ABSTRACT
The tetravalent dengue vaccine (CYD-TDV; Dengvaxia®) is administered on a three-dose schedule, 6 months apart in those aged ≥9 years in a number of dengue-endemic countries in Asia and Latin America. In this study, CYD63 (NCT02824198), participants aged 9–45 years at first vaccination, and who had received three doses of CYD-TDV in the CYD28 study more than 5 years previously, were randomized 3:1 to receive a booster CYD-TDV dose (Group 1) or placebo (Group 2). Dengue neutralizing antibody geometric mean titres (PRNT50 GMTs) for each of the four dengue serotypes were assessed in sera collected before and 28 days after booster injections. Non-inferiority of the booster immune response versus that induced after the third dose was demonstrated for each serotype if the lower limit of the two-sided 95% confidence interval (CI) was >0.5 for the GMT ratios (GMTRs) between post-booster CYD-TDV dose and post-dose 3 in Group 1. Overall, 118 participants received CYD-TDV booster or placebo and 116 (98.3%) completed the study; two participants were withdrawn because of noncompliance. GMTs in the booster CYD-TDV group increased across all serotypes post-booster injection by 1.74- (serotype 1) to 3.58-fold (serotype 4). No discernible increases were observed in the placebo group. Non-inferiority was demonstrated for serotypes 1, 3, and 4, but not for serotype 2 (GMTR; 0.603 [95% CI, 0.439– 0.829]). No safety issues were observed. These data show that the CYD-TDV booster given 5 or more years later tended to restore GMTs back to levels observed post-dose 3.
Introduction
Dengue disease is a viral infection that is widespread throughout the tropics, and is caused by one of four serotypes (DEN 1–4); the virus is transmitted predominantly by Aedes aegypti mosquitoes.Citation1 An estimated 390 million dengue infections occur globally per year, of which about 25% manifest with dengue disease.Citation2 In most cases dengue is a self-limiting illness, but severe dengue (dengue hemorrhagic fever) or dengue shock syndrome may occur in a small proportion of cases, particularly in infants, and following a second dengue infection with another dengue serotype (distinct from that on first exposure).Citation3,Citation4 There is no specific anti-viral treatment for dengue, and disease prevention efforts are currently centered primarily around vector control measures and early diagnosis of severe dengue to decrease fatality rates.Citation5
Singapore has continued to experience periodic dengue epidemics despite largely successful nationwide mosquito control since 1970.Citation6 Epidemiological dengue patterns in the country from 2004 to 2016 show that there have been a number of switches in the predominant serotype between DEN2 to DEN1, and more recently back to DEN2 in 2016.Citation7 The switch in the dominant serotype from DENV2 to DENV1 in 2013 led to a resurgence of cases, with 22,170 and 18,326 cases reported in 2013 and 2014, respectively.Citation7,Citation8
CYD-TDV (Dengvaxia®) has been approved by the Health Sciences Authority (HSA) in Singapore for the prevention of dengue infection in individuals aged 12–45 years since October 2016,Citation9 though the vaccine is not currently part of the national immunization program.Citation10 Recent findings from Sridhar et al. on the effect of dengue serostatus on vaccine safety and efficacyCitation11 have resulted in the HSA further strengthening the warnings and recommendations in the prescribing information of CYD-TDV; to ensure that only individuals with previous dengue infection receive the vaccine and to emphasize the need for serological testing to identify such individuals.Citation10
The immunogenicity and safety of CYD-TDV in Singapore was documented in CYD28, a Phase II randomized controlled trial conducted in five hospitals across the country from April 2009 to October 2014. In CYD28, dengue seropositivity among participants at baseline was 32.4% for those in the control group and 26.5% in the vaccine group;Citation12 figures reflecting Singapore’s lower endemicity compared with other Southeast Asian and Latin American countries, and the South East Asian region in general.Citation13 The CYD28 study showed an overall favorable safety profile for CYD-TDV, increased seropositivity rates, and increased neutralizing antibody titers against all four dengue virus serotypes, post-dose 3.Citation12 Whether a booster dose may be required in certain populations with low dengue endemicity, such as Singapore, remains to be elucidated. This study was undertaken to assess the immune response to a booster CYD-TDV dose, 5 years or more after the completion of the three-dose primary vaccination schedule, among participants of the CYD28 study.
