ABSTRACT
The study aimed to assess the capacity of AEFI surveillance during vaccination campaigns with the new conjugate meningitis vaccine (MenAfrivac). A systematic review of studies on MenAfrivac™ published in English during 2001–2016 was done.AEFIs incidence (I) was estimated and compared between MenAfrivac™ clinical trials and immunization campaigns using incidence difference (Id). Nine studies were included with an overall local AEFI I of 11,496/100,000 doses administered per week in clinical trials and 0.72/100,000 doses in immunization campaigns. An Id of 11,497.92 [11,497.91-11,497.93] and 17,243.20 [17,241.80-17,245.90] per 100,000 doses administered per week for overall local and systemic AEFI, respectively, were observed with highest from clinical trials. The incidence of AEFIs after MenAfrivac™ vaccination was far lower in campaigns than in clinical trial studies. Current capacity of AEFI surveillance during vaccination campaigns requires extensive re-assessment of its structure and capacity.
1. Introduction
1.1 Background
In clinical trials, the monitoring of vaccines and drugs safety is mandatory and harmonized. Phase I clinical trials have as main objective to assess vaccine safety but the number of participants is usually limited and consequently, do not allow the detection of rare adverse events.Citation1 Unlike the marketed phase of vaccines, gradually increasing sample size of participants in phases 2, 3, and 4 clinical trials remains insufficient to detect rare adverse events following immunization (AEFIs).Citation2
AEFIs surveillance after post-licensure is expected to improve vaccines safety by detecting and investigating as well as preventing the following; vaccine quality defect-related reaction, immunization error-related reaction, immunization anxiety-related reaction; to estimate vaccine reaction rates (background rates) in the population and to ensure and facilitate causality assessment of coincidental, serious and unexpected/unusual AEFIs.Citation1,Citation3–Citation5 It provides useful information to anticipate or respond to public concerns about the safety of vaccines and thus contributes to increase the adherence of the public to vaccination.Citation5,Citation6 The currently published studies indicate that AEFIs surveillance is below expectations due to weaknesses such as low detection rates, reporting rates and investigation of AEs.Citation7–Citation10 Comparing the geographical distribution of AEFI surveillance data provided useful information to monitor vaccines safety while mapping some weaknesses of AEFIs surveillance systems.Citation11 An estimation of the magnitude of the difference in incidence and type of AEFI collected during clinical development phases of vaccine and post-marketing surveillance; could be useful to evaluate the amount of AEs not reported by either vaccinees themselves or health personnel during campaigns or routine immunization.
The PSA-TT vaccine (MenAfrivac™) was developed to respond to meningococcal meningitis A outbreaks raging in more than 21 African countries.Citation12 MenAfrivac clinical trials demonstrated the vaccine to be safe and effective thus, the vaccine was licensed in India in 2009 and pre-qualified by WHO a year later.Citation12–Citation15 From the introduction of the vaccine in Burkina Faso in 2010 to 2012, 10 African countries have been targeted by vaccination campaigns, with a total of 100 million individuals vaccinated.Citation12 AEFI surveillance has been an important part of these campaigns with results published in three countries.Citation16–Citation19
Although the number of people vaccinated gradually increases from phase 1 to phases 4 clinical trials and to vaccination campaigns, there is a decrease in AEFIs incidence during vaccination campaigns due to reduced capacity of AEFIs surveillance. There is a public health advantage during mass campaigns as the immunized population during mass campaign does not only have a high sample size power but, the population is also diversified and therefore has the tendency to allow the detection of rare serious AEFI that may only occur in specific populations. These rare events are not expected to be captured during clinical trials because clinical trials do not have sufficient power to assess such rare AEFIs. Moreover, conditions of storage and use of vaccines are less likely to be complied with during mass campaigns and thus, predisposing to a potential increase of AEFIs incidence. With an aim to inform AEFIs surveillance activities for better planning and monitoring of MenAfrivac™ campaigns, this study compared the incidence and distribution of AEFIs reported during clinical trial phases to that reported during immunization campaigns.
2. Results
2.1. Study selection
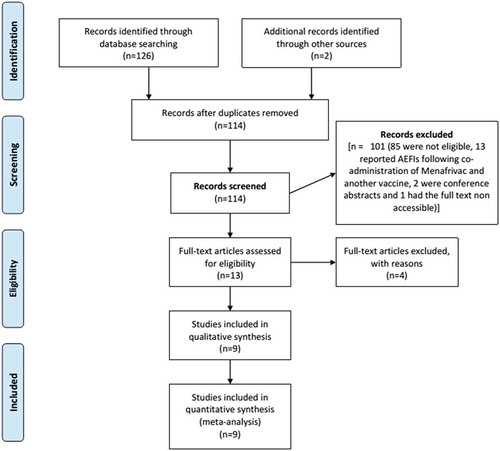
By March 17, 2015, the search identified 128 titles which included 53 (43.1%) from the Meningitis Vaccine Project (MVP) website, 63 (47.2%) from Medline, 10 (8.1%) from Ovid and 2 (1.6%) from references of articles. After eliminating duplicates, a total 114 titles and abstracts were further screened of which 13 full texts were selected and 9 included in the study. Four could not provide needed data to estimate AEFIs incidence and thus, were excluded.Citation20–Citation23
illustrates the selection process.
