ABSTRACT
The quadrivalent meningococcal conjugate vaccine MenACWY-CRM is approved in the Republic of Korea for use in individuals from 2 months of age. This single-arm, open-label, observational, multicenter, post-marketing study (NCT01766206) assessed the safety of MenACWY-CRM vaccine administered according to local clinical practice. A total of 3939 individuals aged 2 months–55 years provided safety data post-vaccination; the analysis was conducted on the per-protocol set (3920 participants). Solicited and unsolicited adverse events (AEs) were collected over 7 days post-vaccination and medically-attended AEs (MAAEs) and serious AEs (SAEs) over 29 days post-vaccination. Among recorded solicited AEs, injection site AEs were reported by 21.38% of participants, with tenderness/pain being most frequent across age groups; systemic AEs were reported in 13.95% of participants, with irritability (in ˂6-year-olds), headache and myalgia (in ≥6 year-olds) being the most frequently reported. Most solicited AEs were mild or moderate in nature. The percentage of participants reporting unsolicited AEs varied in the study population, i.e. 12.56% in participants aged 2–23 months and 3.18% in those ≥2 years of age. Overall, less than 22% of unsolicited AEs were considered as related to vaccination. MAAEs (10.89% of participants) were mostly mild; 2.82% were considered as related to vaccination. Three (0.46%) and 5 (0.15%) SAEs (none vaccination-related) occurred in participants aged 2–23 months and 2–55 years, respectively. No deaths were reported. The safety profile for MenACWY-CRM in this post-marketing surveillance was consistent with observations from studies conducted during the vaccine’s clinical development, with no new safety concerns.
Introduction
Invasive meningococcal disease (IMD) is an acute bacterial infection caused by Neisseria meningitidis, with six meningococcal serogroups (A, B, C, W, Y and more recently, X) accounting virtually for all IMD cases worldwide.Citation1,Citation2 Although a decrease in the incidence of the disease has been noted in the last decade, IMD remains a significant global public health threat: the case fatality ratio is around 10% with treatment, and long-term sequelae are observed in up to 20% of survivors.Citation3,Citation4 Young people are at the highest risk of contracting the disease, with incidence peaks observed in infants, and to a lesser extent in adolescents and young adults.Citation2
The epidemiology of IMD is dynamic, with great variations in the prevalence of disease-causing serogroups observed from one region to another, and over time.Citation1,Citation2,Citation5–Citation7 In the Republic of Korea, the incidence of meningococcal disease is relatively low according to the national surveillance system, with annual estimates of 0.01–0.08 cases per 100,000 persons, although these reports are believed to underestimate the true burden of the disease.Citation7,Citation8 According to the Korea Centers for Disease Control and Prevention data, the annual number of meningococcal meningitis cases was 5–6 between 2013 and 2016, but increased to 17 in 2017 and was estimated at 12 in August 2018.Citation9 Between 2010 and 2016, serogroup B was the most prominent cause of IMD in the Republic of Korea identified in 37% of cases, followed by serogroups W (26%) and C (21%).Citation10
Vaccination remains the most effective approach to prevent IMD.Citation2 Polysaccharide meningococcal vaccines were initially used, but were observed to not be immunogenic in children and unable to elicit immunological memory; the focus therefore shifted toward the development of protein-conjugate formulations. Mass vaccination with the monovalent serogroup C conjugate meningococcal vaccine, first implemented by the United Kingdom, was successful, with a rapid decline in the incidence of serogroup C IMD in vaccinated and unvaccinated populations.Citation1 Subsequently, quadrivalent meningococcal vaccines, targeting serogroups A, C, W and Y, have been developed and licensed worldwide. The meningococcal ACWY quadrivalent CRM197-conjugate vaccine (MenACWY-CRM; Menveo, GSK) is approved for use in different age groups in many countries.Citation11 The vaccine has been shown to be immunogenic, able to prime immunological memory, and well tolerated in infants,Citation12,Citation13 children,Citation14 adolescents and adults.Citation15–Citation17
MenACWY-CRM was initially licensed in the Republic of Korea for use in individuals aged 11–55 years in 2012, and subsequently in children aged 2–11 years and infants ≥2 months of age in 2013 and 2014, respectively. MenACWY-CRM is the first meningococcal vaccine approved for use in infants in this country. This surveillance study was conducted as a post-marketing commitment to the Ministry of Food and Drug Safety (MFDS) of the Republic of Korea, to monitor the safety of marketed MenACWY-CRM in participants aged 2 months–55 years receiving the vaccine according to routine clinical practice and prescribing information.
