ABSTRACT
A number of live-attenuated varicella vaccines are produced globally that provide protection against the varicella zoster virus. In Mexico, varicella vaccination is not included in the national immunization program and is recommended for use only in high-risk subgroups. We developed a budget impact model to estimate the impact of universal childhood immunization against varicella on the national payer system in Mexico. A scenario of no varicella vaccination was compared to scenarios with vaccination with a single dose at 13 months of age, in alignment with the existing program of immunization with the measles-mumps-rubella vaccine. Nine different vaccination scenarios were envisioned, differing by vaccine type and by coverage. Varicella cases and treatment costs of each scenario were computed in a dynamic transmission model of varicella epidemiology, calibrated to the population of Mexico. Unit costs were based on Mexico sources or were from the literature. The results indicated that each of the three vaccine types increased vaccine acquisition and administration expenditures but produced overall cost savings in each of the first 10 years of the program, due to fewer cases and reduced varicella treatment costs. A highly effective vaccine at 95% coverage produced the greatest cost savings.
Introduction
Varicella (chickenpox) is an acute, highly contagious infection that is one of the most common infectious diseases in childhood. Globally, varicella incidence estimates range from 2 to 16 cases per 1,000 persons.Citation1–Citation3 The infection is caused by the varicella zoster virus, and is characterized by an itchy, blister-like rash on the skin. Reactivation of the virus from its latent state results in herpes zoster (shingles).
Varicella vaccines protect against varicella virus infection. Several live-attenuated varicella vaccines are produced worldwide, either as a monovalent OKA or MAV/06 strain vaccine, or as a component of quadrivalent vaccines (OKA strain only), given in combination with the measles-mumps-rubella vaccine. Implementation of routine childhood varicella vaccination with moderately or highly effective OKA vaccines has resulted in decreased varicella-related morbidity and mortality in countries with high vaccine coverage.Citation4–Citation6 In the United States varicella-related hospitalizations and ambulatory visits decreased by 88% and 59%, respectively, over the first 7 years of the vaccination program (1995–2002),Citation7 and the mortality rate due to varicella decreased from 410 to 50 per 1,000 population over 12 years of the vaccination program (1995–2007).Citation8 Reductions in morbidity and mortality as a result of vaccination programs were observed across all ages, with the greatest reduction in infants and young children primarily targeted for vaccination.Citation4,Citation5,Citation7,Citation8 Similarly, publicly funded varicella vaccination in Ontario, Canada resulted in decreasing rates of hospitalizations, emergency department use, and office visits.Citation9 The introduction of a universal varicella vaccination in Uruguay resulted in a 73% reduction in the number of cases of varicella from 1.48 cases per 1,000 in 1989–1998 to 0.39 cases per 1,000 in 2000–2012.Citation10 Furthermore, hospitalizations decreased by 81% in children <15 years of age and 94% among children 1–4 years of age while the number of overall outpatient visits decreased by 87%.Citation10
Varicella prevention has not been a priority in Mexico.Citation11 The varicella vaccine is not included in the country’s national immunization program and is recommended for use only in high-risk groups.Citation12 Although most states in Mexico have adopted strategies to increase varicella vaccination and efforts have been made by private health care providers to extend the use of the vaccine, varicella vaccine coverage remains low.Citation11,Citation13 As a result, the burden of varicella in Mexico has remained high.
The cost-effectiveness of varicella vaccination has been analyzed in several studies.Citation14–Citation18 The results suggest that dynamic transmission models are more accurate than static models at capturing the indirect effects of varicella vaccination (e.g., herd immunity, age distribution of varicella cases, incidence of herpes zoster),Citation14,Citation16,Citation17 and, thus, dynamic transmission models have become the gold standard. However, publications on budget-impact models in low- and middle-income countries are sparse, despite their importance for decision making.Citation19 Recently, Brazil and Columbia have required budget-impact analyses alongside cost-effectiveness analyses for evaluation of new vaccines,Citation20 reflecting the increasing importance of budgetary analyses for Latin American countries. The objective of this study was to use a newly developed dynamic transmission model for varicella infection to estimate the economic impact of a one-dose varicella vaccination program in Mexico, under different scenarios of coverage with available varicella vaccines.
