ABSTRACT
Pre-existing immunity to influenza is dependent on a number of factors and can vary greatly within and across influenza subtypes. In this study, volunteers (aged 18–85 years) were vaccinated with split, inactivated FluzoneTM in four consecutive influenza seasons from 2013 to 2016. The impact of repeat vaccination on breadth and durability of functional antibodies was assessed for total IgG and IgA anti-hemagglutinin (HA) binding antibodies and hemagglutination-inhibition (HAI) activity against both influenza B lineages. Many subjects were able to maintain high seroprotective titers to the vaccine strains in subsequent years, which resulted in low vaccine-induced seroconversion rates. This was especially evident in younger subjects who typically had higher titers and maintained these titers into the following season. In contrast, the HAI titers in elderly subjects were generally lower and more likely to decline prior to the start of the next influenza season. Immunological recall or ‘back-boosting’ to antigenically related viruses was associated with seroconversion. Overall, influenza vaccination in both younger and older people elicited broadly reactive immune responses within a lineage, as well as cross-reactive immune responses between lineages. This study exemplified the impact that age and influenza exposure history have on determining an individual’s ability to respond to future influenza infections.
Introduction
Influenza virus infection is a recurrent health and economic burden as it transmits in the human population.Citation1 Worldwide, there are millions of hospitalizations and thousands of deaths each year due to seasonal epidemics and the occasional pandemic with spillover events that altogether affect between 5% and 30% of the global population. Influenza A virus (IAV) accounts for the majority of these hospitalizations and deaths, as well as every recorded pandemic event, due to its ability to infect a broad range of natural vertebrate hosts, such as avian waterfowl. The virus RNA genome is segmented that results in reassortant events that leads to numerous viral variants and the formation of new subtypes. In contrast to IAV, influenza B virus (IBV) has a limited ability to transmit outside of humans and IBV infections are more regionally contained.Citation2 Nevertheless, IBVs can also cause annual hospitalizations and death, and while uncommon, there have been influenza seasons (i.e. 2002–2003 Northern Hemisphere) where IBV was documented as the predominant, circulating subtype.
IBVs were first isolated in 1940 and circulated as a single antigenic type; however, in the 1980s two distinct lineages of IBVs were identified, Victoria and Yamagata.Citation3 The two different lineages have individual dynamics that are determined by viral transmission, the age of person infected, and the preference for receptor binding.Citation4 The Victoria-lineage dominated global circulation from 1987 to 1989, but was succeeded by Yamagata-lineage dominance in the 1990s, with both lineages continuing to co-circulate each season.Citation3 The Yamagata-lineage evolved into two separate clades,Citation2,Citation3 with the majority of IBV viruses circulating today classified as clade 3.Citation5 The Victoria-lineage has also evolved into clades and subclades, most recently 1A and V1A.1, yet these are not antigenically distinct like the two currently circulating Yamagata-lineage clades.Citation6 On the other hand, Victoria-like viruses do continue to reassort between clades over time, which is a phenomenon not observed thus far in Yamagata-lineage influenza B viruses.Citation6
Each season, the World Health Organization (WHO) recommends components to include in the seasonal influenza vaccine based on recently circulating influenza strains. Prior to 2014, this consisted of three recommended strains for a trivalent influenza vaccine (TIV), two influenza A viruses (H1N1 and H3N2) and one influenza B virus strain chosen from one of the two IBV lineages (Yamagata or Victoria). In 2014, after continued years of IBV mismatch between the vaccine selected strain lineage and the eventual circulating strain, a quadrivalent influenza vaccine (QIV) was developed to cover both Yamagata and Victoria lineages.Citation3 Since some TIV vaccines are still deployed, such as the high-dose (HD) vaccine for the elderly,Citation7 the WHO identifies the IBV strain to be prioritized for TIV formulations.
In this study, we investigated the polyclonal antibody response to influenza B viruses elicited by a split, inactivated influenza virus (IIV) vaccine (Fluzone™, Sanofi Pasteur, Swiftwater, PA, USA) in a cohort of individuals that were vaccinated over three consecutive influenza seasons (2014–2015 to 2016–2017) in the Northern Hemisphere. Serum samples were collected and analyzed for anti-hemagglutinin (HA) immune responses against currently circulating IBVs, as well as predecessor vaccine strains and other historical IBVs. The goal was to determine if vaccine-elicited immune responses were influenced by the age of the individual and/or the pre-existing anti-HA immune status as a result of previous influenza vaccinations or infections. Vaccinated subjects were examined for responses to the HA components in the current vaccine, whether seroconversion to the vaccine strain(s) stimulated immunity against past-historical IBV strains (recently described as the ‘back-boosting’ phenomenon), and if sera collected from individuals with pre-existing hemagglutination-inhibition (HAI) breadth of activity against past vaccine strains varied between age groups. Understanding how responses to influenza vaccination are influenced by pre-existing influenza immunity in people of different ages may be critical for designing successful next-generation ‘universal’ or broadly protective influenza vaccines.
Materials and methods
Ethics statement and role of the funding source
The study procedures, informed consent, and data collection documents were reviewed and approved by the Western Institutional Review Board and the Institutional Review Boards of the University of Pittsburgh and the University of Georgia. The funding source had no role in sample collection nor decision to submit the paper for publication.
Subjects
Eligible volunteers between the ages of 18 and 85 years old (y.o.), who had not yet received the seasonal influenza vaccine, were enrolled beginning in September of each year. All vaccine formulations are based on WHO recommendations for the Northern Hemisphere influenza seasons beginning in the Fall, and as such, all vaccinations and collections occurred each year between September 1st to December 15th. Influenza virus did not circulate widely in the community during the time periods that the volunteers participated, and as such, participants were not monitored for influenza virus infection during that time-period; they were however asked during each visit if they had flu symptoms, and those who did were excluded from the study.
Volunteers were recruited at medical facilities in two sites: Pittsburgh, Pennsylvania and Stuart/Port St. Lucie, Florida. All were enrolled with written, informed consent. Exclusion criteria included documented contraindications to Guillain-Barré syndrome, allergies to eggs or egg products, and an estimated life expectancy <2 years. Anyone with dementia or Alzheimer disease was excluded due to an inability to provide informed consent. And those with current or recent medical treatment or a diagnosis of an immunocompromising condition were excluded due to a potentially limited or skewed response to the vaccine that would not be representative of the general population. Concurrent participation in another influenza vaccine research study where the current seasonal influenza vaccine was already received was another exclusion criterion, but otherwise, previous vaccine history was not a factor nor was this information collected due to the limited reliability of self-reporting.
