ABSTRACT
Influenza contributes to morbidity and mortality worldwide. Children are at a higher risk of influenza-related complications and vaccination promotes direct protection and limits transmission. This study aimed to explore influenza vaccination coverage among children in Australian general practice from 2015 to 2018, and patterns in coverage before and after the implementation of state-funded immunization programs. Data from 196,520 ‘active’ patients (3+ consultations in two consecutive years) aged <5 years from 542 Australian general practices were included (MedicineInsight database). Logistic regression models were used to identify associations between vaccination with patient and practice characteristics. The overall vaccination coverage increased more than five times from 2015 (3.9%) to 2018 (19.6%) and varied among states. Children attending practices located in the wealthiest areas were more likely to receive the vaccine and appeared to benefit most from the funding, as the increase in coverage from 2017 to 2018 was greater among them than those attending practices in the least advantaged areas (17 vs. 11 percentage points, respectively). This relationship was not evident when analyzing the patient’s socioeconomic level. In conclusion, free influenza vaccinations increase coverage in at-risk populations. Promotional campaigns may be required to maintain higher coverage and target practices located in low-income areas.
Introduction
Influenza is a highly contagious viral respiratory infection that contributes to morbidity and mortality especially among children aged < 5 years, the elderly, pregnant women and people with chronic conditions.Citation1–Citation3 The economic consequences of influenza in children are extensive, involving both direct (e.g. hospitalizations) and indirect costs (e.g. productivity losses for their parents).Citation4,Citation5 Global estimates have found that up to 105,000 influenza-associated respiratory deaths occur annually among children aged < 5 years.Citation6 In Australia, the highest rates of laboratory-confirmed influenza and influenza hospitalization from 2006 to 2015 were in this age group.Citation7
Vaccination is the most important measure to prevent influenza infection and influenza-related hospitalization and death.Citation1,Citation4 Studies show that influenza vaccination in children is either cost-saving or cost-effective, as they are also the principal transmitters of influenza.Citation4,Citation8–Citation11 The World Health Organization includes all children aged six months to < 5 years as a priority group to be targeted for annual vaccination.Citation3 Although some countries are following this recommendation, such as the United States (US),Citation12 Canada,Citation13 Brazil,Citation14 MaltaCitation15 and Australia,Citation16 there is still a high number of countries recommending the vaccine for children in restricted age groups (e.g. 6 months-2 years; 2–3 years; 2–4 years).Citation15 Furthermore, funding or payment methods vary across countries. In Europe, only 40% of countries reporting a recommendation for influenza vaccination in children offered the vaccine at no cost through national health services.Citation15 In Australia, until 2017, only Western Australia had funded the vaccine for all children aged 6 months to < 5 years, but from 2018 all states and territories have provided free influenza vaccine for this group, except for Northern Territory which started in 2019.Citation17
Identifying influenza vaccination coverage and understanding what factors are associated with that is essential when trying to develop effective vaccine strategies and reduce influenza-related outcomes. It is therefore unfortunate that influenza vaccination is not required to be routinely recorded on the Australian Immunization Register (AIR), even those administered in children, and unlike other childhood vaccines, there are no incentive payments for immunization providers to report this information.Citation18 For this reason and other issues with the recording process,Citation19 influenza vaccination is expected to be underreported to the AIR. An Australian study showed that almost 70% of influenza vaccinations administered in a sample of children with special risk medical conditions between 2014 and 2015 were not recorded in the AIR.Citation20 However, other sources of primary care data, including MedicineInsight, a large general practice database, have shown good data qualityCitation21 and consistency in estimating influenza vaccination coverage in adultsCitation22 and could be used for the same purpose in children. Therefore, this study aimed to identify influenza vaccination coverage among children aged < 5 years from 2015 to 2018 in Australian general practice using the MedicineInsight database, and patterns in coverage before and after the implementation of state-funded programs that provided free influenza vaccinations to this age group.