Methods
Budget impact model structure
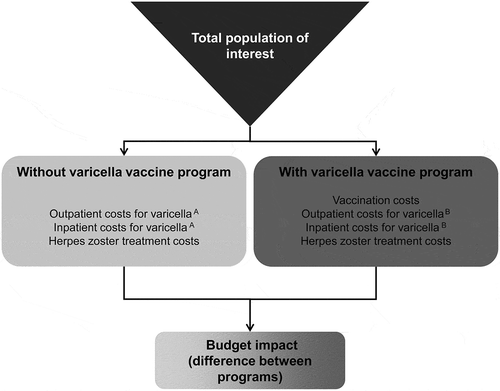
A budget impact model was developed in Microsoft Excel to calculate the impact of universal childhood varicella vaccination on varicella-related treatment costs in Mexico (). A scenario of no varicella vaccination was compared with several different scenarios of universal childhood vaccination, each modeled as a single vaccine dose given at 13 months of age, but differing in vaccine coverage and effectiveness. The budget impact of each vaccination scenario was calculated as the difference in direct medical costs between the vaccination scenario and no vaccination. The time horizon was 10 years, and the cost perspective was that of the national payer system. Annual numbers of varicella cases, deaths, and rates of varicella-related health care resource utilization under the different vaccination scenarios were computed using a dynamic transmission model of varicella infection.
Dynamic transmission model of varicella infection
The model inputs for the budget impact analysis were calculated in a dynamic transmission model for varicella infection that was based on previously reported models.Citation21–Citation23 A full description of the dynamic transmission model is provided elsewhere.Citation24 In brief, the dynamic transmission model has demographic and epidemiologic components. The demographic component describes the age structure of the simulated population, i.e., how persons age, enter and exit the model. It assumes a steady-state age distribution and a constant total population. A set of 45 age groups were defined: two 6-month groups up to 1 year old, 12 1-month groups between 1 and 2 years, 18 1-year groups up to 20 years, 10 5-year groups up to 70 years, and 3 10-year groups to 100 years. The epidemiologic component is a mathematical description of varicella virus transmission and the occurrence of varicella and zoster in the age-structured population. The population is divided into 20 distinct compartments defined according to the host’s susceptibility to infection or the host’s status with respect to infection, immunity, disease, and treatment. Unvaccinated cases are tracked separately from breakthrough cases of varicella because of differences in severity and infectiousness. Infants born to mothers who have had varicella or who have been vaccinated are assumed to benefit from maternal antibodies that last, on average, the first six months of their lives, while all other infants are susceptible from birth. Susceptible persons may acquire latent varicella, which progresses to infectious varicella. After clearing varicella, the host becomes immune to varicella and zoster. The varicella immunity is permanent, but older adults become susceptible to wild-type zoster as cell-mediated immunity wanes. After recovering from herpes zoster, adults are assumed to gain permanent immunity. Of the children that were successfully vaccinated, we assume that a fraction T become immune, and (1-T) are susceptible to breakthrough varicella. Varicella vaccine immunity may also wane over time, leaving vaccinated persons susceptible to breakthrough varicella. The incidence of varicella in the Mexican population in the absence of vaccination, and health care resource model inputs are presented in the Supplemental Material. All computations were performed using Wolfram Research Mathematica, version 10.3.
Population
The budget impact model analyzed the impact of childhood vaccination on the entire population of Mexico, which was 121,005,815 in 2015.Citation25 The age and sex distribution was assumed to remain static over the 10-year period in the model. The age-specific incidence of varicella was modeled to fit the Mexican population for the period 2003–2016, with the assumption that virtually the entire population is eventually infected, consistent with seroprevalence data.Citation26 That is, the epidemiologic model was calibrated to the Mexican population when there was either no varicella vaccination or only high-risk groups were vaccinated. It was assumed that in the absence of vaccination, varicella epidemiology would have remained static over the 10-year period modeled.