These two cohorts spanned four years from 2013 to 2016. The first year enrolled only 127 eligible subjects and the subsequent years included 277 eligible subjects in the 2014 season, 267 eligible subjects in the 2015 season, and 257 eligible subjects in the 2016 final season (). Over years 2-4, there were 177 eligible subjects who participated sequentially in all three seasons of collections. This paper will particularly focus on these returning participants and the 2014 to 2016 seasons.
Table 1. Demographics of volunteers.
Blood (70–90 ml) was collected from each subject at the time of vaccination (D0) and collected again 7–9 days (D7) and 21–28 days (D21) post-vaccination. Blood samples were processed for sera and peripheral blood mononuclear cells (PBMC). For PBMC isolation, blood was collected in CPT tubes at D0, D7, and D21. These samples were processed immediately, within 1–24 h of collection, and stored at −150°C for future analysis. Sera were collected in SST tubes and processed within 24–48 h, storing at 4°C until separated and aliquoted for long-term storage at −30°C. These serum samples were tested for the ability to mediate hemagglutination inhibition (HAI) against a panel of influenza viruses representing historical and current vaccine strains. We previously showed this type of analysis for influenza A subtypes H1N1 and H3N2,Citation8 but this paper will focus on the response to the influenza B lineages Victoria and Yamagata.
Vaccine formulation
Each year, volunteers between the ages of 18–64 years received the standard-dose (15 µg/HA) inactivated influenza vaccine (IIV-SD) and subjects over 65 years of age were offered IIV-SD or a high dose (60 µg/HA) inactivated influenza vaccine (IIV-HD). Both IIV-SD and IIV-HD were split-virion versions of licensed FluzoneTM (Sanofi Pasteur, Swiftwater, PA, USA) and were administered as 500 µl doses. The IIV formulations consisted of three or four strains of influenza virus in accordance with recommendations from the WHO ().
Table 2. Fluzone vaccine formulations for Northern Hemisphere.
For the 2014–2015 season, a trivalent influenza vaccine (TIV) was used that included B/Massachusetts/2/2012 (Yamagata-lineage) along with A/California/7/2009 (H1N1) and A/Texas/50/2012 (H3N2). The 2014–2015 season was the first year the WHO made recommendations for a quadrivalent influenza vaccine (QIV) formulation containing both of the IBV lineages, but the 2015–2016 season was the first season in this study to use the QIV formulation; it was composed of B/Phuket/3073/2013 (Yamagata-lineage) and B/Brisbane/60/2008 (Victoria-lineage) along with A/California/7/2009 (H1N1) and A/Switzerland/9715293/2013 (H3N2). The 2016–2017 year also used QIV and it contained the same strains as the previous year except that the H3N2 component was switched to A/Hong Kong/4801/2014 (H3N2). Subjects aged 65 and older who were vaccinated with the IIV-HD formulation received a TIV version instead of the standard QIV. For those receiving TIV versions in these final two years, the Yamagata-lineage was chosen as the primary strain in 2015, and the Victoria-lineage was chosen as the primary strain in 2016.
Hemagglutination-inhibition (HAI) assay
The hemagglutination inhibition (HAI) assay was used to assess functional antibodies to the HA able to inhibit agglutination of turkey erythrocytes. The protocols were adapted from the WHO Laboratory Influenza Surveillance Manual.Citation9 To inactivate non-specific inhibitors, sera were treated with a receptor-destroying enzyme (RDE) (Denka Seiken, Co., Japan) prior to being tested. Briefly, three parts of RDE were added to one part of sera and incubated overnight at 37°C. RDE was inactivated by incubation at 56°C for 30–45 min and then cooled to room temperature before diluting with 1x PBS to a final sera concentration of 1:10. RDE-treated sera were serially diluted in PBS two-fold across 96-well V-bottom microtiter plates. An equal volume of each influenza virus (25 μl), adjusted beforehand via Hemagglutination (HA) Assay to a concentration of 8 hemagglutination units (HAU)/50μl, was added to each well. The plates were covered and incubated at room temperature for 20 min, and then 0.8% turkey erythrocytes (Lampire Biologicals, Pipersville, PA, USA) in PBS were added. Red blood cells (RBCs) were prepared fresh each week, stored at 4°C, and used within 72 h of preparation. The plates were mixed by agitation and covered, and the RBCs settled for 30 min at room temperature. The HAI titer was determined by the reciprocal dilution of the last well that contained non-agglutinated RBCs. Positive and negative serum controls were included for each plate. Seroprotection was defined as HAI titer ≥1:40 and seroconversion as a 4-fold increase in titer compared to baseline resulting in a titer of ≥1:40, as per the WHO and European Committee for Medicinal Products to evaluate influenza vaccines.Citation10 People were considered seronegative with a titer less than 1:40.
Anti-HA enzyme-linked immunosorbent assay (ELISA)
ELISA was performed to assess the presence of HA-reactive antibodies in the 2016 serum samples. We previously showed with 2014 and 2015 samples for H1N1 and H3N2 that there was no significant difference between years as much as within each year between pre- and post-vaccination.Citation8 Trimeric, recombinant HA (rHA) was captured and purified via a C-terminal histidine tag on HisTrap excel nickel-affinity chromatography columns (GE Healthcare Life Sciences, Marlborough, MA, USA). Full-length rHA proteins were developed for each of the IBV lineages corresponding to the 2016 vaccine strains: B/Phuket/3073/2013 (Yamagata-lineage) and B/Brisbane/60/2008 (Victoria-lineage). Immulon 4 HBX 96-well microtiter plates (Thermo Fisher, Waltham, MA, USA) were coated with 0.5 µg/ml rHA in a solution of carbonate buffer, pH 9.4, and 5 µg/ml fraction V bovine serum albumin (BSA) (Equitech-Bio, Kerrville, TX, USA) and then stored overnight in a humidified chamber at 4°C. Plates were then blocked with a blocking buffer (PBS containing 5% BSA, 2% bovine gelatin, and 0.05% Tween 20) for 60 min at 37°C. Serum samples were added in duplicate, along with positive control serum in columns 11 and 12, and then two-fold serially diluted in blocking buffer and incubated overnight at 4°C. Human sera samples were added at an initial 1:500 dilution for IgG sets and an initial 1:50 dilution for IgA. Additionally, row H columns 1–10 were left blank for background subtraction. Following overnight incubation with serum, plates were washed four times with 1x PBS and then incubated for 90 min at 37°C after adding the appropriate heavy chain-specific secondary antibody (2° Ab), Goat anti-Human IgG-HRP or Goat anti-Human IgA-HRP (Southern Biotech, Birmingham AL USA), at a 1:4000 dilution in blocking buffer. Plates were then washed again four times with 1x PBS, and then an ABTS Diammonium Salt (aMReSCO, Solon, Ohio, USA) substrate solution is added to plates for 20 min before being stopped with a 1% SDS solution. Plates were read using a spectrophotometer (BioTek, Winooski, VT, USA) at 414 nm (OD414). The average of the background blanks was subtracted from the readouts, averaged between the duplicate samples, and then compared to an established end-point value (the average of the background value from the blanks multiplied by the number of blanks plus the standard deviation of these values). End-point titers of each individual is expressed as the Log10 of the last reciprocal serum dilution for which the OD414 readout is still 20% higher than the calculated end-point value.