Results
Study participants
Participants from the CYD28 study who met the inclusion criteria (i.e. those who were aged 9–45 years at the first visit in CYD28, completed all three vaccinations and had remaining serum for post-dose 3 PRNT reanalysis) were identified by the sponsor. When the investigators attempted to contact eligible participants, many were uncontactable, out of the country, did not wish to participate in the study or had insufficient serum volume from post-dose 3 samples from CYD28. Consequently, 118 eligible participants from CYD28 returned to participate in CYD63, and were subsequently randomized (CYD-TDV group, n = 89; placebo group, n = 29). The vaccination period in CYD28 was from April 2009 to Oct 2010. All randomized participants received booster injection or placebo during the period from July 2016 to Feb 2017 in CYD63, and provided a blood sample before injection. At 28 days post-injection, one (1.1%) participant in the CYD-TDV group and one (3.4%) in the placebo group were discontinued from the study because they did not provide a post-dose blood sample. Overall, 116 (98.3%) completed the study, of which 103 participants (87.3%) were included in the per-protocol analysis set (PPAS; CYD-TDV group, n = 75 [84.3%]; placebo group, n = 28 [96.6%]).
Baseline demographics for the enrolled participants are presented in Supplementary Table S1. In the PPAS of this study (CYD63), 19/75 (25.3%) from the CYD-TDV group were dengue seropositive and 56/75 (74.7%) were dengue seronegative at baseline. In the placebo group, 10/28 (35.7%) were dengue seropositive and 18/28 (64.3%) dengue seronegative at baseline. Baseline in CYD63 was defined as Day 0 of the CYD28 study. At enrollment of CYD63, the majority of participants (108 [93.1%]) had remained dengue seropositive (50% plaque reduction neutralization test [PRNT50] titer ≥10) after completing the 3-dose schedule of CYD-TDV in previous CYD28 studies
Immunogenicity
The geometric mean titers (GMTs) for each serotype, pre- and 28 days post-booster injection are presented in . In the CYD-TDV group, GMTs increased across all serotypes by 1.74- to 3.58-fold after the booster dose, whereas, no discernible increases were observed in the placebo group.
Table 1. Summary of geometric mean titers and geometric means, of individual titer ratios, of antibody pre- and post-booster injection (per-protocol analysis set)
Non-inferiority for the booster CYD-TDV dose compared with post-dose 3 was demonstrated for serotypes 1, 3, and 4 (), but not for serotype 2. For serotype 2, the post-booster to post-dose 3 GMT ratio (GMTR) was 0.603 (95% confidence interval [CI], 0.439; 0.829); i.e. the lower 95% CI was below the pre-set threshold of 0.5. As a result, the strict protocol-specified non-inferiority of the CYD-TDV booster could not be demonstrated, and the superiority analysis could not proceed according to protocol. Nonetheless, the GMTs for serotype 2 increased by more than two-fold from pre- to post-booster with CYD-TDV. In addition, analysis of covariance, adjusting for the pre-booster titers, confirmed that CYD-TDV booster increased neutralizing antibody levels for each serotype (). The covariance analysis of post-booster titers against each of the four serotypes was carried out to control for baseline (Day 0 in CYD28) neutralizing antibody levels with the consequent removal of the pre-booster effect. This confirmed that the CYD-TDV booster increased neutralizing antibody levels for each serotype, regardless of pre-booster titers in each group.
Table 2. Ratio of geometric mean titers for CYD-TDV booster in CYD63 compared with the third CYD-TDV dose in the CYD28 study to assess non-inferiority, which was demonstrated if the lower limit of the two-sided 95% confidence interval (CI) for the ratio is greater than 0.5 (per-protocol analysis set, CYD-TVD Group only)
Table 3. Analysis of covariance of post-booster titers against each of the four serotypes strains (per-protocol analysis set)
The seropositivity rates post-dose 3 in CYD28, pre-booster, and 28 days post-booster injection are summarized in . In the CYD-TDV group, seropositivity rates increased post-booster compared to pre-booster and those in the placebo group tended to remain stable from pre- to post-booster injection.