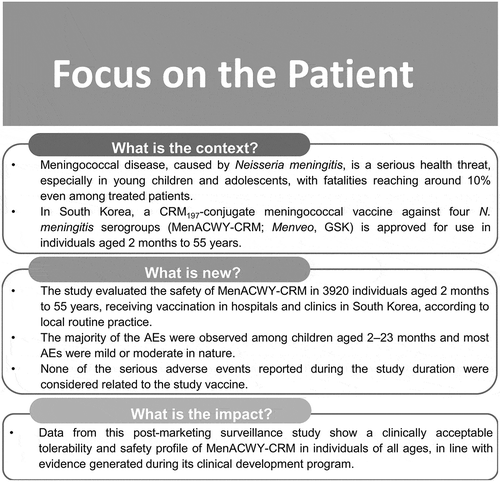
The Focus on the Patient section () summarizes the present research and its clinical relevance and impact on the vaccinated population.
Methods
Study design and participants
This single-arm, open-label, multi-center, observational, post-marketing surveillance study was conducted between May 2012 and May 2018 in the Republic of Korea, and was designed to monitor the safety of MenACWY-CRM administered according to routine clinical practice in healthy participants aged 2 months–55 years.
Safety data were collected for a 29-day period (day 1–day 29) following the receipt of a MenACWY-CRM dose which was administered according to local prescribing information in each age category: as a 4-dose series in children initiating vaccination at 2–6 months of age (three doses administered at least two months apart, with the fourth dose in the second year of life), as a 2-dose series in unvaccinated children 7–23 months of age (with the second dose administered in the second year of life, at least three months after the first dose), or as a single dose in participants aged 2–55 years. Children 2–23 months of age were enrolled at any point in the vaccination series and were followed-up after each dose of MenACWY-CRM received, according to parental consent.
Individuals were considered eligible for enrollment if: (i) they were 2 months–55 years of age at the time of MenACWY-CRM administration, (ii) written informed consent/assent was obtained by them or their parent/legal representative, (iii) they were able to comply with the protocol’s requirements (e.g. completion of the diary card), and (iv) they were in good health as determined by their medical history outcome and the physical assessment and clinical judgment of the investigator. Concomitant routine vaccinations according to the national recommendations in South KoreaCitation18 were allowed. Individuals were excluded if: (i) they had a contraindication, special warnings and/or precautions reported in the MenACWY-CRM prescribing information as evaluated by the investigator, and (ii) they had already been enrolled in the study at another study site for previous MenACWY-CRM vaccination.
A 0.5 mL dose of MenACWY-CRM contains 10 μg of N. meningitidis serogroup A oligosaccharide and 5 μg each of oligosaccharides from serogroups C, W and Y, conjugated to CRM197.The vaccine was reconstituted immediately before administration by mixing the lyophilized A component with the liquid CWY component and administered by intramuscular injection, preferably in the anterolateral aspect of the thigh in infants or into the deltoid muscle (upper arm) in children, adolescents and adults.Citation11
This study was conducted in accordance with International Council for Harmonized Tripartite Guidelines for Good Clinical Practice, the MFDS requirements and regulations, and the Declaration of Helsinki. The study was registered at www.clinicaltrials.gov (NCT01766206) and a protocol summary is available from www.gsk-clinicalstudyregister.com (study ID 205341).
