was directly collected as the final product.
Characterization and immunological activity of pilot-scale rEHc
The character of pilot-scale rEHc antigen was determined by the methods below. SDS-PAGE gel scanning, HPLC (High-Performance Liquid Chromatography), mass spectrometry,N-terminal sequencing and spectral scanning was used for the determination of protein purity, molecular weight, amino acid sequence and characteristic spectrum map of rEHc. The abnormal toxicity of the rEHc protein was evaluated in mice according to Pharmacopoeia of the People’s Republic of China (PPRC, Appendix XII F: the abnormal toxicity test method). Validated antigens were used for further experiments.
The immunogenicity of pilot-scale rEHc was studied by immunization of mice using previously described methods. To further assess the immune protective response against BoNT/E and determine ED50 (median effective dose) of the pilot-scale rEHc, 10 mice of each group were i.m. injected with one or two doses of rEHc antigen ranging from 0.0039 μg to 4 μg by fourfold, respectively. Three weeks after each vaccination, mice were challenged with an active preparation containing 102 or 103 50% lethal doses (LD50) of BoNT/E by i.p. injection, and survival was monitored for 7 days. Mouse sera were collected three weeks after each immunization for serum antibody titer measurement and BoNT/E neutralization assay.
Immunization of horses and collection of horse anti-serum
Ten brown horses with no detectable antibodies against BoNT/E were chosen for rEHc immunization. The horses were first immunized subcutaneously with 2 mg pilot-scale rEHc emulsified with an equal volume of complete Freund’s adjuvant (CFA, Sigma, St. Louis, MO). Subsequent immunizations were carried out using 2 mg, 3mg, 3 mg, 4 mg, 4 mg rEHc emulsified with an equal volume of incomplete Freund’s adjuvant (IFA, Sigma, St. Louis, MO) at 2–3 week intervals. The formulation was injected into the sites near the submandibular and inguinal lymph nodes on the back of the body with less than 2 ml per site. The sera samples were collected 7 days after each immunization and anti-rEHC antibody titer assay and neutralizing potency assay were performed. Then, the horses were booster immunized with 5–6 mg rEHc after a 2-week rest for hyperimmune sera. Based on these results of the neutralizing potency assay, the qualified horses were bled, and large quantities of hyperimmune sera were collected into sterilized pyrogen-free glass containers with anticoagulant acid citrate dextrose (ACD).
The anti-BoNT/E antibody titer of the hyperimmune sera was measured by ELISA as described before. The neutralizing potency assay was carried out according to Pharmacopoeia of the People’s Republic of China (PPRC, Appendix XII H: the potency assay of botulinum antitoxin). Briefly, 0.01 IU of a reference standard antitoxin of BoNT/E was mixed with a gradient-diluted toxin and injected i.p. into mice (four mice per group) to define an L+/50 dose of BoNT/E. The mice were observed for 4 days to record survival numbers. The minimum toxin dose required to kill a mouse was considered to be the L+/50 dose of BoNT/E. After the toxin dose was determined, the L+/50 dose of toxin was mixed with the gradient-diluted horse sera and injected into the mice to determine the neutralizing potency. The potency of the antitoxin was calculated by comparison with the reference standard antitoxin of BoNT/E and reported as IU/ml.