Table 4. Seropositivity rates (titers ≥10) against at least 1, 2, 3, or 4 serotypes with parental dengue virus strains (per protocol analysis set)
Overall, antibody titers against each serotype at post-dose 3 in CYD28, pre-booster, and 28 days post-booster injection were higher in participants seropositive at baseline (Day 0 in CYD28).
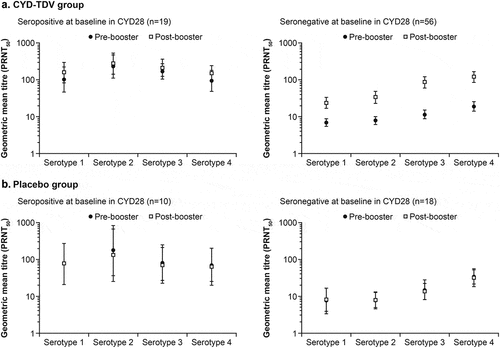
In the PPAS, GMTs pre-booster injection were higher in participants who were dengue seropositive at baseline in the CYD28 study than those who were dengue seronegative, as was also the case for post-booster in the CYD-TDV group (). However, the relative GMT increases between pre-booster and post-booster injection tended to be higher in participants dengue seronegative at baseline compared with those dengue seropositive (Supplementary Table S2). GMTRs (post-booster versus pre-booster) for serotypes 1–4 ranged from 1.16–1.47 for participants in the CYD-TDV booster group who were dengue seropositive at baseline, and from 1.88–5.00 in those who were dengue seronegative. For the placebo group, GMTRs for serotypes 1–4 ranged from 0.688–0.897 for those dengue seropositive, and from 0.58–0.81 for those dengue seronegative.
Figure 1. Geometric mean titers and 95% confidence intervals for each dengue serotype at pre-booster (●) and 28 days post-booster (□) injection in the CYD-TDV group (a) and placebo group (b) by dengue serostatus at baseline in CYD28 (per-protocol analysis set)
Safety assessments
None of the participants experienced any immediate unsolicited adverse events (AEs) within 30 minutes following booster injection. At least one solicited reaction was reported by 51.1% (45/88) of participants in the CYD-TDV group and 32.1% (9/28) in the placebo group. The most frequently reported solicited injection-site reaction was pain in both treatment groups, with headache and myalgia the most frequently reported solicited systemic reactions (). Overall, the proportion of participants who experienced at least one unsolicited non-serious AE (SAE) was 19.1% and 10.3% in the CYD-TDV (17 participants reported 23 AEs) and placebo (three participants reported three AEs) groups, respectively. One SAE was reported in the CYD-TDV group but was considered not related to vaccination; a 23-year-old male participant was hospitalized 10 days after injection following a road traffic accident with a deep cut to his leg. There were four non-serious AEs of special interest (AESIs) reported in three participants within 28 days after booster CYD-TDV; Grade 1 pruritus, which started within 1 day after injection and resolved spontaneously within 4 days; two episodes of Grade 2 urticaria in one participant, which started 2 days and 5 days after injection and both resolved within 1 day with medication; and Grade 2 generalized rash, which started within 1 day after injection and was still on going at Visit 4. There were no deaths, no AEs leading to discontinuation, and no serious AESIs reported. There were no dengue cases reported.
Table 5. Frequency of solicited injection site and systemic reactions by group (safety analysis set)
Discussion
This study was designed to demonstrate the non-inferiority, in terms of anti-dengue antibody GMTRs of a CYD-TDV booster dose administered 5 or more years after a three-dose schedule, compared with after the three-dose schedule CYD-TDV injection, in Singapore. Secondary objectives included description of the immune responses elicited by the CYD-TDV booster compared with placebo and the safety profile of the vaccine.
Non-inferiority of the CYD-TDV booster dose was demonstrated for serotypes 1, 3, and 4, but not for serotype 2. The study being underpowered with the low number of randomized participants may have contributed, in part, to the failure of the CYD-TDV booster to demonstrate non-inferiority for all four dengue serotypes. A subset of participants from CYD28 who were aged <9 years at the time of vaccination were excluded from this study to reflect the global license of the vaccine for use in those aged 9–45 years. In addition, only those who completed all three doses of CYD-TVD in the previous CYD28 study, and had post-dose 3 serum samples available, were eligible for CYD63 participation leading to a limited number of eligible participants for inclusion by definition. Although overall non-inferiority was not demonstrated, an increase in GMTs and seropositivity rates for all serotypes was observed after the CYD-TDV booster injection.