Patients and methods
Sample
This study used data from the MedicineInsight database, a national general practice program developed and managed by the National Prescribing Service (NPS) MedicineWise.Citation1 De-identified clinical information system data is extracted regularly from participating general practices around Australia. Details of data collection are available elsewhere.Citation21–Citation23
To investigate influenza immunization coverage from 2015 to 2018, ‘active’ patients aged <5 years were included for analysis. ‘Active’ patients were defined according to the Royal Australian College of General Practitioners as any person who has at least three consultations in two consecutive years in a single general practice.Citation24
Data extraction
When a child is vaccinated in an Australian general practice, general practitioners (GPs) and registered nurses may select a medical code in the medical record system or type a ‘free’ text to register this procedure. ‘Free’ text records can include synonyms of influenza vaccination (i.e. flu shot or flu injection) as well as brand names. The data extraction process was based on a previous algorithm used to identify influenza vaccination among adults in the MedicineInsight database.Citation22 Therefore, the terms used to identify all recorded influenza vaccinations in the immunization field included the term ‘flu’, brand names available in Australia during the study period, such as Agrippal®, Fluarix®, FluQuadri®, Influvac®, Vaxigrip®, as well as possible misspellings of these words.
Outcome and covariates
Influenza immunization status for each year from 2015 to 2018 for ‘active’ patients aged < 5 years was used to provide annual vaccination coverage among all ‘active’ patients in this age group who had a consultation that year.
Covariates comprise practice and patient’s characteristics. Practice characteristics include state, rurality (major cities; inner regional; outer regional/remote/very remote) and Index of Relative Socio-economic Advantage and Disadvantage (IRSAD) quintile according to practice location. IRSAD is a macroeconomic indicator of relative economic and social advantage/disadvantage position within an area compared with the rest of the country.Citation25 Patient’s variables include gender, ethnicity (Aboriginal and/or Torres Strait Islander: yes/no), IRSAD quintile according to the location of residence, and the presence of some comorbidities requiring influenza immunization (i.e. diabetes, cardiac disease or severe asthma).Citation16 Different fields of the MedicineInsight database were used in for the identification of these comorbidities, including diagnosis, reason for encounter or prescription. Severe asthma was defined as those patients with a diagnosis of asthma requiring the use of prednisolone.Citation22,Citation26
Data analysis
Influenza vaccination coverage from 2015 to 2018 was calculated as the adjusted proportion of ‘active’ patients aged < 5 years vaccinated that year among all active patients in the same age group who had a consultation that year. The association between influenza vaccine coverage and practice or patient’s characteristics was assessed using logistic regression with robust standard errors for taking into account the clustering of patients within the practice. Analyses investigating associations with patient’s characteristics were adjusted for gender and practice variables, while associations with practice characteristics were mutually adjusted. Marginal adjusted prediction of vaccination coverage in each category of the exposure variables was then estimated and presented in a table and graphically with their respective 95% confidence intervals.
All analyses were performed in the statistical software Stata 15.0 (StataCorp, Texas, USA). To minimize selection bias (i.e. the likelihood of receiving medical treatments or diagnosis increase with the number of visits to the practice)Citation27 analyses were conditioned to the inverse of the patient’s probability of being in the sample (1/median number of consultations with the GP). The independent MedicineInsight Data Governance Committee has approved the study (protocol 2017–007) and the Human Research Ethics Committee of the University of Adelaide exempted it of an ethical review, as only non-identifiable data was used.
Results
The number of ‘active’ patients varied from 72,252 in 2015, to 81,648 in 2016, 96,282 in 2017 and 119,806 in 2018 (). Approximately 67% of practices were located in major cities, 30% of them in areas in the top IRSAD quintile and 15% in the bottom quintile. Around 52% of patients were boys, 4.5% were Aboriginal or Torres Strait Islander and 5% had comorbidity.