Study objectives and endpoints
The primary objective of the study was to monitor the safety of MenACWY-CRM in individuals aged 2 months to 55 years in the Republic of Korea, as assessed in terms of number of participants exposed to the study vaccine with reported: solicited local and systemic adverse events (AEs) and unsolicited AEs within the 7-day period (day 1–7) post-vaccination, and medically-attended AEs (MAAEs) and serious AEs (SAEs) within the 29-day period post-vaccination.
Solicited local AEs were injection site erythema, injection site induration and injection site tenderness (children <6 years of age) or pain (participants aged ≥6 years). Solicited systemic AEs were changes in eating habits, sleepiness, irritability, rash, vomiting, diarrhea and fever for children aged <6 years, and chills, nausea, malaise, generalized myalgia, generalized arthralgia, headache, rash and fever for those ≥6 years of age (Supplementary Material, Table S1).
MAAEs were defined as events requiring a physician’s or an emergency room visit.
All AEs were graded by severity, from mild to severe, as shown in Table S1.
All AEs/SAEs were assessed by the investigator as either probably related, possibly related, or unrelated to MenACWY-CRM vaccination. Any AEs resulting in withdrawal from the study were also summarized. Unsolicited AEs were recorded by preferred terms using the most recent Medical Dictionary for Regulatory Activities and grouped by system organ class.
Statistical analysis
A total of 3960 participants were planned for enrollment in this study (assuming a 10% drop-out rate) to obtain approximately 3000 evaluable participants in the 2–55 years of age cohort, and 600 participants in the 2–23 months of age cohort, to provide continued safety monitoring in the Korean population in compliance with post-licensure safety MFDS requirements. All planned analyzes were purely descriptive and were initially conducted by age strata (2–10 years, 11–18 years, 19–34 years and 35–55 years of age). Following a request from the MFDS, post-hoc analyses were performed to present safety data separately for the 2–23 months and 2–55 years age groups.
Results
Demographics
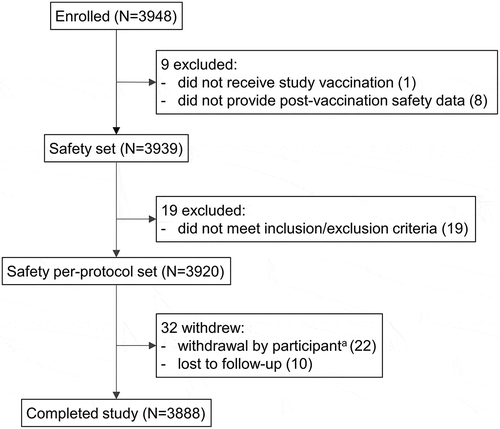
A total of 3948 individuals were enrolled in the study: 3939 provided safety data and 3920 were included in the safety per-protocol set (with no protocol deviations). Failure to meet inclusion/exclusion criteria was the main reason for exclusion from the per-protocol set (). The demographic characteristics of participants at enrollment are presented in . The mean age in the safety per-protocol set was 18.21 years and 83.34% of participants belonged to the 2–55 years age group. There were slightly more female (52.53% in the 2–23 months and 56.01% in the 2–55 years age groups, respectively) than male participants. Most participants in either age group received 1 dose of MenACWY-CRM ().
Table 1. Baseline characteristics of study participants (per protocol safety set; N = 3920).
Safety data
During the surveillance period, a total of 3011 AEs; including solicited, unsolicited and SAEs; were reported in 1376 study participants, with 35.10% of participants experiencing at least one AE (). The percentage of participants with at least one reported AE was higher in the 2–23 months age group (64.47%), compared to the 2–55 years one (29.23%) (). Overall, no increase in the reporting of AEs was observed with subsequent vaccinations in the 2–23 months age group (Table S2).
Table 2. Summary of reported adverse events, overall and by age group (safety per protocol set).