2.2. Study characteristics
presents characteristics of included articles. Three articles which include four studies reported clinical trials;Citation13–Citation15 three articles reported three studies on AEFI surveillance during immunization campaigns and one article reported a phase 4 field trial assessing MenAfrivacTM safety when delivered in a controlled temperature chain.Citation17,Citation18,Citation22,Citation25 With respect to geographical factors, two studies (clinical trials) were conducted in the Asian region precisely in India while the remaining six studies (two clinical trials and four vaccination campaigns) were conducted in the African region representing Mali, Gambia, Senegal, Niger, Burkina Faso, Benin, and Cameroon. For clinical trials, different age groups of participants ranging from 1 to 35 years were included in all of the four trial studies while for mass campaign studies, the same age group (1–29 years) of participants were included. Also, the number of persons vaccinated varied within clinical trials (with a minimum number of 24 and a maximum of 604) and mass campaign studies (minimum of 1999 and maximum of 11,117,555). All included studies had the same number of doses administered (0.5 ml) be it clinical trial or mass campaign and also, the same number of doses administered per person except for one clinical study, which administered a primary dose and a booster dose. All vaccines administered to participants both for trials and campaigns were manufactured by the same manufacturer (Serum Institute India Ltd).
Table 1. Characteristics of included studies.
presents characteristics of AEFIs surveillance among included studies. All included studies employed active surveillance except for one campaign study that used passive surveillance method for case detection. Two campaign studies used a combination of both active and passive surveillance methods. All clinical trial studies and one campaign study did not include case definitions for minor and serious AEFI while the remaining three campaign studies included case definition of AEFI which complied with the WHO case definition. The probability of detecting AEFI for all vaccinated persons was the same in clinical studies but not the same for three mass campaign studies. AEFI case reporting procedure was the same for all studies that had description of the reporting procedure. All included studies investigated serious AEs. The surveillance time for active follow-up varied across studies ranging from 4 to 7 days after vaccination.
Table 2. Characteristics of AEFI surveillance.
2.3 Incidence of AEFI in clinical trials and in immunization campaign studies
In total, 13,919,052 doses of MenAfrivacTM comprising 1190 from clinical trials and 13,917,862 from immunization campaigns were reported to have been administered following manufacturer’s recommendations from articles included in this study. IR of overall AEFI in clinical trials and immunization campaign studies are presented in . IR of AEFIs were lower in immunization campaign studies than in clinical trials. The AEFI IRs of clinical trials, mass immunization campaigns and overall were 10,046.9, 4.5 and 3.7 per 100,000 doses administered per week, respectively.
Table 3. Incidence rate (IR) of overall reported studies in clinical and campaign studies.
2.3.1 AEFI incidence in clinical trials
Most clinical trials recorded details on the type of AEFIs only for 4–7 days of surveillance; and therefore, only this period was considered in estimating IR of AEs per type and characteristics (local or systemic) in clinical trial studies. presents AEFI IR in clinical trial studies. The IR per 100,000 doses administered per week, for local and systemic AEFIs were 11,499, and 17,248, respectively. Among local AEFIs, the highest IR was reported for pain at injection site while the highest among systematic AEFIs was headache.
Table 4. Incidence of different types of AEFIs following MenafrivacTM in clinical trials studies.
2.3.2 AEFI incidence in mass vaccination campaigns
presents IRs of AEFIs reported in studies on vaccination campaigns. The IR per 100,000 doses administered per week of local and systemic AEFIs were 0.72 and 2.63, respectively, for immunization campaigns. The highest IR reported among local reactions was a pain at the injection site and fever among systematic reactions.
Table 5. Incidence of types AEFIs following MenafrivacTM in vaccination campaign studies after 42 days of surveillance.
2.3.3 Serious AEFI in clinical trials and vaccination campaign studies
A total of 51 severe AEFI were reported including 10 (1.56 serious AEFIs per 100,000 doses administered per week) from clinical trials and 41 (0.08 serious AEFIs per 100,000 doses administered per week) from mass vaccination campaign studies. and present types and etiologies of severe AEFIs in clinical trials and vaccination campaigns, respectively. Types of AEFI reported in clinical trial studies differed to that reported during vaccination campaign studies. After causality assessment of all cases reporting with severe AEFI, none of the serious AEFIs reported during clinical trials was related to the vaccine. Causality assessment was conducted for 40 out of 41 reported serious AEFIs in vaccination campaign studies and 5 were probably related to vaccine (12.5%) while 5 (10%) were not classified because of lack of information.
Table 6. Type and etiology of serious MenAfrivacTM AEFIs in clinical trials.