In a similar study conducted in Latin America that also assessed CYD-TDV booster 4–5 years after the three-dose schedule (NCT02623725, CYD64 study), protocol-defined non-inferiority for the CYD-TDV booster was demonstrated for all four serotypes.Citation14 The reasons why the aforementioned study and not the current study demonstrated non-inferiority may be a result of the higher proportion of dengue immune participants at baseline in the former, as that study was undertaken in dengue-endemic countries, as well as the larger number of participants (N = 187).
In the current study (CYD63), participants who were seropositive at baseline in CYD28 had higher GMTs pre- and post-booster injections than those who were dengue seronegative. This is consistent with observations in the original CYD28 study indicating that pre-vaccination serostatus may influence both the persistence of GMTs at pre-booster injection, and the level of GMTs post-booster injection. At 28 days after booster injection, participants who were dengue seronegative at baseline had higher relative GMT increases for each serotype than those dengue immune; which may reflect lower pre-booster GMTs in dengue seronegative participants, and thereby allowing for proportionally greater increases in post-booster GMTs. Of note, higher GMTs post-dose 3 have been observed in other countries, particularly in endemic regions,Citation13 whereas Singapore (CYD28) may be generally considered a country with lower endemicity, despite resurgence in recent years.Citation8
Consideration should also be made for the recent results from Sridhar et al., which used a dengue anti–non-structural protein 1 (NS1) IgG enzyme-linked immunosorbent assay to retrospectively assess the impact of inferred baseline dengue serostatus on safety outcomes following vaccination with CYD-TDV.Citation11 Administration of CYD-TDV to dengue seronegative participants aged 2–16 years resulted in a higher risk of severe virologically confirmed dengue (VCD) and hospitalization for VCD; while in those who were dengue seropositive at baseline, the vaccine was shown to be protective.Citation11 In line with this, the WHO recommends pre-vaccination screening as the preferred strategy in countries using vaccination as part of their dengue control strategy, to ensure that only those who are dengue seropositive are vaccinated.Citation15 In the current study, all participants received a 3-dose CYD-TVD primary series in the previous study, with all but five participants in the CYD-TDV group and one participant in the placebo group dengue immune at the time of pre-booster. Therefore, the impact of dengue pre-booster serostatus on the incidence of AEs post-booster injection could not be assessed nor was it not accounted for in the non-inferiority analysis for immunogenicity. In the previous CYD28 study, participants were followed up for hospitalized dengue cases for 4 years after the completion of primary vaccination. One participant had hospitalized VCD and this participant was not included in the current study; however, one participant who had been hospitalized with suspected dengue was included. Furthermore, reactogenicity and safety profiles after booster injection, 5 years or more after the three-dose primary schedule, were similar to those observed after the first dengue vaccine injection in CYD28. There were no new safety issues with the CYD-TDV booster in this study, and the vaccine was well tolerated. Most of the local and systemic reactions were of mild intensity, and of short duration. The proportion of participants who reported at least one solicited adverse reaction following the CYD-TDV booster tended to be lower than those after any CYD-TDV injection in the original CYD28 study.Citation12
Non-inferiority of the booster dose was demonstrated for serotypes 1, 3 and 4 but not for serotype 2. It is intriguing that vaccine efficacy in the first proof-of-concept study in Thailand (the CYD23 study) was lowest for serotype 2, the predominant serotype circulating at the time despite PRNT titers that were at least similar to the other serotypes.Citation16 However, the threshold PRNT titer for seroprotection has not been established for any dengue serotype and this threshold may vary per serotype depending on multiple factors.