Solicited adverse events
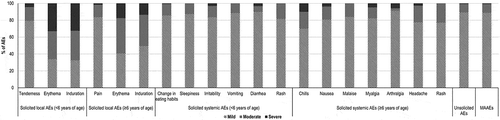
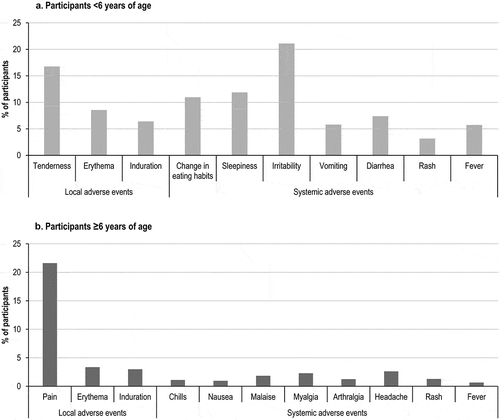
The most frequently reported solicited local AEs were injection site tenderness (in 16.78% of participants <6 years of age) and pain (in 21.61% of participants aged ≥6 years) (). The most frequently reported solicited systemic AEs were irritability (in 21.10% of children aged <6 years), and headache and myalgia (in 2.61% and 2.28% of participants aged ≥6 years of age, respectively) ().
Figure 3. Occurrence of solicited local and systemic adverse events, by age group (safety per protocol set).
Solicited AEs were generally mild or moderate in nature. Among solicited local AEs, 4.46–33.01% in children <6 years and 1.70–17.58% in participants ≥6 years were severe. The proportion of severe events among solicited systemic AEs ranged from 0.00%–3.37% and from 0.00%–10.00% in participants <6 years and ≥6 years of age, respectively ().
Unsolicited adverse events
Overall, unsolicited AEs were reported in 4.74% of participants. Higher percentages of participants with reported unsolicited AEs were observed for the 2–23 months age group (12.56%), compared to the 2–55 year age group (3.18%) ().
In the 2–23 month age group, the most frequently unsolicited AEs were nasopharyngitis (in 3.22% of participants), bronchitis (in 2.14% of participants), pharyngitis and bronchiolitis (each in 1.07% of children). In the 2–55-year-olds, the most frequent AEs were bronchitis (in 1.04% of participants), nasopharyngitis (in 0.70% of participants), pharyngitis (0.64% of participants) and gastroenteritis (0.55% of participants).
Most unsolicited AEs were mild (89.18%) or moderate (10.07%) in nature, with only 0.75% reported as severe (). In total, 21.65% of the unsolicited AEs (occurring in 47 [1.20%] participants of all ages) were considered as possibly or probably related to the study vaccine.
Medically-attended adverse events
In total, 674 MAAEs occurred in 427 (10.89%) participants in the safety per-protocol set within the 29-day post-vaccination period. MAAEs were reported with a higher frequency in children aged 2–23 months (36.45%), compared to participants aged 2–55 years (5.79%) (). In the 2–23 months age group, nasopharyngitis, bronchitis, and pharyngitis were the most frequent MAAEs, reported in 9.49%, 9.34%, and 3.22% of children, respectively. The same MAAEs were also the most frequently reported in the 2–55 years age group, but in a much lower percentage of participants (in 2.26% for nasopharyngitis, 4.08% for bronchitis and 2.25% for pharyngitis) compared to the younger age group.
The majority of MAAEs (88.72%) were considered mild in nature, and only 0.89% were classified as severe (). By study end, 80.56% of MAAEs were resolved and 19.29% persisted beyond the 29-day post-vaccination period, with 0.15% (1) lost to follow-up. Overall, 2.82% of the MAAEs (occurring in 0.46% [18] of participants) were considered as possibly or probably related to the study vaccine.
Serious adverse events
Within the 29-day post-vaccination period, a total of 8 SAEs were reported (). Three SAEs (bronchiolitis, pneumonia and tonsillitis) were reported in the 2–23 months age group, and 5 were reported in participants aged 2–55 years (two cases of pneumonia and one of pyrexia in the 2–10 years age stratum, and a case of pneumonia and one of dizziness in the 11–18 years age stratum). None of the SAEs were considered related to the study vaccine. No fatal events were reported.