Vaccine characteristics
Three vaccine types, characterized as highly, moderately, and weakly effective, were included (). The highly effective vaccine was an OKA strain vaccine with 95% of those vaccinated protected from breakthrough varicella, with average duration of protection of 25 years. Vaccine performance parameters were estimated in a dynamic transmission model,Citation23 fitted to summary clinical trial data.Citation27 The moderately effective vaccine was an OKA strain vaccine with an initial efficacy of 75% and a duration of effect of 5 years. Efficacy parameters were based on those reported in two recent modeling papers.Citation28,Citation29 The weakly effective vaccine was a MAV/06 strain vaccine with an initial efficacy of 50%Citation30,Citation31 and an assumed duration of effect of 1 year.Citation31
Table 1. Vaccine characteristics.
Clinical outcomes and resource utilization
The budget impact model was based on the numbers of varicella cases and rates of varicella-related health care resource use computed by the dynamic transmission model. The following age-specific outputs of the dynamic transmission model were used: number of vaccination doses, number of natural and breakthrough varicella cases, and rates of health care resources used to treat varicella cases (assumed to be the same for natural and breakthrough cases). Health care resources were the numbers of outpatient visits, inpatient days and prescriptions. In addition, the numbers of herpes zoster cases with and without postherpetic neuralgia were included in a scenario analysis.
Costs
Only direct medical costs were considered, as recommended by the ISPOR 2012 Budget Impact Analysis Task Force.Citation32 Unit costs are presented in . The acquisition cost for a unit of a highly effective vaccine was based on the market price, obtained from the Mexican Institute of Social Security (IMSS).Citation33 The unit cost for a moderately effective vaccine was assumed to be 10% less than the cost of a highly effective vaccine, and the cost of a weakly effective vaccine was assumed to be 10% less than the cost of a moderately effective vaccine. An administration cost was included.Citation34
Table 2. Unit costs.
Varicella management costs were the costs for outpatient visits, inpatient visits, and prescription medications. The unit cost for an outpatient visit was obtained from the IMSS 2015 Unit Costs Catalog.Citation35 The cost per day of inpatient care varied by age group and was estimated by dividing the average total costs for a hospitalization stay for varicella by the number of days in the hospital for a specific age group. The total cost of hospitalization was the weighted average of the cost for uncomplicated and complicated cases. The number of days in hospital by age category was based on the opinion of an expert panel (Merck & Co., Inc; unpublished).
The cost of prescription medications to treat varicella was included only for the outpatient setting, as the cost of an inpatient stay included the costs of medications. The unit cost of prescription medications was the average cost of naproxen 250 mg (30 tablets) and diphenhydramine (60 mL), obtained from IMSS. These were assumed to be the most commonly prescribed medications in the outpatient setting, based on opinion of the expert panel (Merck & Co., Inc. unpublished). The unit cost to treat a case of herpes zoster, which was included in an additional scenario analysis,was as reported.Citation36
All costs are presented undiscounted in 2015 Mexican Pesos (MXN).Citation32 Where necessary, costs were converted and/or adjusted to 2015 MXN using a web-based cost converter.Citation37
Model outcomes
The primary outcomes of the budget impact analysis were the total annual cost of varicella treatment, and the component costs of vaccine acquisition, vaccine administration, and inpatient and outpatient treatment of varicella infection. The annual number of cases and disease-related deaths are also presented. Costs and health outcomes were calculated for a no-vaccination scenario compared with 9 different vaccination scenarios i.e., combinations of three varicella vaccine types () and 3 levels of vaccine coverage (10%, 50%, and 95%). The budget impact, for each year over 10 years, was calculated as the difference between the total annual cost for the no-vaccination scenario and the total annual cost for each vaccination scenario.
Results
No vaccination scenario
In the no-vaccination scenario, the model projected a total of 2,010,002 varicella cases and 283 varicella-related deaths per year, with total varicella treatment costs of 4.98 billion ().
Table 3. Health outcome and direct medical costs for 3 vaccination scenarios in year 1A.