Viruses and HA antigens
Influenza viruses were obtained through the Influenza Reagents Resource (IRR), BEI Resources, the Centers for Disease Control (CDC), or were provided by Sanofi Pasteur and Virapur, LLC (San Diego, CA, USA). Viruses were passaged once in the same growth conditions as they were received, in 10-day-old embryonated, specific pathogen-free (SPF) chicken eggs per the protocol provided by the World Health Organization.Citation9 Due to low influenza B virus sensitivity in the HAI test, viruses underwent ether-treatment as recommended by the Influenza Division of the CDCCitation11 in order to increase the sensitivity in detecting antigen-specific human serum antibody and allow more reliable detection of HAI rises following influenza B vaccination.Citation12 Ether-extracted split viruses were created from freshly harvested allantoic fluid and from previously frozen virus lots. Virus was mixed at a 1:1 ratio with anhydrous, diethyl ether (ACROS Organics/Fisher Scientific, Pittsburgh, PA, USA) in a beaker on a stir plate in a fume hood for four or more hours. The mixture was allowed to separate for ether to evaporate and then transferred to a BSC for use in HA and HAI assays. Titrations before and after ether treatment were performed with turkey erythrocytes and the virus was standardized to 8 HAU/50 μl for use in HAI assays.
The Victoria virus panel included the following strains: B/Hong Kong/330/2001 (B/HK/01), B/Malaysia/2506/2004 (B/May/04), B/Victoria/304/2006 (B/Vic/06), B/Brisbane/60/2008 (B/Bris/08), and B/Colorado/06/2017 (B/CO/17). The Yamagata virus panel included the following strains: B/Yamagata/16/1988 (B/Yam/88), B/Harbin/7/1994 (B/Hrb/94), B/Sichuan/379/1999 (B/Sic/99), B/Florida/4/2006 (B/FL/06), B/Wisconsin/01/2010 (B/WI/10), B/Texas/06/2011 (B/TX/11), B/Massachusetts/02/2012 (B/Mass/12), and B/Phuket/3073/2013 (B/Phu/13). Also included is the influenza B ancestral strains B/Lee/1940 (B/Lee/40), B/Maryland/2/1959 (B/MD/59), and B/Singapore/3/1964 (B/Sing/64).
Statistical methods
Statistical significance was calculated using a two-tailed paired Student t-test with Wilcoxon-signed rank test comparing day 0 to day 21. Values were considered significant for p ≤ 0.01. Unless otherwise stated, data are presented from at least three independent experiments.
Results
Demographics of volunteers
Subjects were recruited from Pittsburgh, Pennsylvania and Stuart/Port St. Lucie, Florida during the 2014–2015 to 2016–2017 influenza seasons. In the 2014–2015 season, 277 subjects enrolled in the study with ~70% of the subjects over the age of 50 years (). Comparable numbers of subjects were enrolled in 2015–2016 and 2016–2017 influenza seasons with similar age distributions. Throughout all years, three times more women were enrolled in the study than men. Approximately 70% of the subjects were self-identified as White, with 20% classified as Black/African American and 6–10% self-identified as Hispanic/Latino. Of the 277 volunteers enrolled in 2014, 177 participated in the next two seasons.
Antibody responses to the vaccine strains
During the 2014–2015 season, the vaccine only contained one of the two IBV components, the Yamagata-lineage (B/Mass/12). During the 2015–2016 and 2016–2017 influenza seasons, both Yamagata (B/Phu/13) and Victoria lineages (B/Bris/08) were represented in a quadrivalent (QIV) vaccine, along with the H1N1 and H3N2 influenza A components ().
There were statistically significant increases (2–3 fold) in the total anti-HA IgG and anti-HA IgA antibodies following vaccination against both the Yamagata and Victoria HA components (). There was no significant increase in either IgG or IgA against the Yamagata HA in the elderly subjects, however, there was an increase against the Victoria HA ()). However, there was a significant increase in antibodies with HAI activity in all age groups against both Yamagata and Victoria viruses in each of the three seasons ( and ). Prior to vaccination (D0), most subjects had a HAI titer ≥1:40 against the Yamagata component and continued to maintain that titer in each of the three subsequent seasons ( and ). The youngest age group had the highest percentage (88–98%) of people with a positive HAI titer at D0 and the oldest age group had the lowest percentage (58–85% with SD and HD combined) at D0 (). Similar trends were observed in these same subjects against the Victoria components.
Table 3. Hemagglutination-inhibition (HAI) | Geometric Mean Titer (GMT) ± Standard Error of the Mean (SEM) | Seroprotection at D0 & D21 + Seroconversion (SC) Rates.
Figure 1. Comparison of pre- and post-vaccination titers for IgG and IgA in ELISAs against rHA for influenza B in the 2016–2017 influenza season. The box-and-whisker plots show the lower (Q1) and upper (Q4) quartile representing the IgG and IgA endpoint dilution titer for anti-HA antibodies. The diagram also shows the geometric mean titer (GMT) for day 0 pre-vaccination and day 21 post-vaccination for each age group. The n value per age group is listed on the x-axis. 2016 serum samples were tested for anti-HA IgG antibody against (a) rHA for the B Victoria-lineage component of the vaccine, B/Brisbane/60/2008 (B/Bris/08), and (b) rHA for the B Yamagata-lineage component of the vaccine, B/Phuket/3073/2013 (B/Phu/13). 2016 serum samples were also tested for anti-HA IgA antibody against (c) rHA for the B Victoria-lineage component of the vaccine, B/Brisbane/60/2008 (B/Bris/08), and (d) rHA for the B Yamagata-lineage component of the vaccine, B/Phuket/3073/2013 (B/Phu/13). The y-axis is presented as a log scale and statistical significance shown as such: *p0.05; *p0.01; *p0.001; *p0.0001.
