ABSTRACT
Background We conducted a matched case–control study in China during the 2013/14–2015/16 influenza seasons to estimate influenza vaccine effectiveness (VE) by dose among children aged 6 months to 8 years.
Methods Cases were laboratory-confirmed influenza infections identified through the influenza-like illness sentinel surveillance network in Guangzhou. Age- and sex-matched community controls were randomly selected through the expanded immunization program database. We defined priming as receipt of ≥1 dose of influenza vaccine during the immediate prior season.
Results In total, 4,185 case–control pairs were analyzed. Among children 6–35 months, VE for current season dose(s) across the three seasons during 2013/14–2015/16 were 59% (95% Confidence Interval: 44–71%), 12% (−11%,30%), 54% (32–69%); among unprimed children 6–35 months, VE for 1 vs 2 current season doses were 45% (8–67%) vs 65% (46–78%), −2% (−53%,32%) vs 19% (−11%,40%), and 37% (−24%,68%) vs 61% (32–78%). Among children aged 3–8 years, VE for current season dose(s) across study seasons were 62% (36–78%), 43% (22–58%), 32% (1–53%). VE for unprimed children receiving 1 dose only in current season was insignificant or lower than among all children.
Conclusion Findings support utility of providing second dose (“booster dose”) of seasonal influenza vaccine to unprimed children aged 6–35 months, and the need to study further dose effect of a booster dose among unprimed children aged 3–8 years in China.
Introduction
Since 2003, the Chinese Center for Disease Control and Prevention (China CDC) has recommended annual influenza vaccination for groups at higher risk for severe illness and complications from influenza infection, including children aged 6–59 months.Citation1,Citation2 Still, influenza vaccine coverage in China is very low, with average national estimates between 1.5% and 2.2% during 2004–2014.Citation3,Citation4 Telephone surveys conducted in 2009–2012 estimated influenza vaccine coverage among children aged <5 years were 22–32% in China, but these were likely overestimates.Citation5 Prior studies conducted in China have shown moderate influenza vaccine effectiveness (VE) against laboratory-confirmed influenza illness in young children.Citation6–Citation10 However, VE varies each season due to changes in vaccine components and their antigenic match to dominant circulating strains, highlighting the value of annual VE monitoring to estimate the health impact of influenza vaccination.Citation6–Citation12
In China, the current national pharmacopeia, which serves as a guideline for instructions on domestic vaccine package inserts, recommends administering two doses of seasonal influenza vaccine of 0.25 ml containing 7.5 μg hemagglutinin for each virus strain per dose for children aged 6–35 months who have never received influenza vaccine before, and only one dose of 0.5 ml containing 15 μg hemagglutinin for each virus strain for children aged ≥3 years, regardless of prior vaccine receipt.Citation13,Citation14 These instructions are not consistent with recommendations by the World Health Organization (WHO),Citation15 the Advisory Committee on Immunization Practices, United States (ACIP),Citation16 and China CDC,Citation1,Citation2,Citation17 all of which recommend two standard doses of seasonal influenza vaccine for children aged 6 months–8 years who have never received seasonal influenza vaccine in the past: two doses for children aged 6–35 months with 0.25 ml containing 7.5 μg hemagglutinin for each virus strain per dose, and two doses for children aged 3–8 years with 0.5 ml containing 15 μg hemagglutinin for each virus strain per dose (Supplemental Table 1). To examine whether variations in the number of vaccination doses administered are associated with differences in VE over three influenza seasons, we conducted a matched case–control study in Guangzhou, China during the 2013/14–2015/16 influenza seasons and estimated VE against laboratory-confirmed influenza illness among children aged 6 months - 8 years by age group and doses of vaccination received.
Table 1. Characteristics among study children by age groups in Guangzhou, China, 2013/14–2015/16
Methods
Guangzhou is the capital city of Guangdong Province in southern China, with a population of 13 million.