Vaccination scenarios in year 1
shows varicella outcomes and costs in Year 1 for 3 vaccination scenarios: highly, moderately, and weakly effective vaccines, each at 95% coverage. The model projected vaccine acquisition costs of 381.6–471.7 million MXN and vaccine administration costs of 27.5–27.6 million MXN. The projected budget impact of vaccination was a reduction in varicella cases of 332,859–417,768, a reduction in varicella deaths of 48–56, and a reduction in varicella treatment costs of 836.4–1,060.5 million MXN. The net budget impact of vaccination at 95% coverage with the highly, moderately, and weakly effective vaccines was a reduction in total annual costs of 561.2, 516.0, and 427.3 million MXN, respectively ().
Vaccination scenarios over 10 years
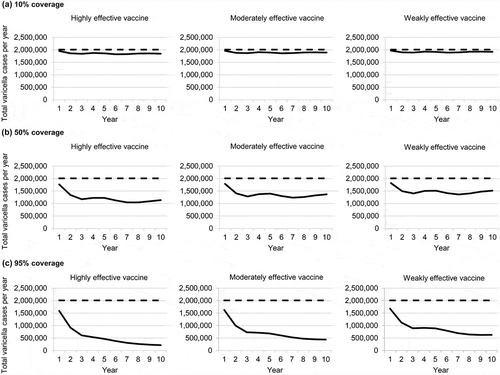
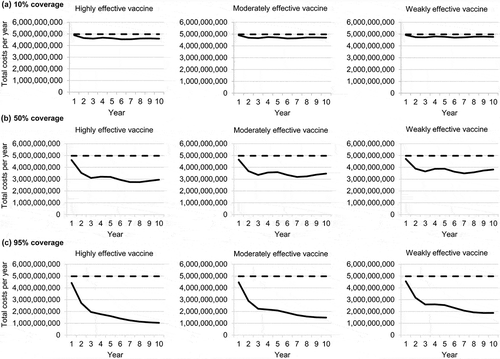
The annual number of varicella cases over 10 years under vaccination scenarios differing in vaccine coverage and effectiveness are compared with the no-vaccination scenario in . The number of varicella cases decreased under all scenarios over the 10-year time horizon. More cases were prevented with the highly effective vaccine than with the moderately effective or weakly effective vaccines, but vaccine coverage had the greatest impact, with higher coverage leading to more cases avoided. Results for the total annual costs of varicella disease were similar ().
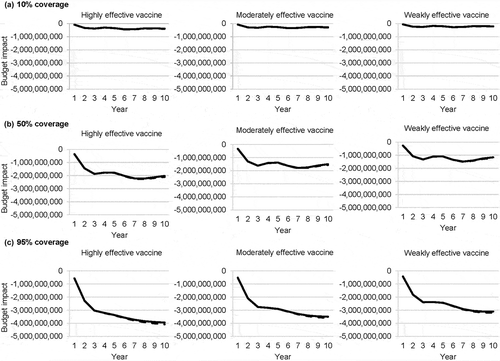
The projected budget impact, shown in , increased dramatically with vaccine coverage. Even with the highest vaccine price, the highly effective vaccine resulted in the greatest cost savings. At Year 10, with 95% coverage of a highly effective vaccine, the projected annual budget impact was approximately −4 billion MXN. Including herpes zoster in the budget impact model resulted in minimal differences in the cost savings ().
Discussion
To our knowledge, this is the first budget impact model for varicella vaccination in a Latin American country to use an underlying dynamic model of varicella transmission. The model projected that increases in vaccine acquisition and administration expenditures were offset by savings in varicella treatment costs, leading to an overall cost saving in each of the first 10 years of the program. However, previous studies have shown that a “bounce-back” in incidence of varicella cases occurs and varies widely by weakly, moderately, and highly effective vaccines.Citation24 More effective vaccines led to greater control of varicella transmission, and subsequently fewer cases and increased cost savings. It is important to choose an optimal strategy that limits bounce-back and therefore reduces future costs.