Table 1. Influenza vaccine coverage among ‘active’ patientsa aged < 5 years according to practice and patient’s characteristics. Australia, 2015–2018
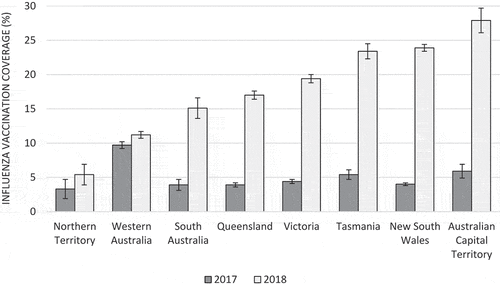
Overall, vaccination coverage was similar in 2015 and 2016 (3.9%), with a slight increase in 2017 (5.1%). However, the coverage in 2018 was four times higher (19.6%) than in previous years, and this pattern was observed in all states except the Northern Territory and Western Australia. Patients from practices located in the highest IRSAD quintiles were more likely to receive the vaccine than those in the lowest quintiles in all years. Until 2017, vaccination coverage was highest among Aboriginal and/or Torres Strait Islanders, but the difference decreased after the implementation of vaccine funding for all children aged <5 years in 2018 (except in the Northern Territory). The coverage in any year was similar in boys and girls, and a patient’s IRSAD did not appear to influence vaccination coverage as did practice IRSAD. In 2018, approximately 20% of children were vaccinated regardless of their area of residence. On the other hand, children with comorbidities had a higher vaccination coverage than their peers in all years, especially in 2018, when the coverage increased 18 percentage points compared to 2017, while among those without comorbidities it increased less than 15 percentage points ().
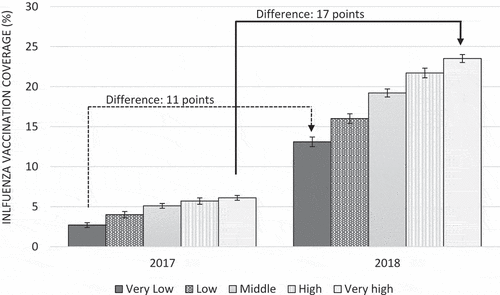
and analyze in detail the main changes in influenza vaccination coverage between 2017 and 2018 (i.e. when funding vaccines were made available in most states) according to state and practice IRSAD quintiles. The Northern Territory continued to have the lowest coverage in 2018, while in Western Australia coverage was less than two percentage points higher than in 2017. Vaccination coverage among children attending practices located in the top IRSAD quintile (i.e. the most advantaged areas) was 17 percentage points higher than in 2017, while among practices in the bottom quintile the difference was of 11 percentage points, reinforcing disparities between practices in these areas.
Discussion
The study aimed to identify, using de-identified individual patient electronic medical records from a general practice database, influenza vaccination coverage in children aged <5 years and vaccination patterns related to practice and patient’s characteristics before and after the implementation of state-funded vaccines. Three main findings can be highlighted based on our results. First, vaccination coverage was at least four times higher in 2018 than previous years in most Australian states, except Western Australia and the Northern Territory. Second, patients attending practices in the wealthiest areas have the highest vaccination coverage in all years and they benefited most from this state-funding program. Finally, children with comorbidities continued to have higher vaccination coverage than their peers, but the same pattern was not observed among Aboriginal and/or Torres Strait Islander children.
Our results showed that coverage was markedly higher in 2018 (~20%) compared to previous years (5%), corroborating data from the AIR for New South Wales (changing from 5% to 25% in the same period).Citation19 According to the mentioned report and other studies, to maintain a higher coverage, influenza vaccination should continue to be funded for all children aged 6 months to 5 years, and strategies should also include providers to enhance knowledge and awareness on the vaccine, as a direct recommendation from a health professional has been associated with a higher likelihood of being vaccinated.Citation19,Citation28,Citation29 A study performed in the United Kingdom (UK) also found a large increase in influenza vaccination after the introduction of a universal vaccination policy for all children aged 2–4 years. According to that study, the vaccination uptake in the UK changed from 1% in 2012–2013 to more than 30% in 2014–2015 among children without comorbidities.Citation30
Western Australia and the Northern Territory were the only two states in our study with less pronounced changes from 2017 to 2018. These findings can be explained by the fact that Western Australia has already funded influenza vaccine for all children since 2008.Citation17 This may also explain why it had the highest coverage from 2015 to 2017 among all states and territories. It is noted, however, that in 2018 the coverage in Western Australia (11%) was lower compared to the other states (15–28%, except by the Northern Territory). This finding suggests that coverage in other states could decrease over time without appropriate promotional vaccine strategies, as the state-funding program may be undermined by the lack of incentive payments.Citation19 On the other hand, the Northern Territory showed the lowest vaccination coverage over the period, and it is probably related to the funding being started in 2019.Citation17 Nevertheless, a subtle increase was observed from 2017, probably because of a higher influenza activity identified in Australia in that year,Citation26 which may have increased awareness among the community. Unlike other states, where community health services deliver only 7% of vaccinations, in the Northern Territory approximately 47% of vaccinations are administered in this setting.Citation18 In this sense, results from this jurisdiction should be analyzed with caution, especially because more than 25% of its population is Aboriginal and/or Torres Strait Islander.