Table 7. Types and causes of serious AEFIs following administration of MenAfrivacTM in vaccination campaigns.
2.4. Comparing AEFI incidence rate in clinical trials and vaccination campaign studies
The IRds of local, systemic, types and serious AEFI are presented in . IR of AEFIs were lower in immunization campaign studies than in clinical trials, with the difference (IRd) for overall AEFIs being 10,043 (95% CI 10,042–10,044) per 100,000 doses per week, for local being 11,498 (95% CI 11,498–11,498), for systemic being 17,243 (95% CI 17,242–17,246) and for serious AEFIs being 1.55 (95% CI 1.54–1.56). In some studies, some types of AEFIs were aggregated and thus, the IRd of these AEFI were not presented in this study. Headache had the highest IRd [3,622 (95% CI 3,622–3,623)] among systemic reactions while pain at injection site had the highest IRd 5,118.90 [95% CI 5,118.86–5,118.94] among local reactions. An attributable fraction of AEFIs IR was more than 99% for overall, local and systemic AEFIs and also for serious AEFIs in clinical trials than immunization campaigns.
Table 8. Incidence rate difference of types AEFI between clinical trials and immunization campaigns.
2.5. Risk of bias within studies
Two review authors independently assessed the risk of bias of each included study against key criteria such as random sequence generation, allocation concealment, surveillance time and minor and serious AEFI case definitions, incomplete outcome data and participant follow-up procedures and selective reporting, in accordance with methods recommended by the Cochrane Collaboration.Citation24 Authors resolved disagreements by consensus and a third author was consulted to resolve disagreements if necessary.
presents the assessment of the risk of bias in included studies. Assessment of the risk of selection bias was limited due to the fact that information on participants’ selection in clinical trial studies was not sufficiently detailed. Case definitions of minor and serious AEFIs were not presented for all clinical trials thus, limiting the assessment of detection bias. There existed an increased risk of detection bias across studies because the duration and detection procedures of AEFI surveillance and of serious AEFI surveillance were different in all included studies. Risk of detection bias also existed among studies conducted during immunization campaigns as all vaccinated populations had varying geographic access to AEFI surveillance. In some of the included studies, AEFI was only presented in syndrome or in systemic organ class without prior presentation of symptom or sign. This predisposed to an increased risk of reporting bias in and across studies. There existed limited information to rule out the risk of attrition bias for 6 out of 7 included studies as nothing was mentioned on followed-up procedures for each study participants till the end of the surveillance period. The causality assessment of serious AEFIs was not clearly described in clinical trial studies meanwhile campaign studies, it was conducted by a multidisciplinary committee. This could increase the risk of bias across studies regarding the classification of serious AEFIs.
Table 9. Risks of bias for included studies.
3. Discussion
To the best of our knowledge, this is the first systematic review comparing incidence rates of AEFIs between clinical trial phases and immunization campaigns with MenafrivacTM, but also with any other vaccines. From prior experience, it was known/expected for AEFI during mass campaigns to be inadequate in comparison to clinical trials, but the level of incompetency was alarming. This study, therefore, provides an insight into the magnitude of difference in incidence rates of overall, local, systemic, serious and types of AEFI when assessed in a controlled setting such as clinical trials, and when assessed in real-life situations during mass immunization campaigns. As expected, the incidence of AEFI was much higher in clinical trials than in mass campaign studies with a decrease of more than 99% AEFI detection for all AEFIs from different phases of clinical trials to immunization campaigns. From this study, it was clear that more than 99% of overall, local and systemic AEFI detected in clinical trials could be attributed to the surveillance system put in place which is not the same during mass campaigns. Due to the rigorous surveillance system used during clinical trial studies for AE detection and reporting, there exist high chances of detecting and reporting more events encountered as opposed to mass campaigns where the surveillance system for AE detection and reporting is not consistent or systematic although a greater population is being served. More of the AEFI reported during trial studies would have been expected to be reported in mass campaign studies plus additional rare and/or new AEs which could not be detected during trials due to its limited sample size, but the reverse was true from our findings. More so, 5 out of 40 serious AEFIs investigated in immunization campaign studies were probably related to the vaccine whereas none of the serious AEFIs reported in clinical trials studies was related to the vaccine. Based on this, it can be deduced that more vaccine-related serious AEs could be detected in mass campaigns provided the surveillance system is strengthened to detect more, rare and new AEFI.
Considering the fact that the objectives of clinical trials are completely different from those of mass immunization campaigns, the former assessing the safety and reactogenicity of a new product with primary endpoints dependent on the phase of the trial while the latter being to protect a whole population from a disease, it is not expected to have equivalent IR of AEFIs. Owing to the fact that, the safety of a vaccine cannot be ascertained by clinical trials only as a result of its limited sample size which consequently hinders detection of significant rare AEFIs, it is highly desirable that a good surveillance system is put in place when a new product is distributed on a large scale. New serious probably vaccine-related AEFIs observed during mass immunization campaigns elucidates the importance and necessity of putting in place an efficient safety monitoring system during such enterprises. The scope of this review was limited in that, it does not reveal the number of such events that were missed during campaigns. There are certainly many since the IR of serious AEFI was more than 99% lower in mass campaigns than in clinical trials. This observation is therefore worrying.