Case selection methods to estimate VE based on Guangzhou’s influenza sentinel surveillance network have been previously described.Citation6–Citation8 In brief, the network covers 19 hospitals, located in all 12 districts of the city. Following the national influenza sentinel surveillance guidelines, hospitals screened for influenza-like illness (ILI) cases among all outpatient visits in surveillance departments year-round, with each new influenza season beginning on October 1. ILI was defined as acute onset of measured fever (temperature ≥38°C) with cough or sore throat. Each week, each hospital was required to collect nasal, throat, or combined nasal and throat swab specimens from a random sample of 5–15 ILI cases that had illness onset in the past 3 days and no antiviral treatment since illness onset. Specimens were transported at a temperature of <4°C within 48 h of sample collection to Guangzhou CDC laboratory and tested for influenza virus by real-time reverse transcription polymerase chain reaction (rRT-PCR).Citation18 Cases were rRT-PCR confirmed influenza infections identified through the ILI sentinel surveillance network in Guangzhou during the 2013/14–2015/16 seasons. Guangzhou experiences year-round influenza circulation, with peaks usually identified between February and July.Citation19 Influenza cases were further designated as type A or B influenza cases. We randomly selected half of all influenza cases reported during the study period by assigning computer-generated random numbers to each case, sorting the random numbers, and selecting the first 50%. We did not exclude cases that occurred in the same child more than once.
We selected random community controls matched by sex and age for comparison. We used records of local children in the Childhood Immunization Information Management System (CIIMS), matching each case 1:1 with controls of the same sex and birth date (±1 month within the influenza case’s birth date). CIIMS registration is completed the first time children seek vaccination services and more than 99% of all children aged 6 months–8 years living in Guangzhou are included in CIIMS.Citation10 Using the combination of name and birth date, we excluded all influenza cases reported during the study period from the sample of potential controls. Then, we identified all remaining children in CIIMS who met the age and sex matching criteria for each influenza case and used a random selection data management module to match one child among all eligible control children to each case.
Vaccine components for the study seasons are listed in Supplemental . Each year, seasonal influenza vaccination was offered at 252 vaccination clinics throughout Guangzhou. We obtained the date of vaccination for both cases and controls through CIIMS. CIIMS has previously demonstrated >97% completeness for number of times vaccinated, and >91% accuracy for items recorded at each vaccination (including vaccine batch and vaccination date and place).Citation20,Citation21
Table 2. Influenza vaccination dose among unprimed a children receiving influenza vaccination for the first time, Guangzhou, 2013/14–2015/16
In the VE analysis, for each case–control pair, a child was only considered vaccinated if they had received seasonal influenza vaccine at least 14 days before the date of the case’s influenza illness onset. In each case–control pair, children vaccinated <14 days prior to or after the case’s influenza illness onset date was considered unimmunized for that dose. In sensitivity analyses, we excluded these children from analysis. A child was considered vaccinated in a prior season if vaccination was recorded at any time for that prior year. To look at the effect of doses on children subject to different dose recommendations, we divided all study children into two age groups: 6–35 months and 36 months–8 years.
VE was estimated as (1-exp(β))*100%, where β was the coefficient estimate associated with the vaccination calculated using an unconditional logistic regression model adjusting for sex and age. We also performed stratified analysis of VE against different types of influenza virus and used the median test and chi-square to analyze group differences when appropriate. For statistical analysis, we used IBM SPSS Statistics 21(NIH) software. All tests were two-tailed, and we considered an alpha-level of 0.05, or 95% Confidence Interval (CI) not overlapping with zero as statistical significance.
To analyze the effect of priming dose(s) on subsequent protection against influenza illness (with or without a current season vaccination), we defined priming as receipt of ≥1 dose of influenza vaccine during the immediate prior season. We also examined receipt of 1 dose or 2 doses of vaccine in the current season (prior to the index illness).Citation22 In calculating VE, the reference group was those who were unprimed and had no current season dose. We calculated VE of different combinations of vaccination history (Supplemental Table 3): (a) prime dose(s) with no current season dose(s) (as an example, for the 2013/14 season analysis, this would include children who received dose(s) in 2012/13 but no dose in 2013/14), (b) no priming and 1 current season dose, (c) no priming and with 2 current season doses, and (d) priming with 1 or 2 current season doses. (Due to small numbers these could not be examined separately.) We did not estimate VE for any exposure categories with <5 influenza cases. We estimated that 81 children in each group were needed to achieve 80% power (α = 0.05) to detect a VE of 50% with an influenza positivity rate of 40% in the unvaccinated group and 20% in the vaccinated group. Despite low statistical power, we presented these estimates to note trends in effects across age groups and seasons. We did not correct for multiple comparisons.