To better understand optimal timing of a booster, there is currently an ongoing study (NCT02628444) that is assessing CYD-TDV booster doses at 1 or 2 years after completion of the three-dose schedule, focusing only on those who are dengue seropositive at baseline. Indeed, in dengue-endemic areas, GMTs tend to decline in the first year after the last injection, but thereafter remain relatively stable up to 5 years after the three-dose schedule with annual fluctuations due to natural exposure to wild-type dengue, or other flaviviruses, contributing to antibody persistence in this setting.Citation17 For children living in endemic countries, including participants from the CYD64 study, the annual incidence of asymptomatic dengue infection was shown to be over four-times higher than for symptomatic dengue, with the incidence 14.8% and 3.4% for asymptomatic and symptomatic infection, respectively.Citation18
The present study had some limitations. It was underpowered due to limits on recruitment as previously described. Other limitations include a lack of recognized PRNT50 threshold for seroprotection against dengue, and the lack of non-vaccinated control group in order to establish a comparative background rate for exposure to wild type dengue, which is unknown.
In summary, this study indicates that anti-dengue antibody titers can generally be restored with a booster injection to levels similar to those initially observed after the three-dose schedule in a population in a low dengue endemicity area. There were no safety concerns with the CYD-TDV booster.
Methods
Study design
This current study, CYD63, was a multi-center, observer-blind, randomized, placebo-controlled, Phase II trial of a booster dose of CYD-TDV, conducted at three sites in Singapore. Participants who received three CYD-TDV doses in the CYD28 study, more than 5 years prior, were recruited into the current study (CYD63 Trial registration: UTN: U1111-1161-2813; BB-IND #: 11219; NCT02824198). CYD28 included participants aged 2–45 years old, who were randomized 3:1 to receive three doses of CYD-TDV or a control vaccine, at 0, 6, and 12 months.Citation12 Both studies were undertaken in compliance with the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol and amendments were approved by applicable Independent Ethics Committees/Institutional Review Boards and the regulatory agency as per local regulations. Informed consent was obtained from the participants or their parents/legal guardians before any study procedures were performed.
Participants
Participants included in the current study were those from CYD28 who had received all three CYD-TDV doses, were aged 9–45 years on the day of first vaccination, in line with the approved global label of CYD-TDV,Citation15 and had remaining post-dose 3 serum samples for PRNT reanalysis. Exclusion criteria included participation in any other clinical trial, receipt of another vaccine 4 weeks before or after the CYD63 trial, moderate or severe acute illness/infection on the day of vaccination, or febrile illness.
Randomization and blinding
Eligible participants were randomized in permuted blocks of four, stratified by site and age group via an interactive voice response system, in a 3:1 ratio to receive CYD-TDV booster (Group 1), or placebo (Group 2) on Day 0 of the study. A designated vaccinator at each study site, who was unblinded but not involved in data collection or safety assessments, reconstituted and administered the assigned vaccine dose or placebo injection. The participants and investigators remained blinded until completion of the trial.
CYD-TDV and placebo
CYD-TDV (five-dose formulation) was presented as a powder for immediate reconstitution in saline (NaCl 0.9%) before use. Each 0.5 mL of reconstituted vaccine contained 4.5–6 log10 cell-culture infectious dose 50% (CCID50) of live, attenuated, recombinant dengue serotype 1, 2, 3, and 4 virus. The vaccine was administered subcutaneously in the deltoid region of the upper arm in a volume of 0.5 mL. The remaining doses of the multi-dose presentation were discarded. Placebo was 0.5 mL NaCl 0.9%.
Immunogenicity
Dengue neutralizing antibody titres were determined using serum samples collected on Days 0 (before study injection) and 28, and Months 6, 12, and 24 after study injection. This report includes data up to Day 28 after booster vaccination compared with post-dose 3, which was given more than 5 years prior during CYD28 study. PRNT50 was used to determine dengue neutralizing antibody levels as described previously by Timiryasova et al.Citation19 For the computation of GMTs, a titer reported as < LLOQ (lower limit of quantification) was converted to a value of 0.5 LLOQ. When calculating GMTR, a titer reported as < LLOQ is converted to 0.5 LLOQ for the numerator, and a titer reported as < LLOQ is converted to LLOQ for the denominator. The LLOQ of the assay was 10 (1/dil).