The magnitude of this difference can be explained by several reasons which can be categorized into two groups; firstly due to methodological differences and secondly due to the surveillance systems put in place. For methodological differences, the duration of observation may have played a role. Indeed, the surveillance period in clinical trials was shorter than in mass campaigns. This might have led to higher overall IR since AEFIs are usually observed in the first or second week post-vaccination and therefore, resulting to have a much higher numerator. Differing vaccine safety monitoring procedures, surveillance periods as well as AEFI reporting and analysis has been identified to contribute in reducing accurate scientific information on vaccine safety.Citation27 Also, another reason which can explain these large differences in IR of AEFI between clinical trials and mass campaigns can be due to the fact that clinical trial participants are probably much more aware of AEs than people who are routinely vaccinated or vaccinated in the community with an existing vaccine. To maximize scientific progress on immunization safety, there is essential need for standardization of AEFI surveillance period in clinical trials and mass campaigns after registration. The second reason for this huge IRd can be linked to the poor performance of the surveillance systems put in place during mass campaigns to capture AEFIs. There exist many challenges which has largely been described in previous studies.Citation28 Under-detection and under-reporting of AEFIs in mass campaigns are well known and can be rooted in that the activity is not focused on safety assessment. In clinical trials, the detection, reporting, and investigation of AEFIs are part of the TOR (Terms of Reference) of a well trained, monitored, supervised and audited research team. Meanwhile, in mass vaccination campaigns, there is limited number of trained health personnel to carryout supervision of AEFI surveillance, detect, report and investigate cases of AEFI. Drawing from experiences in Cameroon, one focal point per health district is designated and trained for AEFI surveillance supervision and one focal point per health facility is designated and trained in case detection, reporting, and investigation of AEFIs. From this perspective, one person to supervise surveillance in a whole district and one in a big hospital is clearly not sufficient. Definitely, within a hospital where many serious cases are treated in different wards at different times of the day and night, this poses difficulty in detecting AEFIs that can result in an SAE. Moreover, consultation registers are mostly not standardized and do not allow the detection of cases of AEFI after the consultation because these registers do not include variables on vaccination history. Thus, cases of AEFIs consulted in the absence of a focal point have very little probability of being detected. Also, training of supervisors and surveillance focal points are often carried out in cascade and integrated with training of other campaign activities due to inadequate or lack of resources. Added to these, the training duration is often very short and decreases drastically when applied from the central to operational level. Consequently, competence of trainers’ decreases as one trainee cannot deliver better training than that received in a shorter time. Improvements can, however, be made which can include: i) revising the patient registration system to include a variable for active case detection, ii) ensuring effective and refresher training to enable them to implement minimum AEFIs monitoring activities at the operational level, iii) sensitizing and emphasizing that chiefs of health facilities include the detection and reporting of AEFI in the TOR of all health personnel involved in care and more importantly in hospitals, iv) to use efficient interventions such as continuous supervision and SMS messages to remind health staff of their surveillance duty.Citation22
Poor performance of AEFI surveillance during mass campaigns is worrying in terms of vaccine safety. This is because i) local AEFIs such as abscesses, for example, that may be a key indicator of program error (such as compliance with vaccine storage, transportation, and administration procedures) are missed ii) preventable severe local AEFIs are missed which can likely draw public attention by spreading rumors which might resort in vaccination refusals by the population, iii) serious AEFIs are missed thereby compromising causality assessment and appropriate response possibilities.
To understand whether such differences of AEFIs reporting between clinical trials and mass vaccinations were inherent to MenAfrivacTM or were common to all vaccines, we made a rough estimate of the magnitude of the difference in IR of AEFIs between clinical trials and post-registration immunization using the new pneumococcal 13-valent conjugate vaccine. For 5308 doses of the vaccine administered during clinical trials, 5004 AEFIs were reported comprising 1803 local and 3201 systemic.Citation29–Citation32 While in post-registration, 871 vaccine doses were administered and 550 AEFIs were reported of which 236 were local and 314 systemic.Citation33 IRd per 100 000 doses per week between clinical trials and post-registration vaccination were 19ʹ089, 5ʹ423, and 13ʹ666 for overall, local, and systemic AEFIs reported, respectively. Thus, AEFI incidence decreased from clinical trials to post-registration vaccination studies by 52%, 37%, and 53% for overall, local, and systemic AEFI, respectively. This relatively low difference can be explained by the fact that in the post-registration study, AEFI monitoring was more rigorous, which increased the chance of detecting AEFIs than during studies conducted during the vaccination campaigns with MenAfrivacTM. More so, vaccinees were closely monitored for AEFIs by telephone throughout the monitoring period. Lastly, the vaccinated population was made up of people of older age from a European country.