The results of additional analyses by age strata (including the 11–55 yeas age group) are presented in Table S3.
Discussion
This is the first study assessing the safety of MenACWY-CRM administered according to routine local immunization practices in the Republic of Korea. Overall, 3948 participants aged 2 months – 55 years were enrolled, and 3920 were followed up for approximately one month after MenACWY-CRM vaccination and included in the study analysis. Overall, AEs were reported in 35.10% of study participants. The majority of the AEs were observed among children aged 2–23 months and most AEs were mild or moderate in nature. Eight SAEs were reported during the study but none were considered as related to the study vaccine.
The safety data observed in this post-marketing surveillance, observational study is consistent with the current safety profile of MenACWY-CRM as observed during its clinical development program.Citation19 Overall, a slightly lower occurrence of AEs was observed in this study conducted in the Republic of Korea, when compared with safety data collected in other geographical areas, in line with what was previously noted in pivotal clinical trials in Asian countries, such as South Korea,Citation20 TaiwanCitation21 and India.Citation22
As already shown in other clinical trials in which MenACWY-CRM was administered alone or with routine pediatric vaccines, injection site tenderness and irritability were the most frequently reported solicited local and systemic AEs for young children<2 years of age.Citation12,Citation13,Citation23–Citation25 Overall, in pre-licensure trials, solicited AEs were observed in ≥50% of children receiving vaccination until 23 months of age, in line with observations made in our study. Unsolicited AEs occurred with similar or lower frequency to that reported in a large phase 3 multi-country pivotal safety study in 5772 infants, who received MenACWY-CRM co-administered with routine vaccines, with overall incidences of AEs observed up to 84%.Citation12 A relatively lower frequency of SAEs (0.6%) was observed in our study compared with data from pre-licensure trials, in which rates of up to 3.6% were reported in infants and toddlers receiving MenACWY-CRM at 2, 4, 6 and 12 months of age.Citation11 This frequency was also lower than that observed in the pivotal safety study, where SAEs were reported in 6% of participants aged below 2 years.Citation12 Of note, in these clinical trials, SAEs were collected over the entire study (from first vaccination to approximately six months post last vaccination), which constitutes a longer follow-up interval than the 29-day period post each vaccination in our study.
As expected, the incidence of reported AEs was lower in the 2–55 year-olds than in children <2 years of age during this post-marketing surveillance. Moreover, this incidence was similar or even lower than that emerging from clinical studies in children, adolescents and adults in this age group, for whom an acceptable clinical safety profile was shown.Citation11 Safety data are available for children 2–10 years of age from various trials conducted in Europe, the United States (US), Latin AmericaCitation13,Citation17,Citation22 and Asia.Citation21,Citation22 Studies in adolescents and adults aged 11–55 years on MenACWY-CRM administered alone or with other vaccines (tetanus-diphtheria-acellular pertussis or human papillomavirus vaccines) are also available from the US,Citation17,Citation26 ItalyCitation27 and Latin America (Argentina, ColombiaCitation28 and Costa RicaCitation15).