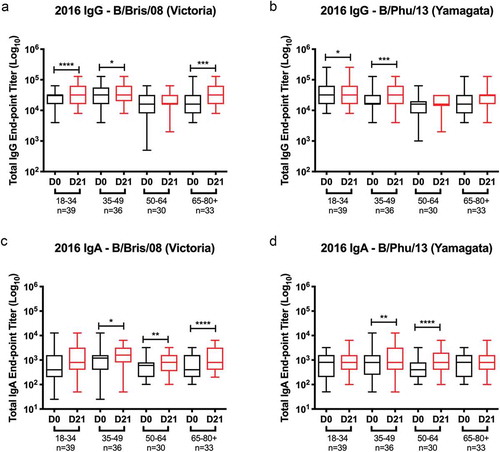
Figure 2. Hemagglutination inhibition (HAI) activity in serum antibody induced by FluzoneTM. HAI titers were determined from pre- (D0) and post-vaccination (D21) serum samples against the 2014–2016 influenza B viruses found in the Fluzone™ seasonal influenza vaccine. The vaccine strains consisted of B Yamagata-lineage strains (a) B/Massachusetts/2/2012 (B/Mass/12), and (b–c) B/Phuket/3073/2013 (B/Phu/13), and B Victoria-lineage strain (D-F) B/Brisbane/60/2008 (B/Bris/08). Values of each individual titer are the geometric mean titers (GMT) plus or minus the standard errors of the means (SEM), seen by the error bars. The box-and-whisker plots show the lower (Q1) and upper (Q4) quartile representing the ability of antibody to block viral attachment and the variability amongst the grouped data. The data are split into four different age groups for each component of the vaccine. The age group and n-value per age group is listed on the x-axis. Serum samples were tested (a and d) 2014 season, (b and e) 2015 season, (c and f) 2016 season. The y-axis is presented as a log scale and statistical significance shown as such: *p0.05; *p0.01; *p0.001; *p0.0001. (^^ note: The 2014 vaccine did not contain a Victoria component, so these increases seen are most likely a result of crossprotection/crossboosting from the Yamagata vaccine strain).
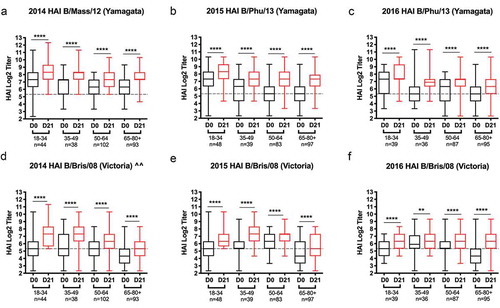
Figure 3. Seroconversion vs. seroprotection. Stacked bar graphs represent the seroconversion to the Yamagata- and Victoria-lineage vaccine strains over three consecutive influenza seasons: (a–c) 2014–2015, (d–f) 2015–2016, and [(g–i]) 2016–2017.The number of individuals for each age group per season are listed on the x-axis. For panels A, D, G, subjects were determined to be seroconverters (blue) following a 4-fold rise in HAI titer with a titer ≥1:40 at day 21 or non seroconverters (red). Individuals were then broken down based on their pre-vaccination response: panels B, E, H represent those seronegative (<1:40) at day 0, and panels C, F, I represent those seropositive (≥1:40) at day 0. For both data sets, red bars are indicative of those that are seronegative (<1:40) at day 21. For the middle set, blue bars represent volunteers that were seronegative at day 0 and then seropositive (≥1:40) at day 21. The last set has blue bars to show seroconverters (a titer of ≥1:40 at day 21 with at least a four-fold rise in titer from day 0) and green bars to show volunteers that are non-converters, but still seroprotected (a titer of ≥1:40 at day 21 with less than a four-fold rise in titer from day 0).
![Figure 3. Seroconversion vs. seroprotection. Stacked bar graphs represent the seroconversion to the Yamagata- and Victoria-lineage vaccine strains over three consecutive influenza seasons: (a–c) 2014–2015, (d–f) 2015–2016, and [(g–i]) 2016–2017.The number of individuals for each age group per season are listed on the x-axis. For panels A, D, G, subjects were determined to be seroconverters (blue) following a 4-fold rise in HAI titer with a titer ≥1:40 at day 21 or non seroconverters (red). Individuals were then broken down based on their pre-vaccination response: panels B, E, H represent those seronegative (<1:40) at day 0, and panels C, F, I represent those seropositive (≥1:40) at day 0. For both data sets, red bars are indicative of those that are seronegative (<1:40) at day 21. For the middle set, blue bars represent volunteers that were seronegative at day 0 and then seropositive (≥1:40) at day 21. The last set has blue bars to show seroconverters (a titer of ≥1:40 at day 21 with at least a four-fold rise in titer from day 0) and green bars to show volunteers that are non-converters, but still seroprotected (a titer of ≥1:40 at day 21 with less than a four-fold rise in titer from day 0).](/cms/asset/a1049895-5240-4bb7-bf19-021a5731640b/khvi_a_1642056_f0003_c.jpg)
Following vaccination (D21), subjects in the youngest age group had the lowest percentage of people that seroconverted to each IBV component (; )). The seroconversion rates correlated with the highest pre-vaccination HAI titers (D0) each season and were generally sustained each subsequent season (). Even though the Victoria HA component was not included in 2014–2015 vaccine formulation, there were seroconverters in all age groups to the Victoria HA component. The ability of the vaccine to elicit antibodies with HAI activity were assessed based on the titer prior to vaccination. Subjects were categorized as seronegative (defined as a HAI titer <1:40) ()) or seropositive (defined as a HAI titer ≥1:40) ()). Almost all young subjects that were seronegative at D0 were seropositive at D21 following vaccination to both IBV strains in all three seasons. There was an age-dependent decrease in the number of individuals that were seropositive on D21 with 65–85 y.o. subjects most likely seronegative following vaccination. Regardless of age, all subjects that were seropositive on D0 remained seropositive on D21 to both Yamagata and Victoria vaccine components, and many seroconverted to one or both of these ()). Overall, the rates of seroconversion are dependent on the HAI titers to the vaccine component prior to vaccination with people seronegative prior to vaccination having higher seroconversion rates to the vaccine components.