Despite the strength of this study, the study was limited in the methods of estimating AEFI frequency per 100,000 doses which is unlikely to be true in clinical trial studies with limited number of doses administered due to its limited sample size unlike in mass campaigns.
4. Conclusion/recommendations
This systematic review highlights the magnitude of the difference between IR of AEFI as evaluated in the controlled setting of clinical trials and more pragmatic approach of mass vaccination campaigns. IR of AEFIs was more than 99% lower in vaccinations campaigns than in clinical trials, including reporting of serious ones. Although safety surveillance is an integral part of both clinical trials and mass vaccination campaigns, IRs of AEFI are not expected to be equivalent in both settings due to differences in how AEs are collected. However, there is need to improve the surveillance system put in place during mass campaign and post-registration implementation at both national and international levels. At national levels, this could include aspects such as standardization of hospital registries to capture information on vaccination status, variables that will enhance case detection in hospitals thereby allowing better investigation of causality, sufficient number of trainees and dedicated health staff in health facilities and hospitals, and adequate supervision, coupled with new technological methods for reminders such as SMS. For these to be practically achievable, the following strategies could be put in place. i) Inclusion of specific indicators in consultation registers to capture information on vaccination status of participants during routine and mass immunizations ii) the need to include surveillance aspects as part of campaign communication strategy. This will aid in conveying surveillance messages to the population and informing them to report any AEs post vaccination at health facilities so as to improve AE reporting rates during campaigns iii) recruitment and training of specific human resources to handle key responsibilities such as active case finding of any AEs within the communities, keen attention at health facilities to consult and record information of any participants reporting at health facilities due to events arising from mass campaigns. This strategy can be very effective as it eliminates the possibility of every non-trained hospital staff at the out-patient station consulting participants visiting the hospital due to AEFI in the population iv) Pasting of AEFI case definitions in consultation rooms v) Using SMSs and telephone beeps to report AEFI in the community vi) Pasting posters and distribution of flyers illustrating signs and symptoms of AEFI in the communities to improve AEFI knowledge within the population and improve reporting status during campaigns.
At International levels of low and middle-income countries, feasible approaches which are sustainable, flexible, affordable and timely for international collaborative vaccine safety monitoring and particularly suitable for a consortium of upper, middle and low-income countries have been suggested in previous studies. Some have proposed a two-pronged approach which consists of an integrated coordinated passive surveillance and international collaborative epidemiological efforts.Citation34 A study involving LMIC suggested the development of a global network of hospital-based sentinel sites for vaccine safety signal verification and hypothesis testing.Citation35 This study confirmed the feasibility and demonstrated the validity and utility of large collaborative international studies to monitor the safety of new vaccines introduced in LMICs. Another study has proven that, an international hospital-based network for the investigation of rare vaccine adverse events, using common standardized procedures and with high participation of LMICs, is feasible, can produce reliable results, and has the potential to characterize differences in risk between vaccine strains.Citation36
5. Material and methods
5.1. Study design
This was a systematic review study in which all studies published that reported on adverse events following MenAfrivac™ administration during clinical trials and immunization campaigns were identified. An estimated incidence rate (IR) of overall, local, systemic, serious and types of reported AEFIs was compared between clinical trials and immunization campaigns studies using the incidence rate difference (IRd).
5.2. Study settings
This study covered clinical trials or mass campaign studies on MenAfrivac™ in seven (7) African countries and one (1) Asian country. Hence, studies from a total of eight countries were included namely; India, Mali, Gambia, Senegal, Niger, Burkina Faso, Benin, and Cameroon.
5.3. Study population
The study population comprised individuals vaccinated with MenAfrivac while enrolled in clinical trials or during vaccination campaigns. Studies that reported on adverse events following immunization with meningococcal group A conjugate vaccine were included. These studies reported AEFI from male and female subjects’ age 1–35 years because these age groups are usually targeted during clinical trials and vaccination campaigns with MenAfrivac™.
5.4. Literature search
The following sources were searched from February 15 – March 17, 2015 and cross-checked in August 29, 2016 to detect any recently published papers: meningitis vaccineCitation26 project website, Medline (PubMed) and Embase (Ovid). The search consisted of keywords relating to the disease of interest combine with other key terms using Boolean operators. The following terms were therefore used for the literature search: ‘’meningococcal meningitis A’’AND‘’conjugate vaccine’’AND‘’safety’’ in Medline; and ‘’meningococcus’’ OR “Neisseria meningitidis group A’’ AND ‘’conjugate vaccine’’ AND ‘’safety’’ in Ovid. The reference lists of all included primary and review articles were examined to identify cited articles not captured by electronic searches. The search was restricted to articles published in English Language. The database was scrutinized by two reviewers and full articles likely to meet the selection criteria were obtained. The reviewers made the final inclusion/exclusion decisions according to adherence to the eligibility criteria.