Preparation of equine IgG and F(ab’)2 fragments
Total equine IgG and F(abʹ)2 were prepared as previously described.Citation27,Citation33,Citation34 Briefly, the hyperimmune sera were diluted with pyrogen-free distilled water (1:2 v/v) and adjusted to pH 3.0 with 1 N HCl. The IgG was digested with pepsin (6 U/ml) at 37°C for 30 min. Next, add 15% (w/v) ammonium sulfate to the mixture and adjusted the pH to 5.4, then incubate the mixture at 58°C for 30 min to precipitate the undesired proteins. After incubation, the mixture was cooled to 45°C and 0.8% perlite was added, stirred and press-filtered to collect the filtrate. Then, the pH of the filtrate was adjusted to 7.2, and a final concentration of 20% (w/v) ammonium sulfate was added, and incubated for 45 min. Then, 0.8% perlite was added, stirred and press-filtered to collect the sediment, which was dissolved in 2 volume of pyrogen-free distilled water. A final concentration of 0.8% alum was added to the mixture, and the pH was adjusted to 7.8 ± 0.1. The mixture was stirred for 60 min to adsorb the heat-denatured Fc fragments and press-filtered. The filtered F(ab’)2 fragment was concentrated and further separated from the low molecular weight byproducts with a centrifugal filtration device (Millipore; MW cut-off 50 kDa).Citation35–Citation37
The F(ab’)2 thus obtained was equilibrated to PBS (pH 7.0) and filtered using a 0.22 μm filter (Millipore, USA). The final F(ab’)2 product was stored at 4°C and its specificity, potency, protein concentration and purity were further determined.
Potency assay of the antitoxin against BoNT/E
The neutralizing potency of the F(ab’)2 antitoxin was assayed as described before. To further determinate the potency of the new antitoxin, the in vivo protective efficacy was conducted in mouse model.
To evaluate the therapeutic role of the new antitoxin against post-exposure to BoNT/E, Balb/c mice (18–22 g) were injected i.p. with different doses of BoNT/E (i.e., 1, 5, or 20 LD50 of BoNT/E), followed by i.v. injection of the F(ab’)2 antitoxin (0.25, 1 or 5 μL) at 1, 3 or 6 h later. The mice (a group of four mice) were observed for 2 weeks, and survival was determined for each group.
To investigate the preventive role of the new antitoxin against BoNT/E challenge, Balb/c mice (18–22 g) were injected with the F(ab’)2 antitoxin (2.5 or 10 μL). The mice (a group of four mice) were challenged i.p. with different doses of BoNT/E (i.e., 5, 20, or 100 LD50 of BoNT/E) 1, 3 or 7 days later, and were observed for 2 weeks. The F(abʹ)2 did not cause anaphylactic reaction in mice.
Statistical analysis
Differences in the antibody immune responses were analyzed statistically by ANOVA one-way variance test or using the Student’s t-test for the analysis of differences between groups. Fisher’s exact test was used to determine statistical differences in survival between the treatment groups. For all tests, only data resulting in P values < .05 were regarded as statistically significant. Fifty percent effective concentrations (ED50) were determined by probit analysis at the 95% confidence level with IBM SPSS Statistics (version 20.0, Armonk, NY: IBM Corp).
Disclosure of potential conflicts of interest
The authors declare no financial or commercial conflict of interest.
Supplemental Material
Download MS Word (25.9 KB)Acknowledgments
This work was supported by grants from the Special Key Project of Biosafety Technologies (2017YFC1200902) for the National Major Research and Development Program of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Dembek ZF, Smith LA, Rusnak JM. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep. 2007;1:122–34. doi:10.1097/DMP.0b013e318158c5fd.
- Smith LA. Botulism and vaccines for its prevention. Vaccine. 2009;27(Suppl 4):D33–D39. doi:10.1016/j.vaccine.2009.08.059.
- Sundeen G, Barbieri JT. Vaccines against Botulism. Toxins. 2017;9:268.
- Shukla HD, Sharma SK. Clostridium botulinum: a bug with beauty and weapon. Crit Rev Microbiol. 2005;31:11–18. doi:10.1080/10408410590912952.
- Rusnak JM, Smith LA. Botulinum neurotoxin vaccines: past history and recent developments. Hum Vaccin. 2009;5:794–805. doi:10.4161/hv.9420.
- Turton K, Chaddock JA, Acharya KR. Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. Trends Biochem Sci. 2002;27:552–58.
- Smith LA, Rusnak JM. Botulinum neurotoxin vaccines: past, present, and future. Crit Rev Immunol. 2007;27:303–18.
- Schiavo G, Rossetto O, Santucci A, DasGupta BR, Montecucco C. Botulinum neurotoxins are zinc proteins. J Biol Chem. 1992;267:23479–83.