Table 3. Influenza vaccine effectiveness (VE) by influenza season and age group in Guangzhou, China, 2013/14–2015/16
Results
2013/14 influenza season
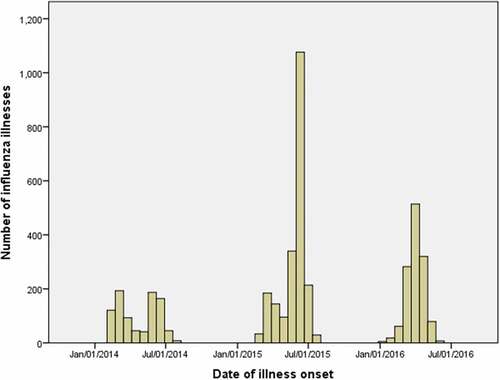
The 2013/14 season included 819 age- and sex-matched case–control pairs (). Two peaks of influenza activity were observed, the first from February–April 2014, and the second from May–July 2014 (). Among all study children, influenza type A viruses accounted for 79% (650) of infections; type B viruses were more common among older children (aged 36 months–8 years) than younger children (aged 6–35 months) (25% vs. 17%, p = .008) (not shown in table).
Figure 1. Laboratory-confirmed influenza infections among children aged 6 months to 8 years who were enrolled during the 2013/14–2015/16 seasons in Guangzhou, China
Among all study children, the proportion receiving one or more vaccine dose(s) in the current season was higher among controls than influenza cases (23% vs. 13%, p = .0002). Influenza vaccination coverage was higher among children aged 6–35 months than those aged 36 months–8 years among both community controls (26% vs. 19%, p = .01) and influenza cases (14% vs. 9%, p = .03) (). Among unprimed children (no influenza vaccination in the season prior to 2013/14) receiving influenza vaccination for the first time in the 2013/14 season, the receipt of the universally recommended two doses for children aged 6–35 months was 62% (109/175), and was 30% (13/44) for children aged 36 months–8 years ().
Among all study children, overall VE of current 2013/14 season dose(s) (regardless of priming) was 58% (95% CI: 45–68%), with VE 57% (43–68%) against influenza type A and 61% (33–77%) against influenza type B. Overall VE by age group was 59% (44–71%) in children aged 6–35 months and 62% (36–78%) in children aged 36 months–8 years ().
Among unprimed children aged 6–35 months, either one or two doses of influenza vaccine in the current season offered statistically significant protection against influenza (); VE point estimates were higher for children with recommended two current season doses (65%, 95% CI: 46–78) compared to children with one current season dose (45%, 95% CI: 8–67) ().
Among unprimed children aged 36 months–8 years, VE point estimates were also higher for children with two current season doses (79%, 95% CI: 20–94) compared to children with one current season dose (57%, 95% CI: 4–81) ().
2014/15 influenza season
The 2014/15 season included 2,080 age- and sex-matched case–control pairs (). One major peak was observed, from March–July (). Influenza type A viruses accounted for 60% (1,257) of cases; as in the prior season, influenza type B infections were more common among children aged ≥36 months than those aged 6–35 months (33% vs. 19%, p < .001) (not shown in table). The pattern of vaccine coverage was also similar to the previous season, with higher coverage among the youngest children (). Among unprimed children receiving vaccination for the first time, 64% (184/287) of those aged 6–35 months received two doses, while 5% (5/99) of children aged 36 months–8 years received 2 doses ().
Among all study children, overall VE for ≥1 current season dose was 24% (9–37%), with VE 22% (3–36%) against influenza type A and 20% (−9%,41%) against influenza type B. The VE estimate was lower with a confidence interval that included zero for children aged 6–35 months (12%, 95% CI: −11,30), but remained statistically significant at 43% (22–58%) for children aged 36 months–8 years ().
Among children aged 6–35 months, there was no statistically significant VE among unprimed children who received 1 or 2 doses in the current season. However, the VE point estimate was higher for 2 doses than 1 dose: 19% (−11%,40%) vs. −2% (−53%,32%) ().
Among unprimed children aged 36 months–8 years, we observed statistically significant VE for children with 1 current season dose (36%, 95% CI: 2–58), though the VE point estimate was lower in this group than among children with 2 current season doses (77%, 95% CI:-106, 98) and was lower than among all vaccinated children in this age group ().
2015/16 influenza season
The 2015/16 season included 1,286 age- and sex-matched case–control pairs (). One major peak was observed from March–May (). Influenza type B viruses accounted for 49% (634) of infections, type A viruses accounted for 30% (380), and influenza type information was not recorded for 19% (246) (data not shown). Again, vaccine coverage was higher among the youngest children and higher among controls than cases (). Among unprimed children, 63% (62/99) aged 6–35 months received the recommended two doses, while 1% (1/69) aged 36 months–8 years received two doses ().