5.5. Eligibility criteria
Phase 1, 2, 3 clinical trial studies, and mass immunization campaign studies reporting AEFIs after exposure to MenAfrivac™ were considered eligible to be included in this study. The review only included studies/reports published in English.
5.6. Selection criteria
Inclusion criteria: Reports and studies published in English during 2001–2016 which reported AEFI with MenAfrivac were included in this study. Study participants exposed to MenAfrivac™ were included. The WHO definition of AEFI was used in this study.Citation1,Citation37
Exclusion criteria: i) unpublished reports, reports not published in peer-reviewed scientific journal, ii) publications reporting AEFIs associated concomitant exposure of participants to MenAfrivac™ and other vaccines or drugs; iii) duplicates (case where more than one publication reports AEFIs from the same population over the same period); iv) publications with AEFIs reported in preclinical phase of MenAfrivac™ development; v) publications for which the full text was not available; and vi) abstracts of conferences. Also, studies with participants to whom the vaccine administered were not done following manufacturer’s dose, administration or storage procedures were excluded from this study.
The selection process was conducted by two independent reviewers using a two-step process: the first step included assessing the title and abstract of articles and reports, and the second step involved assessing the full text using the selection criteria. All disagreements arising were resolved by consulting the full text of articles.
5.7. Data extraction
This study adapted a data extraction grid that was used in a previous study.Citation11 Data were extracted and compiled in an excel table by a reviewer. A second reviewer cross-checked all extracted data one after another while comparing with data in the excel table in order to fill data extraction forms and full texts of articles. Wherever discrepancies were observed, corrections were made from the full-text article. From each article, the following characteristics were extracted: study characteristics which included the study design, year of publication, study country, health-care setting, type of resource available, source of the report, and name of the first author; characteristic of the study population such as size, age group, inclusion criteria, exclusion criteria and number of pregnancies exposed; phase of the vaccine development (phase 1, 2, 3, 4 clinical trials, mass immunization campaign, routine EPI); characteristics of the vaccination such as antigen, dose, administration procedure, other vaccine or drugs concomitantly administered and first or second dose; characteristics of the AEFI surveillance system including case definition of AEFI, case definition of serious AEFI, type of surveillance (active, passive stimulated or not), surveillance duration, type of AEFI investigated); characteristics of reported AEFIs, namely, total number, number of serious, local and systemic reaction, number of AEFIs per age group, number of AEFI per type, number of cluster AEFIs, number and type of AEFIs among pregnant women, number of serious AEFIs investigated, number of vaccine product-related reaction, number of vaccine quality defect-related reaction, number of immunization error-related reaction (formerly “program error”), number of immunization anxiety-related reaction, number of coincidental events].
5.8. Outcomes
The primary outcome of this study is the incidence rate difference (IRd) of overall AEFIs between clinical trials and mass campaign studies. Secondary outcomes included the IRd comparing local, systemic, serious and types of AEFIs between clinical trials and mass campaign studies.
5.9. Quality assessment
This study assessed the procedures of participants’ selection, the adopted case definitions of AEFIs, procedures of AEFIs detection, reporting and investigation to ensure the methodological quality of studies. The quality assessment was performed by two independent reviewers who resolved any arising discrepancy through discussions.
5.10. Data analysis
IR (incidence rate) per 100ʹ000 doses administered per week of overall, local, systemic, and serious and types of AEFIs in clinical trials and mass campaigns were estimated. The sum of AEFI cases reported was defined as the numerator of the incidence rate and its denominator was defined as the sum of persons exposed to the vaccine multiplied by the duration of follow up. AEFI IRs in clinical trials were compared to that of post-registration phases by estimating the IRd at 95% confidence interval and the attributable fraction of AEFI IR in clinical trials and mass campaigns. Data were entered in Microsoft excel 2010 analyzed using the same software and STATA version 10 (Texas, 2009).
Authors’ contributions
JA designed the study, implemented the study, analyzed data and drafted the manuscript; BS, ACB, BG revised the protocol, AMT finalized the draft manuscript. All authors read and approved the final version.
Data availability
Data obtained from this review will be made available upon request
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We sincerely thank: The country WHO office of Cameroon and Cameroon Ministry of Public Health who supported activities.
Additional information
Funding
References
- WHO. Immunization safety surveillance guidelines for immunization programme managers on surveillance of adverse events following immunization 2nded. Manila, World Health Organization Western pacific region; Manila, 2013.
- Vaccine development, testing, and regulation. [ accessed 2015 Feb 28] http://www.historyofvaccines.org/content/articles/vaccine-development-testing-and-regulation.
- Abdelnour A, Silas PE, Lamas MRV, Aragón CFG, Chiu N-C, Chiu C-H, Acuña TH, Castrejón TDL, Izu A, Odrljin T, et al. Safety of a quadrivalent meningococcal serogroups A, C, W and Y conjugate vaccine (MenACWY-CRM) administered with routine infant vaccinations: results of an open-label, randomized, phase 3 b controlled study in healthy infants. Vaccine. 2014;32(8):965–72. doi:10.1016/j.vaccine.2013.12.034.