To examine the potential of the TIV vaccine to elicit cross-reactive antibodies with HAI activity against the IBV component not included in the vaccine, serum was assessed post-vaccination from subjects that were vaccinated with the HD vaccine in 2014–2015 and 2015–2016. The HD vaccine elicited higher seroconversion rates when the specific IBV lineage strain was included in the vaccine. When one of the two IBV HA lineage components was omitted, the post-vaccination titers and seroconversion rates were lower to the missing component in these subjects compared to people that received the SD QIV vaccine formulation ().
Seroprotective HAI titers analyzed by age group over multiple seasons
In order to determine the effect of pre-existing antibodies on the elicitation of HAI activity against current IBV vaccine strains, we categorized the HAI responses to the two IBV vaccine components over three consecutive Northern Hemisphere seasons: 2014–2015, 2015–2016, and 2016–2017 (). Subjects immunized over these three consecutive seasons were categorized according to their seroprotection status at each of the six-time points or instances analyzed (days 0 and 21 in all 3 years) for these IBV lineages like was previously described for IAV.Citation8 For example, individuals in each age group that had HAI titers <1:40 at all six-time points were assigned a value of “0”. If an individual had a HAI titer of ≥1:40 at one of the six instances, their response was scored as a “1”, at two of the six instances as a “2”, at three of the six instances as a “3”, at four of the six instances as a “4”, at five of the six instances as a “5”, and if the titer was ≥1:40 all six times, this response was scored as a “6”.
Figure 4. Seropositivity over multiple influenza seasons. One hundred and seventy-seven volunteers that took part in all three consecutive seasons (2014–2015, 2015–2016, and 2016–2017) were assessed for seropositive titers to the (a) Yamagata and (b) Victoria components of the vaccine at day 0 and day 21. Each volunteer was scored 0–6 according to their seroprotection status at each of the 6-time points analyzed (days 0 and 21 in all 3 years). Subjects in each age group that had HAI titers less than 1:40 at all 6-time points were assigned a value of “0”. Subjects with a HAI titer of 1:40 or greater at 1/6 time points were categorized as a “1” and so forth until “6” represents those that had a HAI titer of 1:40 or greater at 6/6 timepoints. The n-value for each age group is listed beneath each pie chart. The 65–85 y.o. group is shown as a collective and further divided based on receivers of high dose (HD) trivalent vaccine versus the standard dose (SD) quadrivalent vaccine. *Note: 2014–2015 season was with a trivalent vaccine that included no Victoria component, but data against the WHO-recommended strain is shown for consistency.
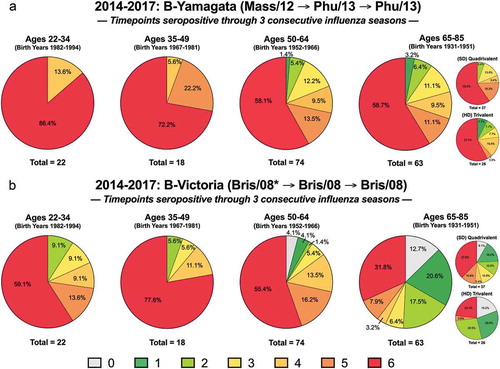
One hundred and seventy-seven individuals were enrolled and vaccinated over these three influenza seasons. In regards to the Yamagata-lineage component of the vaccine, 100% of the two youngest age groups (18–49 y.o.) had seropositive HAI activity against 4–6 timepoints whereas around 80% of the two oldest age groups (50–85 y.o.) did ()). There were lower seropositive rates overall against the Victoria-lineage component, but this was especially evident for the oldest age group (65–85 y.o.) that had 43% seropositive at 4–6 timepoints, compared to 82–89% between the other ages ()). Only the two oldest groups had volunteers that scored a “0” but this was higher for the elderly, particularly those that received the HD TIV formulation where no Victoria component was included in the 2016–2017 season. Overall, more elderly subjects vaccinated with the SD QIV vaccine maintained a 1:40 titer against the Victoria-lineage component across the three seasons compared to those elderly subjects that received the HD TIV vaccine ()). Differences between these formulations in seropositivity to the Yamagata component were not nearly as stark ()).
HAI activity against a panel of historical influenza B vaccine strains
Sera collected pre- and post-vaccination from individuals that participated in the three consecutive seasons were tested for HAI activity against a panel of 16 IBV isolates: three strains representing the ancestral, pre-lineage split (“pre-split”) time period between 1940 and 1964, and 13 strains post-split from 1988 to 2017 that are further subdivided by lineage, eight Yamagata and five Victoria-lineage strains (). For the most part, subjects in all age groups had high levels of antibodies with HAI activity against the panel of Yamagata strains, with the highest titers observed in the youngest subjects. In contrast, these younger subjects had lower HAI titers against both Victoria-lineage and pre-split IBV strains (). Subjects in their 40s and early 50s had the highest HAI titers against the Victoria-lineage strains ( and ). Interestingly, subjects within all age groups showed some HAI activity against the older, pre-split IBV strains, but as expected, older people between 50 and 70 years of age had sera with broadly cross-reactive HAI titers against these three viruses isolated between 1940 and 1964 ().
Figure 5. Heat map of HAI results. Serum samples were collected from volunteers at pre-vaccination (D0) and post-vaccination (D21) and tested for HAI activity against a panel of influenza B viruses. The volunteers shown here are a subset of those that took part three consecutive years, and as such, these heat maps show each subject’s HAI titer against each strain for 3 consecutive years. The subtypes of the tested viruses are listed on the upper x-axis, beginning with those prior to the formation of influenza B lineages, referred to as “Pre-Split” (3 viruses), followed by Yamagata-lineage (8 viruses), and then Victoria-lineage (5 viruses). Within each subtype the season the serum was collected is shown (2014–2016 start), and below that the year of the virus strain tested (listed in chronological order from left to right going from oldest to most recent strain within each subtype). Years in red indicate the vaccine strain for a given year (note: 2014 season was a trivalent vaccine that did not include a Victoria component). The y-axis shows the volunteers, color-coded by age group (listed from youngest to oldest going down). HAI titers greater than or equal to 1:40 are highlighted on a color scale and those less than 1:40 are not colored (1:20, 1:10, and 1:5).