Consistent with observations in our study, in a pre-licensure trial conducted in the United States and Canada in children aged 2–5 years, injection site tenderness/pain (in 28–33% of participants) was the most frequently reported solicited local AE, while irritability (in 16–22% of children) and sleepiness (12–17%) were the most frequent systemic AEs following a MenACWY-CRM vaccination.Citation14 Severe reactions were rarely reported.Citation14 Results from our study were also consistent with previous reports in individuals aged 6 years and above: pain (in 13.3–39%) was the most commonly reported solicited local AEs, while headache, malaise and myalgia were the most frequent systemic AEs for children aged 6–10 years in trials conducted in the US, CanadaCitation14 and Asia.Citation21,Citation22 In adolescents and adults, pain (for ≤54% of participants), headache and myalgia (for ≤41% of participants) were the most common local and systemic AEs, respectively, and very few severe reactions occurred.Citation15,Citation17,Citation20,Citation26–Citation28
Following MenACWY-CRM vaccination in pre-licensure studies, unsolicited AEs were observed in 19%Citation28 to 42%Citation26 of participants aged 2–55 years, including in children aged 2–10 years (up to 26%).Citation14 A lower incidence of unsolicited AEs (in <3.2% of participants) was observed in the current surveillance. Similarly, SAEs were reported in up to 0.7% of participants 2–55 years of age within a 6–12 months follow-up period in previous trials,Citation11 compared to 0.15% within 29-day period post-vaccination in our study. When compared with results from a previous clinical study in adolescents and adults aged 11–55 years in South Korea,Citation20 a lower incidence of solicited local (20.65% versus 28%) and systemic AEs (6.01% versus 28%) was observed in the current surveillance for the same age group. Moreover, a substantially lower incidence of unsolicited AEs (1.26% versus 12%) was reported in participants aged 11–55 years in this post-marketing surveillance compared to the clinical trial conducted in South Korea.Citation20
The current study results are also in line with those from post-licensure surveillance studies in the US, based on data from the Vaccine Adverse Event Reporting System (VAERS) available for a 5-year period in all age groups.Citation29 Most of the US reports emerged from the 11–18 years age group as routine meningococcal vaccination is recommended in adolescents; the most frequently reported AEs were related to local reactions, dizziness, pyrexia and headache. Among the 2614 US reports received following MenACWY-CRM vaccination, 67 were identified as serious, with anaphylaxis and syncope being the most frequent. However, in line with the present Republic of Korean study, VAERS data were aligned with the already known pre-licensure safety data, and no new safety concerns were identified for MenACWY-CRM.Citation29
The observational design of this post-marketing safety surveillance study, including the administration of the vaccine according to field practice – therefore allowing for concomitant vaccination – represents a potential limitation. However, the safety profile of MenACWY-CRM in the routine clinical settings of our study is consistent with other studies conducted in the Asian population,Citation18–Citation20 including one study conducted in 450 Korean adolescents and adults aged 11–55 years.Citation20 In addition, although the study has been carried out in full compliance with the national guidelines on post-marketing safety surveillance, the size of the study may not allow the capture of very rare or uncommon AEs, and there was no control group.
Conclusion
Data from this post-marketing surveillance study in participants aged 2 months to 55 years showed a clinically acceptable tolerability and safety profile of MenACWY-CRM, in line with the evidence generated across its clinical development program.
Abbreviation list
| AE | = | Adverse event |
| IMD | = | Invasive meningococcal disease |
| MAAE | = | Medically-attended adverse event |
| MenACWY-CRM | = | Meningococcal serogroup A, C, W and Y quadrivalent CRM197-conjugate vaccine |
| MFDS | = | Ministry of Food and Drug Safety |
| SAE | = | Serious adverse event |
| US | = | United States |
| VAERS | = | Vaccine Adverse Event Reporting System. |
Data sharing statement
The results summary for this study (GSK study number 205341 – NCT01766206) is available on the GSK Clinical Study Register and can be accessed at www.gsk-clinicalstudyregister.com. For interventional studies that evaluate our medicines, anonymized patient-level data will be made available to independent researchers, subject to review by an independent panel, at www.clinicalstudydatarequest.com within six months of publication. To protect the privacy of patients and individuals involved in our studies, GSK does not publicly disclose patient-level data.
Disclosure of potential conflicts of interest
CC, LM, SJK, CB, MP and YM are employees of the GSK group of companies. MP reports holding shares in the GSK group of companies. BWY, HLJ, YSB, DKH and NYJ have nothing to disclose.
Trademark
Menveo is a trademark owned by the GSK group of companies.