Among all study children, overall VE against influenza for ≥1 dose(s) was 44% (26–57%), with VE 71% (51–83%) against influenza type A and 24% (−5%,45%) against type B. The VE point estimates among children aged 6–35 months were higher (54%, 95% CI: 32–69) than among those aged 36 months–8 years (32%, 95% CI: 1–53) ().
Among unprimed children aged 6–35 months, there was no statistically significant protection with one current season dose alone (37%, 95% CI: −24,68), but significant VE for two current season doses (61%, 95% CI: 32–78).
Among unprimed children aged 36 months–8 years, there was no statistically significant protection with one current season dose alone (31%, 95% CI: −14,59) ().
The results of the sensitivity analyses, which excluded children in each case–control pair who were vaccinated <14 days prior to or after the case’s influenza illness onset date, were similar to the results presented above (data not shown).
Discussion
Among study children aged 6 months–8 years in Guangzhou, China, less than a quarter received at least one dose of influenza vaccine during the 2013/14–2015/16 seasons, and vaccine coverage decreased over study years. The overall VE against influenza ranged from 24% to 58%. Across seasons, only 30%, 5% and 1% of unprimed children aged 36 months–8 years received two doses of vaccine in the first season they were vaccinated, as recommended by WHO,Citation15 China CDCCitation1 and ACIP;Citation16 most received only one dose, as recommended by the Chinese pharmacopeia. Even among children aged 6–35 months for whom there is a universal recommendation for two doses of vaccine among unprimed children, only 62–64% received two doses in the first season they were vaccinated.
In the 2013/14 season, the influenza vaccine provided substantial protection against influenza with a VE of 58%, similar to the VE reported in the United States for the season, where point estimates against the main circulating virus, A(H1N1)pdm09, were 54–62% in health-facility-based studies,Citation23,Citation24 and 66% in a household-based study.Citation25 In 2014/15, the VE (24%) was lower. During this season, both national and global influenza surveillance data demonstrated a mismatch between the predominant circulating influenza A(H3N2) virus and the influenza A(H3N2) vaccine strain.Citation12,Citation26 In the 2015/16 season, VE for influenza was moderate although the circulating viruses matched the vaccine strains. According to national influenza surveillance data, in 2015/16, influenza B Victoria was the predominant circulating influenza B lineage. Influenza B Victoria lineage strain was not included in the 2015/16 northern hemisphere trivalent inactivated influenza vaccine,Citation27 the only vaccine licensed in China. This likely decreased VE against type B influenza illness during the season, with higher impact among older children who had a higher proportion of influenza type B infections than younger children.
Our study demonstrated that VE differed among unprimed children with different doses of current season influenza vaccine. Among unprimed children aged 6–35 months, VE point estimates suggest that two influenza vaccine doses in the current season may have provided higher protection against influenza illness across influenza seasons than one dose alone, though confidence intervals overlapped. These findings may suggest that the booster effect of the second dose was needed to generate sufficient antibody response in younger children, supporting current dosing recommendations for unprimed younger children by WHO, China CDC, ACIP, and the Chinese pharmacopeia. Among unprimed children aged 36 months–8 years, one dose of influenza vaccine in the current season showed insignificant or lower protection against laboratory-confirmed influenza illness compared to two doses. Despite known differences in immune responses to the same vaccination by age group, our findings suggest the need to re-evaluate the current Chinese pharmacopeia instructions, which recommend one dose of seasonal influenza vaccine irrespective of prior vaccine history for children aged 36 months and older.Citation13
The optimal age ceiling for recommending two doses of influenza vaccine among unprimed children requires further study. Given current Chinese pharmacopeia instructions, our study included very few unprimed children aged 36 months–8 years who received two doses of influenza vaccine in the current season, and therefore we were not able to sufficiently evaluate the effect of receiving two doses in the current season among unprimed children in this age group. Although WHO,Citation15 ACIPCitation16 and China CDCCitation1 all recommend administering two doses of seasonal influenza vaccine for unprimed children aged 6 months through 8 years, the evidence supporting this recommendation is limited.Citation28
This study is subject to several limitations. First, children in our study without official household registrations in Guangzhou may have received influenza vaccination in the prior year without documentation in the Guangzhou CIIMS. Fortunately, Guangzhou CDC has access to CIIMS records for all children vaccinated in Guangdong Province, enabling review of records of children who may have been vaccinated in other cities within the province. Second, as our cases were detected through the ILI surveillance system, we did not have the ability to assess VE in preventing more serious illness, such as influenza-associated severe acute respiratory illness. We also lacked complete data on influenza subtypes or lineages. Third, as the number of unprimed children aged ≥36 months who received two doses of influenza vaccine in the current season was low, we did not have the power to sufficiently evaluate the booster dose effect for unprimed children in this age group. Fourth, we cannot confirm that all control children did not have influenza illness during the study period. Some patients with mild illness may not have sought medical care. Random community controls with ILI prior to the ILI onset of influenza cases would bias the true VE estimate toward null.