- Gu S, Jin R. Assembly and function of the botulinum neurotoxin progenitor complex. Curr Top Microbiol Immunol. 2013;364:21–44. doi:10.1007/978-3-642-33570-9_2.
- Lee K, Gu SY, Jin L, Le TTN, Cheng LW, Strotmeier J, Kruel AM, Yao G, Perry K, Rummel A, et al. Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog. 2013;9:e1003690. doi:10.1371/journal.ppat.1003690.
- Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–66. doi:10.1152/physrev.2000.80.2.717.
- Matak I, Lackovic Z. Botulinum toxin A, brain and pain. Prog Neurobiol. 2014;119–120:39–59. doi:10.1016/j.pneurobio.2014.06.001.
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, et al. Botulinum toxin as a biological weapon – medical and public health management. Jama-J Am Med Assoc. 2001;285:1059–70. doi:10.1001/jama.285.8.1059.
- Rossow H, Kinnunen PM, Nikkari S. Botulinum toxin as a biological weapon. Duodecim. 2012;128:1678–84.
- Pittman PR, Hack D, Mangiafico J, Gibbs P, McKee KT, Friedlander AM, Sjogren MH. Antibody response to a delayed booster dose of anthrax vaccine and botulinum toxoid. Vaccine. 2002;20:2107–15.
- Cieslak TJ, Christopher GW, Kortepeter MG, Rowe JR, Pavlin JA, Culpepper RC, Eitzen EM. Immunization against potential biological warfare agents. Clin Infect Dis. 2000;30:843–50. doi:10.1086/313812.
- Torii Y, Tokumaru Y, Kawaguchi S, Izumi N, Maruyama S, Mukamoto M, Kozaki S, Takahashi M. Production and immunogenic efficacy of botulinum tetravalent (A, B, E, F) toxoid. Vaccine. 2002;20:2556–61.
- Froude JW, Stiles B, Pelat T, Thullier P. Antibodies for biodefense. MAbs. 2011;3:517–27. doi:10.4161/mabs.3.6.17621.
- Mayers CN, Holley JL, Brooks T. Antitoxin therapy for botulinum intoxication. Rev Med Microbiol. 2001;12:29–37. doi:10.1097/00013542-200101000-00004.
- Yu YZ, Gong ZW, Ma Y, Zhang SM, Zhu HQ, Wang WB, Du Y, Wang S, Yu W-Y, Sun Z-W. Co-expression of tetanus toxin fragment C in Escherichia coli with thioredoxin and its evaluation as an effective subunit vaccine candidate. Vaccine. 2011;29:5978–85. doi:10.1016/j.vaccine.2011.06.039.
- Moreira GM, Cunha CE, Salvarani FM, Gonçalves LA, Pires PS, Conceição FR, Lobato FC. Production of recombinant botulism antigens: a review of expression systems. Anaerobe. 2014;28:130–36. doi:10.1016/j.anaerobe.2014.06.003.
- Holley JL, Elmore M, Mauchline M, Minton N, Titball RW. Cloning, expression and evaluation of a recombinant sub-unit vaccine against Clostridium botulinum type F toxin. Vaccine. 2000;19:288–97.
- Yu YZ, Li N, Wang RL, Zhu HQ, Wang S, Yu WY, Sun ZW. Evaluation of a recombinant Hc of clostridium botulinum neurotoxin serotype F as an effective subunit vaccine. Clin Vaccine Immunol. 2008;15:1819–23. doi:10.1128/CVI.00239-08.
- Yu YZ, Li N, Zhu HQ, Wang RL, Du Y, Wang S, Yu W-Y, Sun Z-W. The recombinant Hc subunit of Clostridium botulinum neurotoxin serotype A is an effective botulism vaccine candidate. Vaccine. 2009;27:2816–22. doi:10.1016/j.vaccine.2009.02.091.
- Yunzhou Y, Danyang S, Si L, Zheng-Wei G, Shuang W, Zhiwei S. Production and evaluation of a recombinant subunit vaccine against botulinum neurotoxin serotype B using a 293E expression system. Hum Vaccin. 2015;11:468–73. doi:10.4161/hv.29714.