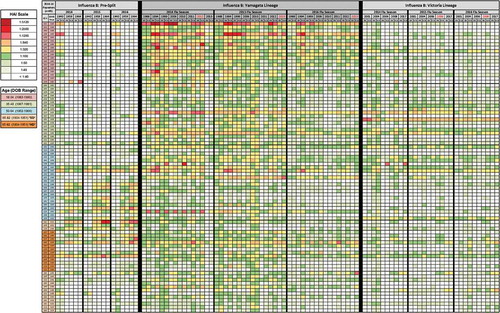
The overall breadth of the HAI responses appeared to decline over time with a decrease in the average HAI titer ( and ). In order to help rule out whether this decline could be a causation of repeat vaccination, the data were compared to that of new volunteers in 2015–2016 (n = 19) and 2016–2017 (n = 47) of relatively equal age distribution who did not take part in the study previously. Previous vaccination status is unknown, but it is assumed that some of this population, if not most, were unvaccinated the year prior. Comparing the pre- and post-vaccination titers of these to the 85 three-year repeaters (), there was no significant difference in fold change, with a very minimal decline or gain observed for the Victoria-lineage strain, ranging from −0.45 GMT to +0.14 GMT depending on the age group (data not shown). The Yamagata-lineage strain declined more, but this was also not significant, ranging from −0.78 GMT to −0.33 GMT depending on the age group (data not shown). Therefore, something else unrelated to repeat vaccination is probably pushing this decline.
Sera collected from subjects in all ages groups had antibodies with HAI activity against the B/Lee/40 virus (). Few subjects under the age of 50 years had antibodies with HAI activity against the 1959 or 1964 pre-split influenza B viruses. In contrast, subjects over the age of 50 years had more intense HAI titers against these two viruses.
Breadth of back boosting response post-vaccination as a function of influenza exposure history or age
Individuals were assigned to groups based on serological response to the Yamagata component () and the Victoria component (). Vaccine responses of pre-vaccination (D0) and post-vaccination (D21) sera were determined by HAI and subsequently individuals were classified into one of four different seroreactivity groups: Group 1 = seronegative (SN) at D0 and D21; Group 2 = seronegative at D0 and seropositive (SP) at D21; Group 3 = seropositive at D0 and D21, without seroconverting (SC); Group 4 = seroconverting at D21 from a seropositive status at D0. Increased responses to Yamagata () indicate “back-boosting” to other Yamagata lineage viruses and cross-reactivity to Victoria lineage viruses. Increased responses to Victoria strains () indicate cross-reactivity to Yamagata lineage viruses and “back-boosting” to Victoria lineage viruses.
Table 4. Broadly reactive seroprotection and cross-reactivity between IBV lineages induced by the Yamagata component.
Table 5. Broadly reactive seroprotection and cross-reactivity between IBV lineages induced by the Victoria component.
For either analysis, and regardless of age, few subjects were classified as Group 1. The majority of subjects fell into Group 3, and in all seasons, the serum samples collected post-vaccination from this group had HAI activity against nearly all of the viruses in the panel. Subjects classified in Group 2 had the biggest increases in the number of people with HAI activity. There was a 38% increase in the number of subjects that recognized the Yamagata virus in the vaccine () and a 24% increase in people that recognized the Victoria component (). The youngest subjects (18–34 and 35–49 y.o.) were more likely to be seropositive at D0 prior to vaccination to either of the Yamagata () or Victoria () components. As such, they were more prevalent in Groups 3 and 4. The opposite was generally the case for the oldest subjects (50–64 and 65–85 y.o.), and so fewer subjects were categorized in these groups, particularly Group 4.
Discussion
In this study, people representing different age groups were vaccinated in three consecutive influenza seasons with a split, inactivated influenza vaccine (Fluzone™). Each vaccine contained one or two influenza B strains (Yamagata and Victoria lineages) along with two influenza A strains (H1N1 and H3N2). Following vaccination, the antibody responses to influenza B viruses were correlated with age and pre-existing immune status in a manner similar to our IAV analysis.Citation8 During this analysis, the Yamagata lineage component, B/Mass/2012, shifted following the 2014–2015 season to B/Phu/13 for the 2015–2016 and 2016–2017 seasons. The vaccine switched from including a Yamagata clade 2 strain to a Yamagata clade 3 virus, and while titers did drop, seroprotection rates were still high. This may be a result of previous exposure to these two clades prior to the start of this study through natural infection, or via recent split, inactivated influenza vaccines containing representatives of these clades, such as B/Wisc/10, a Yamagata clade 3, and B/FL/06, a Yamagata clade 2 (). The Victoria lineage component, B/Bris/08, stayed consistent and was recommended over these three seasons, however, it was not present in the 2014–2015 SD vaccine or 2015–2016 HD vaccine. But given that it has been circulating for some time and was included in three consecutive split, inactivated influenza vaccines beginning with the 2009–2010 season, it is unlikely many of these volunteers have not been exposed to it previously.
In each of the three seasons, the average HAI titer against both IBV components increased following vaccination, irrespective of age ( and ). And from one year to the next when comparing post-vaccination of a year to the following year’s pre-vaccination titer (i.e. 2014 D21 – 2015 D0), titers drop. The lowest rate of seroprotection following vaccination is observed in the elderly, which is similar to the IAV results,Citation8 that shows the elderly population consistently having fewer seroprotected individuals both prior to and following vaccination. Because of this, the waning effect following a season is more significant in the elderly population as they are more likely to drop to a seronegative status (<1:40). This is reflected in the listed GMTs and seroprotective titers for each age group () as well as via the differences for ages in scoring for seropositivity across the three seasons (). It is clear that vaccinations are providing a boost in HAI titers, a correlation of increased immunity to help individuals become more protected during flu season. Whether it is a short-term or long-lasting boost in immunity, this data nonetheless strongly emphasizes the increased importance of elderly individuals being vaccinated.