Conclusions
Annual seasonal influenza vaccination is effective at preventing influenza illness among children aged 6 months to 8 years. Our findings suggest the utility of providing a “booster dose” of seasonal influenza vaccine to children aged 6–35 months, and suggest the need to study further the effect of the booster dose among older children.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to disclose.
Declarations
This study was approved by the Guangzhou CDC ethics committee.
Authors’ contributions
Chuanxi Fu and Suizan Zhou designed the study and wrote the manuscript; Qing He, Ying Liao, Yanmin Wan, Jichuan Shen, and Chao Rong collected the data and built the dataset, and Carolyn Greene revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the US CDC.
Supplemental Material
Download MS Word (31.1 KB)Acknowledgments
We thank Mark G. Thompson for his data analysis guidance and for his thoughtful review of the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Feng L, Yang P, Zhang T, Yang J, Fu C, Qin Y, Zhang Y, Ma C, Liu Z, Wang Q, et al. Technical guidelines for the application of seasonal influenza vaccine in China (2014–2015). Hum Vaccin Immunother. 2015;11(8):2077–101. doi:10.1080/21645515.2015.1027470.
- Guidelines for the application of seasonal influenza vaccine in China (2003-9-4): Chinese center for disease control and prevention; 2003 [accessed 2017 May 18]. http://www.chinacdc.cn/jkzt/crb/bl/lxxgm/ymjz/200509/t20050908_24123.html?from=singlemessage&isappinstalled=1.
- Feng L, Mounts AW, Feng Y, Luo Y, Yang P, Feng Z, Yang W, Yu H. Seasonal influenza vaccine supply and target vaccinated population in China, 2004–2009. Vaccine. 2010;28(41):6778–82. doi:10.1016/j.vaccine.2010.07.064.
- Yang J, Atkins KE, Feng L, Pang M, Zheng Y, Liu X, Cowling BJ, Yu H. Seasonal influenza vaccination in China: landscape of diverse regional reimbursement policy, and budget impact analysis. Vaccine. 2016;34(47):5724–35. doi:10.1016/j.vaccine.2016.10.013.
- Zhou L, Su Q, Xu Z, Feng A, Jin H, Wang S, Feng Z. Seasonal influenza vaccination coverage rate of target groups in selected cities and provinces in China by season (2009/10 to 2011/12). PLoS One. 2013;8(9):e73724. doi:10.1371/journal.pone.0073724.
- Fu C, He Q, Li Z, Xu J, Li Y, Lu J, Li K, Yang Q, Dong Z, Liu X, et al. Seasonal influenza vaccine effectiveness among children, 2010–2012. Influenza Other Respir Viruses. 2013;7(6):1168–74. doi:10.1111/irv.12157.
- Yang Z, Dong Z, Fu C. Seasonal influenza vaccine effectiveness among children aged 6 to 59 months in southern China. PLoS One. 2012;7(1):e30424. doi:10.1371/journal.pone.0030424.
- Fu C, Xu J, Lin J, Wang M, Li K, Ge J, Thompson MG. Concurrent and cross-season protection of inactivated influenza vaccine against A(H1N1)pdm09 illness among young children: 2012–2013 case-control evaluation of influenza vaccine effectiveness. Vaccine. 2015;33(25):2917–21. doi:10.1016/j.vaccine.2015.04.063.
- Yang P, Thompson MG, Ma C, Shi W, Wu S, Zhang D, Wang Q. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012–2013 season in Beijing, China. Vaccine. 2014;32(41):5285–89. doi:10.1016/j.vaccine.2014.07.083.
- Wang Y, Zhang T, Chen L, Greene C, Ding Y, Cheng Y, Yang C, Zeng S, Hua J, Zhou S, et al. Seasonal influenza vaccine effectiveness against medically attended influenza illness among children aged 6–59 months, October 2011-September 2012: A matched test-negative case-control study in Suzhou, China. Vaccine. 2016;34(21):2460–65. doi:10.1016/j.vaccine.2016.03.056.