One of the most important determinants of vaccination coverage in our study was practice IRSAD. Children from practices located in the wealthiest areas had higher coverage than those in the poorest areas before and after the implementation of the free-vaccine scheme. Moreover, the former seemed to benefit most from the funding, as the increase in coverage from 2017 to 2018 was greater among patients from these practices than those in the poorest areas, increasing absolute disparities. This relationship was not evident when analyzing patient’s socioeconomic level (IRSAD quintile), which suggests that interventions to improve vaccination coverage would get a greater benefit from promotional campaigns targeting practices and GPs in low socioeconomic areas.
We identified a similar vaccination coverage in boys and girls, and this finding is consistent with reports from the UK,Citation30 ChinaCitation31 and Australia.Citation32 On the other hand, vaccination coverage between 2015 and 2017 was slightly higher among Aboriginal and/or Torres Strait Islander children, and similar figures were reported in a study conducted in New South Wales.Citation19 Influenza vaccine has been nationally-funded for these groups since 2015 because of their higher vulnerability to respiratory illnesses and influenza-related hospitalizations.Citation7,Citation33 However, the increase in vaccination coverage in 2018 reduced the differences between Indigenous (19%) and non-Indigenous children (20%), with similar results being identified in the study from New South Wales (26% and 25%, respectively).Citation19 These findings should be interpreted cautiously, as these groups are more likely to be vaccinated at Community and Indigenous Health Services rather than in general practices.Citation18
Considering that the AIR still has several limitations in reporting influenza vaccination (e.g. lack of incentive payments, technical problems in the recording process) and surveys are expensive and may overestimate vaccination coverage,Citation20 the use of general practice data may be a simple and low-cost method to monitor annual influenza vaccination coverage in children. The study strengths include its very large sample of Australian patients, from all jurisdictions and socioeconomic areas. Moreover, the database has information on the whole medical history of each patient, so it is possible to identify comorbidities and any other relevant medical information.Citation21
Despite its strengths, the study is not free of limitations. Because of ethical restrictions, MedicineInsight only provides the age in years. Therefore, children under six months (i.e. with no recommendation for influenza vaccination) are included in the study, which may have underestimated coverage estimates. However, it is unlikely that associations with practice or patient’s characteristics were affected by this limitation. Second, the database is populated during the encounters by GPs or registered nurses, and depending on the practice routine or burden, data can have its accuracy and completeness compromised. However, we used algorithms for data extraction including medical codes and ‘free’ text in a systematic process and considering different fields to increase data quality.Citation34 Finally, despite most vaccinations are given in Australian general practice (81%),Citation18 some are administered in Community and Indigenous Health Services, local councils or pharmacies, which are not captured by MedicineInsight.
In conclusion, free access to influenza vaccination has increased the coverage among children aged <5 years. The higher coverage in children with comorbidities indicates GPs and/or parents are aware of the benefits of vaccination among these children. Interventions to promote increased vaccination coverage should target practices or GPs in low socioeconomic areas, as practice/GP location was identified as an important determinant of vaccine coverage. Finally, promotional and educational strategies should be placed in practice to maintain higher influenza vaccination coverage over time, as the lack of incentive payments might undermine the benefits of this funded program.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- NPS Medicinewise. General practice insights report July 2016-June 2017: A working paper. New South Wales, Australia: NPS MedicineWise, 2018; 2018.