Safety assessment
Clinical site personnel recorded immediate AEs that occurred within 30 minutes after injection. Participants or their parents/legally acceptable representatives recorded the following in the diary card with all AEs graded on a three-point scale: solicited injection-site reactions for 7 days; solicited systemic reactions for 14 days; and unsolicited AEs for 28 days. Serious AESIs were collected within defined time windows following study injections, according to the type of serious AESI (hypersensitivity/allergic reactions): serious hypersensitivity/allergic reactions within 7 days; viscerotropic or neurotropic disease within 30 days; and dengue disease requiring hospitalization at any time during the study. Information on non-serious AESIs (hypersensitivity/allergic reactions) was collected for up to 7 days after vaccination. Serious AEs were collected throughout the trial. The study investigators assigned the causal relationship between each unsolicited AE and SAE to study injections.
Sample size and statistical analyses
Although participants were to be followed up for 2 years after booster vaccine administration, the results presented here represent up to Day 28 post-study injections. The planned sample size was 195 participants in Group 1 and 65 participants in Group 2; the assumption was that 10% of participants would not provide valid immunogenicity results, and therefore the resultant evaluable population would be 176 and 59 participants in the two groups, respectively. With 176 evaluable participants in Group 1, for each serotype, there would be 80.2% overall power using the paired t-test to reject the four individual null hypotheses simultaneously, assuming a non-inferiority margin (delta) = 2, one-sided type I error = 0.025, and correlation between the responses post-dose 3 and post-booster dose of the same serotype in the same participant = 0.6. Since four individual null hypotheses were required to be rejected simultaneously to reject the overall null hypothesis, no alpha multiplicity adjustments were required. With the proposed sample size of 176 participants in the CYD-TDV group, the probability of observing at least one AE with a true incidence of 1.7% would be approximately 95%.
The primary objective was to demonstrate the non-inferiority, in terms of GMTRs, of a CYD-TDV booster compared with the post-third CYD-TDV dose assessed in Group 1. Non-inferiority was demonstrated for each serotype if the lower limit of the two-sided 95% CIs of the GMTR was >0.5. The 95% CIs were calculated on log10 (titers/titers ratio) transformed titers using the usual calculation for normal distribution, and then anti-log transformed to compute GMTs, GMTRs, and their 95% CIs on their original scale. If non-inferiority of the four serotypes was shown, a secondary objective was planned to test if the titers post-CYD-TDV booster were superior to those observed after the three primary CYD-TDV doses. Analysis of covariance was used to compare the post-booster neutralizing antibody levels against each dengue virus serotype of Groups 1 and 2 adjusting for baseline (Day 0 in CYD28) neutralizing antibody levels against each dengue virus serotype based on the least squares means.
The primary immunogenicity (non-inferiority) analyses were performed on the PPAS, which comprised all participants who had no protocol deviations from the CYD63 study, who met all protocol-specified inclusion criteria, and who did not have any protocol-specified exclusion criteria. The FAS included participants who received either CYD-TDV or placebo, had blood samples drawn, and valid post-injection serology result for at least one dengue serotype. The safety analysis set (SafAS) included all participants who received either CYD-TDV booster or placebo, and this set was analyzed according to the study injection received. Safety data were summarized using point estimates and 95% CIs calculated using the exact binomial distributions (Clopper–Pearson method) for proportions.
Statistical analyses were conducted with SAS version 9.4 (Cary, NC, USA).
Data sharing
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
Disclosure of potential conflicts of interest
JP, MB, AB, and CF are employees of Sanofi Pasteur. JJ is an employee of Sanofi. AB has a planned, pending or issued, patent broadly relevant to the work in the manuscript. MB holds stocks in Sanofi Pasteur. SA, HO, JJ, and LS have no other conflict of interest to declare.
Supplemental Material
Download MS Word (28.1 KB)Acknowledgments
Editorial assistance with the preparation of the manuscript was provided by Nwoza Eshun, inScience Communications, Springer Healthcare, UK. Funding for this assistance was provided by Sanofi Pasteur. The authors would like to thank both the participants, investigators and research coordinators of the CYD28 and CYD63 studies who contributed to the conduct of the study. The authors also thank Jean-Sébastien Persico for editorial assistance and manuscript coordination on behalf of Sanofi-Pasteur and Mayan Lumandas for pharmacovigilance, and collaborating in the review of the safety data and in the review of the manuscript.
Additional information
Funding
References
- World Health Organization. Dengue and severe dengue [Fact sheet]; 2019. https://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature. 2013;496:504. doi:10.1038/nature12050.