- Qin Y, Zhang Y, Wu P, Feng S, Zheng J, Yang P, Pan Y, Wang Q, Feng L, Pang X, et al. Influenza vaccine effectiveness in preventing hospitalization among Beijing residents in China, 2013–15. Vaccine. 2016;34(20):2329–33. doi:10.1016/j.vaccine.2016.03.068.
- Zhang Y, Wu P, Feng L, Yang P, Pan Y, Feng S, Qin Y, Zheng J, Puig-Barbera J, Muscatello D, et al. Influenza vaccine effectiveness against influenza-associated hospitalization in 2015/16 season, Beijing, China. Vaccine. 2017;35(23):3129–34. doi:10.1016/j.vaccine.2017.03.084.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China 2015. 2017.
- Influenza vaccine (Split Virion), inactivated: medlive; 2012 [accessed 2017 May 18]. http://drugs.medlive.cn/drugref/html/127993.shtml.
- World Health Orgnization. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87(47):461–76.
- Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices, United States, 2015–16 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64(30):818–25. doi:10.15585/mmwr.mm6430a3.
- Guidelines for the application of seasonal influenza vaccine in China (2005-10-26): Chinese center for disease control and prevention; 2005 [accessed 2017 May 18]. http://www.chinacdc.cn/jkzt/crb/bl/lxxgm/tbtj/200511/t20051103_24112.html?from=singlemessage&isappinstalled=1.
- National influenza surveillance guideline: national health and family planning committee 2010 [accessed 2017 May 18]. http://www.moh.gov.cn/jkj/s3577/201009/3fa356d0f4834d408fde6c12891a6482.shtml.
- Guan WD, Gong XY, Mok CK, Chen TT, Wu SG, Pan SH, Cowling BJ, Yang ZF, Chen DH. Surveillance for seasonal influenza virus prevalence in hospitalized children with lower respiratory tract infection in Guangzhou, China during the post-pandemic era. PLoS One. 2015;10(4):e0120983. doi:10.1371/journal.pone.0120983.
- Du H, Xiao Y. Defect analysis of registration documents for childhood immunization. Chin Nurs Manage. 2013;13:64–65.
- Li L, Yuan P, Cao L, Zheng J, Qi Q, Zhang F, Liang X, Cao L, Zhang G, Yin D. Analysis on accuracy of data from immunization unit clients of child immunization registration information system project in earthquake area in China. Chin J Vaccines Immunization. 2012;4:293–96.
- Thompson MG, Clippard J, Petrie JG, Jackson ML, McLean HQ, Gaglani M, Reis EC, Flannery B, Monto AS, Jackson L, et al. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013: the importance of two priming doses. Pediatr Infect Dis J. 2016;35(3):299–308. doi:10.1097/INF.0000000000001006.
- Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, Chung JR, Piedra PA, Avadhanula V, Nowalk MP, et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis. 2016;213(10):1546–56. doi:10.1093/infdis/jiv577.
- Flannery B, Thaker SN, Clippard J, Monto AS, Ohmit SE, Zimmerman RK, Nowalk MP, Gaglani M, Jackson ML, Jackson LA, et al. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness - United States, February 2014. MMWR Morb Mortal Wkly Rep. 2014;63(7):137–42.
- Ohmit SE, Petrie JG, Malosh RE, Johnson E, Truscon R, Aaron B, Martens C, Cheng C, Fry AM, Monto AS. Substantial influenza vaccine effectiveness in households with children during the 2013–2014 influenza season, when 2009 pandemic influenza A(H1N1) virus predominated. J Infect Dis. 2016;213(8):1229–36. doi:10.1093/infdis/jiv563.
- Xie H, Wan XF, Ye Z, Plant EP, Zhao Y, Xu Y, Li X, Finch C, Zhao N, Kawano T, et al. H3N2 mismatch of 2014–15 Northern Hemisphere influenza vaccines and head-to-head comparison between human and ferret Antisera derived Antigenic maps. Sci Rep. 2015;5:15279. doi:10.1038/srep15279.
- Chinese influenza weekly report: Chinese national influenza center; 2017 [accessed 2017 May 18]. http://www.chinaivdc.cn/cnic/en/Surveillance/WeeklyReport/201609/t20160925_134340.htm.
- Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ. Prevention and control of influenza. Recommendations of the advisory committee on immunization practices (ACIP), 2007. MMWR Recomm Rep. 2007;56:1–54.