- Newman LP, Bhat N, Fleming JA, Neuzil KM. Global influenza seasonality to inform country-level vaccine programs: an analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLoS One. 2018;13(2):e0193263. doi:10.1371/journal.pone.0193263.
- World Health Organization (WHO). Influenza (Seasonal); 2018 [accessed 2018 Aug 10]. http://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal).
- Nichol KL. Cost-effectiveness and socio-economic aspects of childhood influenza vaccination. Vaccine. 2011;29(43):7554–58. doi:10.1016/j.vaccine.2011.08.015.
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, Krishnan A. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–30. doi:10.1016/S0140-6736(11)61051-9.
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. doi:10.1016/S0140-6736(17)33293-2.
- Li-Kim-Moy J, Yin JK, Patel C, Beard FH, Chiu C, Macartney KK, McIntyre PB. Australian vaccine preventable disease epidemiological review series: influenza 2006 to 2015. Commun Dis Intell Q Rep. 2016;40:E482–E495.
- Peasah SK, Azziz-Baumgartner E, Breese J, Meltzer MI, Widdowson M-A. Influenza cost and cost-effectiveness studies globally – A review. Vaccine. 2013;31(46):5339–48. doi:10.1016/j.vaccine.2013.09.013.
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2018;2(2):CD004879–CD. doi:10.1002/14651858.CD004879.pub5.
- Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527–e. doi:10.1371/journal.pmed.1001527.
- Sullivan SG, Price OH, Regan AK. Burden, effectiveness and safety of influenza vaccines in elderly, paediatric and pregnant populations. Ther Adv Vaccines Immunother. 2019;7:2515135519826481.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017–18 influenza season. MMWR Recomm Rep. 2017;66(2):1–20. doi:10.15585/mmwr.rr6602a1.
- Zhao L, Young K, Gemmill I. Summary of the NACI seasonal influenza vaccine statement for 2019–2020. Can Commun Dis Rep. 2019;45(6):149–55. doi:10.14745/ccdr.v45i06a01.
- Jamotte A, Clay E, Macabeo B, Caicedo A, Lopez JG, Bricks L, Romero Prada M, Marrugo R, Alfonso P, Moreno Arévalo B, et al. Public health impact and economic benefits of quadrivalent influenza vaccine in Latin America. Hum Vaccin Immunother. 2017;13(4):877–88. doi:10.1080/21645515.2016.1256928.
- European Centre for Disease Prevention and Control. Seasonal influenza vaccination in Europe. Vaccination recommendations and coverage rates in the EU Member States for eight influenza seasons: 2007–2008 to 2014–2015. Stockholm County, Sweden: ECDC; 2017.
- Australian Immunisation Handbook (online); 2019. [accessed 2019 Aug 08]. https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/influenza-flu.
- National Centre for Immunisation Research and Surveillance (NCIRS). History of immunisation in Australia. Significant events in influenza vaccination practice in Australia; 2019 [accessed 2019 Aug 08]. http://ncirs.org.au/sites/default/files/2019-07/Influenza-history-July%202019.pdf.
- Hull BP, Hendry AJ, Dey A, Beard FH, Brotherton JM, McIntyre PB Annual immunisation coverage report. National centre for immunisation research and surveillance. [Online report]. 2017[accessed 2019 Aug 08] http://www.ncirs.org.au/sites/default/files/2018-12/2017%20Coverage%20Report_FINAL_2.pdf.
- National Centre for Immunisation Research and Surveillance (NCIRS). Evaluation of the NSW influenza vaccination program for children aged 6 months to <5 years. Final report; 2009 [accessed 2019 Aug 08]. https://www.health.nsw.gov.au/immunisation/Documents/evaluation-2018-flu.pdf.