- Thein S, Aung MM, Shwe TN, Aye M, Zaw A, Aye K, Aaskov J. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. doi:10.4269/ajtmh.1997.56.566.
- Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NTP, Lien LB, Quy NT, Hieu NT, Hieu LTM, et al. Dengue in Vietnamese infants–results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–24. doi:10.1086/590117.
- World Health Organization. Dengue vaccine research. Immunization, vaccines and biologicals; 2019. https://www.who.int/immunization/research/development/dengue_vaccines/en/.
- Ooi EE, Goh KT, Gubler DJ. Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–93. doi:10.3201/10.3201/eid1206.051210.
- Rajarethinam J, Ang L-W, Ong J, Ycasas J, Hapuarachchi HC, Yap G, Chong C-S, Lai Y-L, Cutter J, Ho D, et al. Dengue in Singapore from 2004 to 2016: cyclical epidemic patterns dominated by serotypes 1 and 2. Am J Trop Med Hyg. 2018;99:204–10. doi:10.4269/ajtmh.17-0819.
- Hapuarachchi HC, Koo C, Rajarethinam J, Chong CS, Lin C, Yap G, et al. Epidemic resurgence of dengue fever in Singapore in 2013–2014: A virological and entomological perspective. BMC Infect Dis. 2016;16:300. doi:10.1186/s12879-016-1987-z.
- Health Sciences Authority, Singapore Government. HSA approves dengvaxia vaccine; 2016. https://www.hsa.gov.sg/content/hsa/en/News_Events/HSA_Updates/2016/hsa-approves-dengvaxiavaccine.html.
- Health Sciences Authority, Singapore Ministry of Health. HSA further updates on dengvaxia®. Singapore, Asia; 2017. https://www.hsa.gov.sg/content/hsa/en/News_Events/HSA_Updates/2017/dengvaxiafurtherupdates.html.
- Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, Moodie Z. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327–40. doi:10.1056/NEJMoa1800820
- Leo YS, Wilder-Smith A, Archuleta S, Shek LP, Chong CY, Leong HN, Low CY, Oh M-LH, Bouckenooghe A, Wartel TA, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2–45 y: phase II randomized controlled trial in Singapore. Hum Vaccin Immunother. 2012;8:1259–71. doi:10.4161/hv.21224.
- Vigne C, Dupuy M, Richetin A, Guy B, Jackson N, Bonaparte M, Hu B, Saville M, Chansinghakul D, Noriega F, et al. Integrated immunogenicity analysis of a tetravalent dengue vaccine up to 4 y after vaccination. Hum Vaccin Immunother. 2017;13:2004–16. doi:10.1080/21645515.2017.1333211.
- Coronel D, García E, Rivera-Medina MD, Arredondo JL, Dietze R, Villar L, Perroud A, Cortes M, Bonaparte M, Hawk W, et al. Dengue vaccine booster in healthy adolescents and adults 4 to 5 years after a 3-dose primary schedule in Latin America. Cancún (México): SLIPE congress; 2017.
- World Health Organization. Dengue vaccine: WHO position paper – September 2018. Wkly Epidemiol Record 2018;36:457–76.
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai school children: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–67. doi:10.1016/S0140-6736(12)61428-7.
- Capeding MR, Laot TM, Boaz M, Wartel TA, Crevat D. Immunogenicity and safety of a tetravalent dengue vaccine during a five-year follow-up period. Trials Vaccinol. 2015;4:19–23. doi:10.1016/j.trivac.2015.03.002.
- Olivera-Botello G, Coudeville L, Fanouillere K, Guy B, Chambonneau L, Noriega F, Jackson N. Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue virus infections in healthy children and adolescents aged 2–16 years in Asia and Latin America. J Infect Dis. 2016;214:994–1000. doi:10.1093/infdis/jiw297.
- Timiryasova TM, Bonaparte MI, Luo P, Zedar R, Hu BT, Hildreth SW. Optimization and validation of a plaque reduction neutralization test for the detection of neutralizing antibodies to four serotypes of dengue virus used in support of dengue vaccine development. Am J Trop Med Hyg. 2013;88:962–70. doi:10.4269/ajtmh.12-0461.