- Liu B, Shi D, Chang S, Gong X, Yu Y, Sun Z, Wu J. Characterization and immunological activity of different forms of recombinant secreted Hc of botulinum neurotoxin serotype B products expressed in yeast. Sci Rep. 2015;5:7678. doi:10.1038/srep07678.
- Yu YZ, Zhang SM, Ma Y, Zhu HQ, Wang WB, Du Y, Zhou X-W, Wang R-L, Wang S, Yu W-Y, et al. Development and evaluation of candidate vaccine and antitoxin against botulinum neurotoxin serotype F. Clin Immunol. 2010;137:271–80. doi:10.1016/j.clim.2010.07.005.
- Shi DY, Chen BY, Mao YY, Zhou G, Lu JS, Yu YZ, Zhou XW. Development and evaluation of candidate subunit vaccine against botulinum neurotoxin serotype B. Hum Vaccin Immunother. 2019;15:755–60. doi:10.1080/21645515.2018.1547613.
- Jones RG, Alsop TA, Hull R, Tierney R, Rigsby P, Holley J, Sesardic D. Botulinum type A toxin neutralisation by specific IgG and its fragments: a comparison of mouse systemic toxicity and local flaccid paralysis assays. Toxicon. 2006;48:246–54. doi:10.1016/j.toxicon.2006.05.007.
- Yu YZ, Guo JP, An HJ, Zhang SM, Wang S, Yu WY, Sun ZW. Potent tetravalent replicon vaccines against botulinum neurotoxins using DNA-based semliki forest virus replicon vectors. Vaccine. 2013;31:2427–32. doi:10.1016/j.vaccine.2013.03.046.
- Sinha J, Inan M, Fanders S, Taoka S, Gouthro M, Swanson T, Barent R, Barthuli A, Loveless BM, Smith LA, et al. Cell bank characterization and fermentation optimization for production of recombinant heavy chain C-terminal fragment of botulinum neurotoxin serotype E (rBoNTE(Hc): antigen E) by Pichia pastoris. J Biotechnol. 2007;127:462–74. doi:10.1016/j.jbiotec.2006.07.022.
- Byrne MP, Titball RW, Holley J, Smith LA. Fermentation, purification, and efficacy of a recombinant vaccine candidate against botulinum neurotoxin type F from Pichia pastoris. Protein Expr Purif. 2000;18:327–37. doi:10.1006/prep.2000.1200.
- Bing NI, Wang XL, Wang L, Zhao GY, Shi XF, Zhang SL, Zhang LY. Protection of mammalian cells from severe acute respiratory syndrome coronavirus infection by equine neutralizing antibody. Immunol J. 2005;10:681–90.
- Yu YZ, Zhang SM, Wang WB, Du Y, Zhu HQ, Wang RL, Zhou X-W, Lin J-B, Wang S, Yu W-Y, et al. Development and preclinical evaluation of a new F(ab′)2 antitoxin against botulinum neurotoxin serotype A. Biochimie. 2010;92:1315–20. doi:10.1016/j.biochi.2010.06.010.
- Kittipongwarakarn S, Hawe A, Tantipolphan R, Limsuwun K, Khomvilai S, Puttipipatkhachorn S, Jiskoot W. New method to produce equine antirabies immunoglobulin F(ab’)2 fragments from crude plasma in high quality and yield. Eur J Pharm Biopharm. 2011;78:189–95. doi:10.1016/j.ejpb.2011.02.018.
- Morais V, Massaldi H. Effect of pepsin digestion on the antivenom activity of equine immunoglobulins. Toxicon. 2005;46:876–82. doi:10.1016/j.toxicon.2005.08.006.
- Rial A, Morais V, Rossi S, Massaldi H. A new ELISA for determination of potency in snake antivenoms. Toxicon. 2006;48:462–66. doi:10.1016/j.toxicon.2006.07.004.