Usually, the first influenza virus infection as a child (typically under 5 y.o.) imprints on the immune memory the antigenic type of the infecting influenza virus.Citation13 This first infection may differentially imprint an antibody repertoire that can later effect immune responses to subsequent influenza virus exposures.Citation13-Citation15 Most studies on imprinting have focused on IAV viruses, but this condition most likely also occurs during IBV infections. It is not currently well understood if high HAI titers to a specific IBV lineage reflects the dominate circulating IBV strains at the time of first IBV infection. It is unclear if IBV imprinting is independent of IAV imprinting or if IAV imprinting interferes with IBV imprinting. Additional studies need to be performed to tease out these answers. But in our study, there is a clear preference for influenza B viruses of specific lineages or eras, which appears to be associated with the age of the subjects. Historically, IBV strains of the Victoria lineage dominated global circulation from 1987 to 1989, but declined as the Yamagata lineage increased in the 1990s.Citation3 The Victoria lineage re-emerged as the dominant strains in 2001, yet for the next decade, strains from both IBV lineages co-circulated and traded off dominant seasons. The Victoria lineage strains dominated in six of these northern hemisphere influenza seasons, while Yamagata lineage strains dominated the other four.Citation3 In many previous influenza seasons, there was a mismatch between the IBV strain selected for inclusion in the vaccine and the lineage of the dominant IBV strain circulating in the human population.Citation3 As a result, the FDA approved a quadrivalent SD influenza vaccine in 2012. Nevertheless, even when one lineage is omitted from the vaccine, the HAI titers still significantly increase following vaccination. This may be due to cross-reactive epitopes that efficiently recall memory B cells with broadly reactive antibodies to increase the titers against antigenically related, but distinct co-circulating strains. This phenomenon has been observed with ferret reference sera. Ferrets infected with an IBV from one lineage has antibodies with HAI activity against both the infecting virus, but also strains from the opposite lineage, as well as the pre-split, B/Lee/40 IBV.Citation16-Citation20 Therefore, younger subjects born decades after the lineage split in 1987 may have cross-reactive antibodies to these older IBV strains. This phenomenon was observed in this study during the 2014–2015 season. Subjects administered a TIV vaccine formulation with the Yamagata-lineage component also elicited a rise in antibodies with HAI activity against the Victoria-lineage strain not included in the vaccine (). Similarly, during the 2016–2017 season, elderly subjects administered the HD TIV vaccine containing only the Victoria-lineage component had a rise in antibodies with HAI activity against the Yamagata IBV strain (). However, immunological priming is often needed for this cross-reactive binding.Citation19-Citation21 Previous exposures to IBV via infection or vaccination is most effective in the elicitation of cross-protective antibodies. This is especially the case with the Victoria lineage where studies have shown that children without detectable antibodies against Victoria-lineage strains prior to vaccination failed to exhibit a rise in these antibodies following vaccination with TIV vaccines containing only a Yamagata strain.Citation19,Citation20
Annual, repeated vaccination may have negative effects on the vaccine-elicited immune responses by accelerating antibody re-focusing towards prior or historical epitopes instead of newly evolved epitopes.Citation22 This phenomenon may select for cross-reactive, non-neutralizing antibodies, instead of cross-reactive, neutralizing antibodies.Citation22 However, there is little evidence equating repeated annual vaccination with the ability to elicit antibodies that recognize past influenza strains. And despite any differences from one year to the next in the boosting effect of these vaccines, it is clear that influenza vaccines are still boosting titers. Altogether, this exemplifies the need for annual vaccinations to boost waning titers, especially for the elderly who are more likely to be seronegative prior to vaccination.
Overall, the responses to the Yamagata component were higher in magnitude and duration with all age groups having over 50% of the subjects retain antibodies to this component at titers ≥1:40 over the three seasons. For both TIV and QIV vaccine, the Yamagata component was included in most of the past six seasons (), but for three consecutive seasons (2012–2014) prior to the start of this study, the Victoria component was absent from the influenza vaccine. The lack of the Victoria lineage strain in the vaccine for the 2014–2015 season most likely explains the lower HAI titers and duration of responses to the Victoria lineage component in vaccinated subjects, but these results could also be a contribution of individual genetic and host factor responses, or even the differential receptor binding properties that vary between the viruses in each lineageCitation23,Citation24 and in young children.Citation25 Future research will examine IBV vaccinations in infants and children.
There were similarities in the elicited immune responses to IAV and IBV following vaccination.Citation8 Similar to IAV, repeated vaccination increased the total IgG anti-hemagglutinin (HA) binding antibody titer, as well as the hemagglutination-inhibition (HAI) activity, but there was no significant year to year differences in the responses. Following vaccination, there was a 1–2 log increase in total IgG titer and no pattern of decreased anti-HA antibody by age. However, the elderly had significantly lower HAI titers for both IAV and IBV, while maintaining similar, higher rates of seroconversion following vaccination. Subjects born between 1964 and 1980 had higher HAI titers to the Victoria component than people born before or after this time period (). In contrast, the same subjects had sera with distinct patterns of HAI activity against the H1 and H3 components of the vaccine.Citation8 Young adult subjects had a strong bias to the most recent 20 years of H1 influenza viruses, whereas people over the age of 35 had sporadic H1N1 activity to strains isolated in the past 100 years. All age groups had strong HAI activity against H3N2 viruses isolated from 2005 to 2014, but people under 40 years of age also had strong HAI activity against H3N2 viruses from 1985 to 2004. Overall, this cohort of vaccinated subjects had differential recognition of both IAV and IBV vaccine components in the same vaccine, likely a result of the different exposure histories to past influenza viruses and vaccinations. Compared to IAV, there was considerable HAI activity across all age groups and both IBV lineages, including older IBV strains, thus indicating strong antibody cross-reactivity across IBV strains and lineages. The serum HAI titers against influenza B viruses could be inflated due to the use of ether-treated IBV antigens, as described in the methods. The ether-treated IBVs used in the HAI panel increases the sensitivity of the assay without interfering with the specificity of the assay. The fold increases in the pre- and post-vaccination HAI titers were similar between the ether-treated and non-treated influenza B viruses (data not shown).
While vaccine effectiveness is important for protection in the given season, it may differentially alter protection to past viruses should they recirculate or mutate into related viruses in the future. We previously reported that ‘back-boosting’ to antigenically related strains are associated with seroconversion to the vaccine strain,Citation8 and while this still holds true here for IBV, there is a clear differentiation between seroconversion from a seronegative state versus seroconversion from a seropositive state and more analysis of this should be performed, for seroconversion in general as well as in relation to IBV antibody cross-reactivity. In this study, the hypothesis that individuals with a breadth of pre-existing immunity against historical IBV strains benefit from immunization with the currently licensed split IIV influenza vaccines is supported. The ability to seroconvert to the vaccine is a primary determinant in a rise in antibodies to past influenza variants and most likely to co-circulating variants in any given season. The mechanism behind this ‘back-boosting’ phenomenon is currently unknown, but in older people, is most likely due to the recalling of memory B cells that results in the rise of anti-influenza HA antibodies against many epitopes and viral variants.Citation25 For young adults though, who may not necessarily have been exposed to these past-historical strains, it may be that some HA immunogens induce novel antibodies with extensive cross-reactivity. As such, influenza exposure history by infection and through previous vaccination influences the breadth of immunity elicited through an annual vaccination. Future studies are needed to examine the vaccine-induced antibody in infants and children, since they are often easily infected with influenza B viruses.