Supplemental Material
Download Zip (37.9 KB)Acknowledgments
The authors thank the study participants, the entire study staff and Dokyuing Lee, Sunil Swamy, Venere Basile, Puneet Vir Singh (GSK), for their contribution to the study. The authors are grateful to all study investigators, including Joon Sup Yeom (Konkuk University Hospital,Seoul), Sang Keun Hahm, Ki Wook Yun and Soo Hyun Cho (Kyunghee University Hospital, Seoul), Jacob Lee (Hallym University Kangnam Sacred Heart Hospital, Seoul), Yoon Seon Park (National Health Insurance Coporation Ilsan Hospital, Gyeonggi-do), Jae Kyoung Park (Seoul Bom Internal Medicine Clinic), Seung Beom Han (The Catholic University of Korea, Seoul St.Mary’s Hospital) and Seong Yeol Choi (Yeonsei Kids Pediatrics Clinic, Seoul). The authors would also like to thank Modis, c/o GSK, for editorial assistance and manuscript coordination (provided by Pauline De Berdt) and medical writing support (provided by Petronela M. Petrar and Sonia Norris).
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echaniz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–28. doi:10.1080/14760584.2017.1258308.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- Meningococcal vaccines. WHO position paper, November 2011. Wkly Epidemiol Rec. 2011;86(47):521–39.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. In: Hamborsky J, Kroger A, Wolfe S, editors. Chapter 14: meningococcal disease. Washington (D.C): Public Health Foundation; 2015. p. 231–246.
- European Centre for Disease Prevention and Control. Invasive meningococcal disease. In: ECDC. Annual epidemiological report for 2015. Stockholm (Sweden): ECDC; 2017. [accessed 2019 Jan 10]. http://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-meningococcal-disease.pdf
- Centers for Disesease Control and Prevention. Enhanced meningococcal disease surveillance report. 2016. [accessed 2019 Jan 14]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf
- Borrow R, Lee JS, Vazquez JA, Enwere G, Taha MK, Kamiya H, Kim HM, Jo DS. Global meningococcal initiative. Meningococcal disease in the Asia-Pacific region: findings and recommendations from the global meningococcal initiative. Vaccine. 2016;34(48):5855–62. doi:10.1016/j.vaccine.2016.10.022.
- Heo JY. Meningococcal disease in Korea: an epidemiologic study of the underestimated infectious disease. Infect Chemother. 2016;48(1):51–53. doi:10.3947/ic.2016.48.1.51.
- Korea Centers for Disease Control and Prevention. Disease surveillance statistics. 2018. [accessed 2018 Aug 8]. http://www.cdc.go.kr/CDC/cms/cmsFileDownload.jsp?fid=9145&cid=123024&fieldName=attach1&index=1
- Lee H, Seo Y, Kim KH, Lee K, Choe KW. Prevalence and serogroup changes of Neisseria meningitidis in South Korea, 2010–2016. Sci Rep. 2018;8(1):5292. doi:10.1038/s41598-018-23365-8.
- Highlights of prescribing information. Menveo. 2018. [accessed 2019 July 31]. http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf
- Abdelnour A, Silas PE, Lamas MR, Aragon CF, Chiu NC, Chiu CH, Acuna TH, Castrejon Tde L, Izu A, Odrljin T, et al. Safety of a quadrivalent meningococcal serogroups A, C, W and Y conjugate vaccine (MenACWY-CRM) administered with routine infant vaccinations: results of an open-label, randomized, phase 3b controlled study in healthy infants. Vaccine. 2014;32(8):965–72. doi:10.1016/j.vaccine.2013.12.034.
- Klein NP, Reisinger KS, Johnston W, Odrljin T, Gill CJ, Bedell L, Dull P. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J. 2012;31(1):64–71. doi:10.1097/INF.0b013e31823dce5c.
- Halperin SA, Gupta A, Jeanfreau R, Klein NP, Reisinger K, Walter E, Bedell L, Gill C, Dull PM. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age. Vaccine. 2010;28(50):7865–72. doi:10.1016/j.vaccine.2010.09.092.
- Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010;28(18):3171–79. doi:10.1016/j.vaccine.2010.02.045.
- Gill CJ, Baxter R, Anemona A, Ciavarro GL, Dull PM. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6(11):881–87. doi:10.4161/hv.6.11.12849.
- Jackson L, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, Dull P. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49(1):e1–e10. doi:10.1086/599117.
- Korea Centers for Disease Control and Prevention. National Immunization Program & International Cooperation 2017. [accessed 2019 Jul 31]. http://www.ghsagenda.org/docs/default-source/default-document-library/seventieth-world-health-assembly/national-immunization-program-international-cooperation—kcdc.pdf
- Keshavan P, Pellegrini M, Vadivelu-Pechai K, Nissen M. An update of clinical experience with the quadrivalent meningococcal ACWY-CRM conjugate vaccine. Expert Rev Vaccines. 2018;17(10):865–80. doi:10.1080/14760584.2018.1521280.
- Lee HJ, Chung MH, Kim WJ, Hong YJ, Choi KM, Lee J, Oh CE, Welsch JA, Kim KH, Hong KB, et al. Immunogenicity and safety of a novel quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) in healthy Korean adolescents and adults. Int J Infect Dis. 2014;28:204–10. doi:10.1016/j.ijid.2014.06.008.
- Huang LM, Chiu NC, Yeh SJ, Bhusal C, Arora AK. Immunogenicity and safety of a single dose of a CRM-conjugated meningococcal ACWY vaccine in children and adolescents aged 2–18 years in Taiwan: results of an open label study. Vaccine. 2014;32(40):5177–84. doi:10.1016/j.vaccine.2014.07.063.
- Lalwani S, Agarkhedkar S, Gogtay N, Palkar S, Agarkhedkar S, Thatte U, Vakil H, Jonnalagedda R, Pedotti P, Hoyle M, et al. Safety and immunogenicity of an investigational meningococcal ACWY conjugate vaccine (MenACWY-CRM) in healthy Indian subjects aged 2 to 75 years. Int J Infect Dis. 2015;38:36–42. doi:10.1016/j.ijid.2015.07.003.
- Klein NP, Shepard J, Bedell L, Odrljin T, Dull P. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine administered concomitantly with measles, mumps, rubella, varicella vaccine in healthy toddlers. Vaccine. 2012;30(26):3929–36. doi:10.1016/j.vaccine.2012.03.080.
- Nolan TM, Nissen MD, Naz A, Shepard J, Bedell L, Hohenboken M, Odrljin T, Dull PM. Immunogenicity and safety of a CRM-conjugated meningococcal ACWY vaccine administered concomitantly with routine vaccines starting at 2 months of age. Hum Vaccin Immunother. 2014;10(2):280–89. doi:10.4161/hv.27051.
- Tregnaghi M, Lopez P, Stamboulian D, Grana G, Odrljin T, Bedell L, Dull PM. Immunogenicity and safety of a quadrivalent meningococcal polysaccharide CRM conjugate vaccine in infants and toddlers. Int J Infect Dis. 2014;26:22–30. doi:10.1016/j.ijid.2014.03.1390.
- Jackson LA, Jacobson RM, Reisinger KS, Anemona A, Danzig LE, Dull PM. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J. 2009;28(2):86–91. doi:10.1097/INF.0b013e31818a0237.
- Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull PM, Ceddia F. Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol. 2010;17(4):537–44. doi:10.1128/CVI.00436-09.
- Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, Karsten A, Dull PM. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM(197) conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis. 2010;14(10):e868–75. doi:10.1016/j.ijid.2010.03.017.
- Myers TR, McNeil MM, Ng CS, Li R, Lewis PW, Cano MV. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010–2015. Vaccine. 2017;35(14):1758–63. doi:10.1016/j.vaccine.2017.02.030.