- Tuckerman JM, Crawford NWP, Lynch JP, Marshall HM. Are children with special risk medical conditions receiving influenza vaccination? Validity of parental and provider report, and to a National Immunisation Register. Hum Vaccin Immunother. 2019;15(4):951–58. doi:10.1080/21645515.2018.1554966.
- Busingye D, Gianacas C, Pollack A, Chidwick K, Merrifield A, Norman S, Mullin B, Hayhurst R, Blogg S, Havard A, et al. Data resource profile: medicineInsight, an Australian national primary health care database. Int J Epidemiol. 2019. doi:10.1093/ije/dyz147.
- De Oliveira Bernardo C, Gonzalez-Chica DA, Chilver M, Stocks N. Influenza immunisation coverage from 2015 to 2017: A national study of adult patients from Australian general practice. Vaccine. 2019;37(31):4268–74. doi:10.1016/j.vaccine.2019.06.057.
- MedicineInsight. MedicineInsight data book version 2.1. New South Wales, Australia: NPS MedicineWise; Oct 2018 [accessed 2019 Feb 04]. https://cdn0.scrvt.com/08ab3606b0b7a8ea53fd0b40b1c44f86/0253b46d9cb7393e/7f0620452bc8/MedicineInsight-databook-2.1-4-Dec-2018.pdf.
- The Royal Australian College of General Practitioners. Standards for general practices. 4th ed. East Melbourne, Australia: The Royal Australian College of General Practitioners. 2015. [accessed 2019 Aug 09]. https://www.racgp.org.au/FSDEDEV/media/documents/Running%20a%20practice/Practice%20standards/4th%20edition/Standards-4th-edtion.pdf.
- Australian Bureau of Statistics (ABS). Census of population and housing: Socio-Economic Indexes for Areas (SEIFA), Australia. [accessed 2019 Jan09]. http://www.abs.gov.au/ausstats/[email protected]/mf/2033.0.55.001.
- Bernardo CDO, Gonzalez-Chica D, Stocks N. Influenza-like illness and antimicrobial prescribing in Australian general practice from 2015 to 2017: a national longitudinal study using the MedicineInsight dataset. BMJ Open. 2019;9(4):e026396. doi:10.1136/bmjopen-2018-026396.
- Goldstein BA, Bhavsar NA, Phelan M, Pencina MJ. Controlling for informed presence bias due to the number of health encounters in an electronic health record. Am J Epidemiol. 2016;184(11):847–55. doi:10.1093/aje/kww112.
- Schmid P, Rauber D, Betsch C, Lidolt G, Denker M-L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One. 2017;12(1):e0170550–e. doi:10.1371/journal.pone.0170550.
- Rizzo C, Rezza G, Ricciardi W. Strategies in recommending influenza vaccination in Europe and US. Hum Vaccin Immunother. 2018;14(3):693–98. doi:10.1080/21645515.2017.1367463.
- Rajaram S, Steffey A, Blak B, Hickman M, Christensen H, Caspard H. Uptake of childhood influenza vaccine from 2012–2013 to 2014–2015 in the UK and the implications for high-risk children: a retrospective observational cohort study. BMJ Open. 2016;6(8):e010625. doi:10.1136/bmjopen-2015-010625.
- Lau JTF, Ng CSM, Wu AMS, Ma YL, Lau MMC. Low coverage of influenza vaccination among Chinese children aged 12–23 months: prevalence and associated factors. PLoS One. 2018;13(10):e0205561. doi:10.1371/journal.pone.0205561.
- Tuckerman J, Misan S, Salih S, Joseph Xavier B, Crawford NW, Lynch J, Marshall HS. Influenza vaccination: uptake and associations in a cross-sectional study of children with special risk medical conditions. Vaccine. 2018;36(52):8138–47. doi:10.1016/j.vaccine.2018.09.039.
- Naidu L, Chiu C, Habig A, Lowbridge C, Jayasinghe S, Wang H, McIntyre P, Menzies R. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia 2006–2010. Commun Dis Intell. 2013;37:S1–95.
- Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi:10.1371/journal.pmed.1001809.