Disclosure of potential conflicts of interest
TMR and RKZ have research funding from Sanofi Pasteur, Inc. RKZ has additional support from Merck & Co, Inc., Pfizer Inc. TMR is also supported, in part, by the Georgia Research Alliance as an Eminent Scholar.
Acknowledgments
The authors would like to thank Ivette A. Nuñez, Thomas M. Rowe, James D. Allen, Spencer R. Pierce, Jeff W. Ecker, Bradford C. Lefoley, Simon O. Owino, Krissy K. Moehling, Patricia Nowalk, Michael Susick, Kensington Diagle, and Kristen Sweeney for technical assistance. We would also like to thank Greg A. Kirchenbaum and Anne G. Bebin-Blackwell for helpful discussions and comments. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: MDCK; Kidney (Canine), Working Cell Bank, NR-2628.
Additional information
Funding
References
- Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356:1861–70. doi:10.1098/rstb.2001.0999.
- Osterhaus AD, Rimmelzwaan GF, Martina BE, Bestebroer TM, Fouchier RA. Influenza B virus in seals. Science. 2000;288:1051–53. doi:10.1126/science.288.5468.1051.
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8:81–88. doi:10.4161/hv.8.1.17623.
- Vijaykrishna D, Holmes EC, Joseph U, Fourment M, Su YC, Halpin R, Lee RT, Deng YM, Gunalan V, Lin X, et al. The contrasting phylodynamics of human influenza B viruses. Elife. 2015;4:e05055. doi:10.7554/eLife.06416.
- Hadfield J, Megill C, Bell SM, Huddleston J, Potter B, Callender C, Sagulenko P, Bedford T, Neher RA. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–23. doi:10.1093/bioinformatics/bty407.
- Langat P, Raghwani J, Dudas G, Bowden TA, Edwards S, Gall A, Bedford T, Rambaut A, Daniels RS, Russell CA, et al. Genome-wide evolutionary dynamics of influenza B viruses on a global scale. PLoS Pathog. 2017;13:e1006749. doi:10.1371/journal.ppat.1006749.
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–45. doi:10.1056/NEJMoa1315727.
- Nunez IA, Carlock MA, Allen JD, Owino SO, Moehling KK, Nowalk P, Susick M, Diagle K, Sweeney K, Mundle S, et al. Impact of age and pre-existing influenza immune responses in humans receiving split inactivated influenza vaccine on the induction of the breadth of antibodies to influenza A strains. PLoS One. 2017;12:e0185666. doi:10.1371/journal.pone.0185666.
- Organization WH, Network WGIS. Manual for the laboratory diagnosis and virological surveillance of influenza. Geneva, Switzerland: World Health Organization; 2011.
- Agency EM. 2014. Guideline on influenza vaccines: Non-clinical and clinical module [Draft], London E14 4HB (UK).
- Organization WH. The 2017–2018 WHO influenza reagent kit for identification of influenza isolates, on who collaborating center for surveillance, epidemiology and control of influenza; 2017 Nov 12. https://www.internationalreagentresource.org/Portals/6/2017-2018%20WHO%20Kit%20Insert%20FINAL%20FINAL_Oct17_1.pdf?ver=2017-10-06-144117-843.
- Kendal AP, Cate TR. Increased sensitivity and reduced specificity of hemagglutination inhibition tests with ether-treated influenza B/Singapore/222/79. J Clin Microbiol. 1983;18:930–34.
- Monto AS, Malosh RE, Petrie JG, Martin ET. The doctrine of original antigenic sin: separating good from evil. J Infect Dis. 2017;215:1782–88. doi:10.1093/infdis/jix173.
- Zhang A, Stacey HD, Mullarkey CE, Miller MS. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J Immunol. 2019;202:335–40. doi:10.4049/jimmunol.1801149.
- Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–78.
- Camilloni B, Neri M, Lepri E, Iorio AM. Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. Vaccine. 2009;27:4099–103. doi:10.1016/j.vaccine.2009.04.078.
- Asahi-Ozaki Y, Yoshikawa T, Iwakura Y, Suzuki Y, Tamura S, Kurata T, Sata T. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol. 2004;74:328–35. doi:10.1002/jmv.20173.
- Pyhala R, Kleemola M, Kumpulainen V, Vartiainen E, Lappi S, Ponka A, Cantell K. Immune response to inactivated influenza virus vaccine: antibody reactivity with epidemic influenza B viruses of two highly distinct evolutionary lineages. Vaccine. 1992;10:631–36.
- Levandowski RA, Regnery HL, Staton E, Burgess BG, Williams MS, Groothuis JR. Antibody responses to influenza B viruses in immunologically unprimed children. Pediatrics. 1991;88:1031–36.
- Levandowski RA, Gross PA, Weksler M, Staton E, Williams MS, Bonelli J. Cross-reactive antibodies induced by a monovalent influenza B virus vaccine. J Clin Microbiol. 1991;29:1530–32.
- Liu Y, Tan HX, Koutsakos M, Jegaskanda S, Esterbauer R, Tilmanis D, Aban M, Kedzierska K, Hurt AC, Kent SJ, et al. Cross-lineage protection by human antibodies binding the influenza B hemagglutinin. Nat Commun. 2019;10:324. doi:10.1038/s41467-018-08165-y.
- Huang KY, Rijal P, Schimanski L, Powell TJ, Lin TY, McCauley JW, Daniels RS, Townsend AR. Focused antibody response to influenza linked to antigenic drift. J Clin Invest. 2015;125:2631–45. doi:10.1172/JCI81104.
- Wang Y-F, Chang C-F, Chi C-Y, Wang H-C, Wang J-R, Su I-J. Characterization of glycan binding specificities of influenza B viruses with correlation with hemagglutinin genotypes and clinical features. J Med Virol. 2012;84:679–85. doi:10.1002/jmv.23219.
- Kumlin U, Olofsson S, Dimock K, Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respi Viruses. 2008;2:147–54. doi:10.1111/j.1750-2659.2008.00051.x.
- de Bruijn IA, Remarque EJ, Beyer WE, le Cessie S, Masurel N, Ligthart GJ. Annually repeated influenza vaccination improves humoral responses to several influenza virus strains in healthy elderly. Vaccine. 1997;15:1323–29.