- Iskander JK, Miller ER, Chen RT. The role of the Vaccine Adverse Event Reporting system (VAERS) in monitoring vaccine safety. Pediatr Ann. 2004 Sep;33(9):599–606. doi:10.3928/0090-4481-20040901-11.
- WHO, Policy Perspectives on Medicines. Pharmacovigilance: ensuring the safe use of medicines. World Health Organization; Geneva, 2004.
- Alicino C, Merlano C, Zappettini S, Schiaffino S, Della Luna G, Accardo C, Gasparini R, Durando P, Icardi G. Routine surveillance of adverse events following immunization as an important tool to monitor vaccine safety. Hum Vaccin Immunother. 2014;7:e34360.
- McNeil MM, Li R, Pickering S, Real TM, Smith PJ, Pemberton MR. Who is unlikely to report adverse events after vaccinations to the Vaccine Adverse Event Reporting System (VAERS)? Vaccine. 2013 May;31(24):2673–79. doi:10.1016/j.vaccine.2013.04.009.
- Monteiro SA, Takano OA, Waldman EA. Evaluation of the Brazilian surveillance system for adverse events following vaccination. Rev Bras Epidemiol. 2011 Sep;14(3):361–71.
- Muehlhans S, Richard G, Ali M, Codarini G, Elemuwa C, Khamesipour A, Maurer W, Mworozi E, Kochhar S, Rundblad G, et al. Safety reporting in developing country vaccine clinical trials-a systematic review. Vaccine. 2012 May;30(22):3255–65. doi:10.1016/j.vaccine.2012.02.059.
- Breugelmans JG, Lewis RF, Agbenu E, Veit O, Jackson D, Domingo C, Böthe M, Perea W, Niedrig M, Gessner DB, et al. Adverse events following yellow fever preventive vaccination campaigns in eight African countries from 2007 to 2010. Vaccine. 2013 April;31(14, 3):1819–29. doi:10.1016/j.vaccine.2013.01.054.
- Guoa B, Pagea A, Wangb H, Taylorc R, Peter M. Systematic review of reporting rates of adverse events following immunization: an international comparison of post-marketing surveillance programs with reference to China. Vaccine. 2013;31:603–17. doi:10.1016/j.vaccine.2012.11.051.
- Frasch CE, Preziosi M-P, Marc LaForce F. Development of a group A meningococcal conjugate vaccine, MenAfriVacTM. Hum Vaccin Immunother. 2012 June;8(6):715–24. doi:10.4161/hv.19619.
- Kshirsagar N, Murb N, Thatte U, Gogtay N, Viviani S, Préziosi M-P, Elie C, Findlow H, Carlone G, Borrow R, et al. Safety, immunogenicity, and antibody persistence of a new. Vaccine. 2007;25S:A101–A107. doi:10.1016/j.vaccine.2007.04.050.
- Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, Tapia M, Akinsola AK, Arduin P, Findlow H, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364:2293–304. doi:10.1056/NEJMoa1003812.
- Hirvea S, Bavdekara A, Pandita A, Juvekar S, Patil M, Preziosi M-P, Tang Y, Marchetti E, Martellet L, Findlow H, et al. Immunogenicity and safety of a new meningococcal A conjugate vaccine in Indian children aged 2–10 years: a Phase II/III double-blind randomized controlled trial. Vaccine. 2012;30:6456–60. doi:10.1016/j.vaccine.2012.08.004.
- WHO. Meningococcal disease in countries of the Africanmeningitis belt, 2012 emerging needs and future perspectives. Weekly Epidemiol Record 2013;12:129–36.
- Chaiboua MS, Bako H, Salisou L, Yaméogo TM, Sambo M, Kim SH, Djingarey MH, Zuber PLF, Perea WA, Pezzoli L. Monitoring adverse events following immunization with a new conjugate vaccine against group A meningococcus in Niger 2010. Vaccine. 2012 Sept;30:5229–34. doi:10.1016/j.vaccine.2012.06.006.
- Ouandaogo CR, Yaméogo TM, Diomandé FV, Sawadogo C, Ouédraogo B, Ouédraogo-Traoré R, Pezzoli L, Djingarey MH, Mbakuliyemo N, Zuber PLF. Adverse events following immunization during mass vaccination campaigns at first introduction of a meningococcal A conjugate vaccine in Burkina Faso, 2010. Vaccine. 2012;30(suppl 2):B46–51. doi:10.1016/j.vaccine.2011.12.112.
- Ankrah DNA, Darko DM, Sabblah G, Mantel-Teeuwisse A, Leufkens HMG. Reporting of adverse events following immunizations in Ghana - Using disproportionality analysis reporting ratios. Hum Vaccin Immunother. 2018;14:172–78. doi:10.1080/21645515.2017.1384105.
- WHO. Meeting of the global advisory committee on vaccine safety, june 2011. Weekly Epidemiol Record. 2011;86(30):321–24.
- WHO. Review of safety profile on meningococcalA. Weekly Epidemiol Record. 2010;5(85):29–36.
- Ateudjieu J, Stoll B, Nguefack-Tsague G, Tchangou C, Genton B. Vaccines safety; effect of supervision or SMS on reporting rates of adverse events following immunization (AEFI) with meningitis vaccine (MenAfriVac™): A randomized controlled trial. Vaccine. 2014 Sep 29;32(43):5662–68. doi:10.1016/j.vaccine.2014.08.012.
- Vannice KS, Keita M, Sow SO, Durbin AP, Omer SB, Moulton LH, Yaméogo TM, Zuber PLF, Onwuchekwa U, Sacko M, et al. Active surveillance for adverse events after a mass vaccination campaign with a group A meningococcal conjugate vaccine (PsA-TT) in Mali. Clin Infect Dis. 2015;61(S5):S493–S500. doi:10.1093/cid/civ497.
- Higgins JPT, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC. Research methods & reporting: the cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928.
- Steffen C, Tokplonou E, Jaillard P, Dia R, Alladji MNB, Gessner B. A field based evaluation of adverse events following MenAfriVac®vaccine delivered in a controlled temperature chain (CTC) approach in Benin. Pan Afr Med J. 2014;18:344. doi:10.11604/pamj.2014.18.226.4800.
- Meningitis vaccine project. Meningitis vaccine project. Meningitis vaccine project. [ Accessed 2015 Mar 07]. http://www.meningvax.org/.
- Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, Jefferson T, Loupi E. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine. 2002;21:298–302. doi:10.1016/s0264-410x(02)00449-8.
- Nzolo D, Ntetani Aloni M, Mpiempie Ngamasata T, Mvete Luemba B, Bazundama Marfeza S, Bothale Ekila M, Ndosimao Nsibu C, Lutete Tona N. Adverse events following immunization with oral poliovirus in Kinshasa, Democratic Republic of Congo: preliminary results. Pathog Glob Health. 2013 Oct;107(7):381–84. doi:10.1179/2047773213Y.0000000113.
- Frenck RW Jr., Gurtman A, Rubino J, Smith W, van Cleeff M, Jayawardene D, Giardina PC, Emini EA, Gruber WC, Scott DA, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19(8):1296–303. doi:10.1128/CVI.00176-12.
- Yeh SH, Gurtman A, Hurley DC, Block SL, Schwartz RH, Patterson S, Jansen KU, Love J, Gruber WC, Emini EA, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010;126:3. doi:10.1542/peds.2009-3027.
- Schwarza FT, Flamaing R, Rümke HC, Penzes J, Juergens C, Wenz A, Jayawardene D, Giardina P, Emini EA, Gruber WC, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged. Vaccine. 2011;29:5195–202. doi:10.1016/j.vaccine.2011.05.031.
- Bryant KA, Stan L, Baker SA, Gruber WC, Scott DA. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:5. doi:10.1542/peds.2009-1405.
- Durando P, Rosselli R, Cremonesi I. Safety and tolerability of 13-valent pneumococcal conjugate vaccine in the elderly. Hum Vaccin Immunother. 2015;11(1):172–77. doi:10.4161/hv.34420.
- Izurieta HS, Zuber P, Bonhoeffer J, Chen RT, Sankohg O, Laserson KF, Sturkenboom M, Loucq C, Weibel D, Dodd C, et al. Roadmap for the international collaborative epidemiologic monitoring of safety and effectiveness of new high priority. Vaccines. 2013;31:3623–27.
- Guillard-Maure EV, Black S, Black S, Perez-Vilar S, Castro JL, Bravo-Alcántara P, Molina-León HF, Weibel D, Sturkenboom M, Zuber PLF. Operational lessons learned in conducting a multi-country collaboration for vaccine safety signal verification and hypothesis testing: the global vaccine safety multi country collaboration initiative. Vaccine. 2018 Jan 8;36(3):355–62. doi:10.1016/j.vaccine.2017.07.085.
- Perez-Vilar S, Weibel D, Sturkenboom M, Black S, Maure C, Castro JL, Bravo-Alcántara P, Dodd CN, Romio SA, de Ridder M, Nakato S, Molina-León HF, Elango V, Zuber PLF. WHO Global Vaccine Safety-Multi Country Collaboration. Enhancing global vaccine pharmacovigilance: proof-of-concept study on aseptic meningitis and immune thrombocytopenic purpura following measles-mumps containing vaccination. Vaccine. 2018;36(3):347–54. doi:10.1016/j.vaccine.2017.05.012.
- WHO. Immunization safety surveillance: guidelines for managers of immunization programmes on reporting and investigating adverse events following immunization. Manila: World Health Organization Western Pacific Regional Office; Manila, 1999.