The budget impact model assumed that the current standard of care was the absence of childhood varicella vaccination. In reality, live-attenuated varicella vaccines are available in Mexico, and varicella vaccination occurs at low levels for at-risk populations.Citation13 No official records of vaccination coverage are available in Mexico, but prior to 2012 an estimated 1–2% of children were vaccinated against varicella.Citation38 Since this analysis showed that the cost savings of 10% coverage was minimal in comparison to the savings of 50% and 95% coverage, it is likely that the impact of the current status quo of vaccination of certain high-risk groups approximates the no-vaccination scenario modeled.
Whereas budget impact analyses of drug therapies typically have time horizons of 1–5 years,Citation39 the time horizon of the varicella vaccination budget impact model was 10 years. Childhood vaccination against varicella may take 5–10 years for the effect on varicella incidence in the general population to approach its maximum.Citation40 The 10-year time horizon includes consideration of a transient increase in herpes zoster cases in older adults, predicted under the exogenous boosting hypothesis to peak 15–35 years after the introduction of varicella vaccination. Analysis of observational data in the United States, however, has found no evidence of such an exogenous boosting effect.Citation41
The model considered only a one-dose vaccination strategy, in order to align with the existing measles-mumps-rubella vaccination schedule in Mexico. A one-dose vaccination schedule exists in several Latin American countries, including Ecuador, Argentina, Paraguay, and Brazil.Citation10 Conversely, Panama, Colombia, and Uruguay have adopted a two-dose schedule, as exists in the United States. A two-dose vaccination strategy could potentially lead to greater control of transmission.Citation24
Some of the assumptions of the budget impact model were arbitrary, including the acquisition cost of the moderately and weakly effective vaccines, and were not subjected to sensitivity analysis. Lower acquisition costs for the moderately and weakly effective vaccines would result in increased overall cost savings. Set at the same level as that of a highly effective vaccines, the acquisition cost of a moderately or weakly effective vaccine would still represent only approximately half of the projected reduction in varicella treatment costs. The assumption of 95% vaccination coverage approximates an optimum scenario. Coverage of infants in the United States with ≥1 dose of varicella vaccine is 90–92%, and we did not investigate coverage levels in this range.Citation42
Per-case resource utilization for breakthrough varicella cases was assumed to be equal to resource use for natural varicella cases, despite evidence of lower severity and potentially reduced medical resource use (e.g., fewer outpatient visits, shorter hospital length of stay). If reduced resource use for breakthrough cases was assumed, cost savings would be greater. The budget impact model did not include vaccine-specific adverse reactions and their management. However, adverse reactions to varicella vaccine are minimal, without a significant difference between vaccine types, and would have a minimal impact on cost savings.
The budget impact model inherited some of the limitations of the dynamic transmission model used to simulate varicella incidence. The dynamic transmission model assumed a constant population size and age distribution, whereas the population structure is expected to change over time. In addition, the age-group-mixing matrix, which determines the transmission rates between people by age group, assumed proportionate mixing, which may overrepresent contacts in some age groups.Citation43,Citation44 The force of infection was assumed to remain constant, which may not accurately reflect the influence of the vaccine on real-world populations.
In conclusion, a one-dose vaccination program for the prevention of varicella in Mexico is predicted to reduce direct medical costs and result in cost saving in each year of the first 10 years of the program. Further, more effective vaccines led to greater control of disease transmission, fewer cases, and lower total costs, despite the higher vaccine costs compared with sub-optimal vaccines. It is possible that a bounce-back in the incidence of varicella cases could occur, and, thus, it is important to choose the optimal vaccine to minimize the economic and health impact in Mexico.
Disclosure of potential conflicts of interest
Jonathan Graham and Sandra Talbird (and/or their institutions) received research funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA to develop the budget-impact estimates and for other research studies. Lara J. Wolfson, Vincent J. Daniels, and Matthew Pillsbury are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Jeffrey Kyle is an employee of Atlas, contracted by Merck & Co., Inc. Diana Guarneros-DeRegil and Carlos Perez Bolde-Villarreal are employees of MSD Mexico. Homero Monsanto is an employee of MSD (IA) LLC. All MSD employees may own stock and or/hold stock options in the company.
Supplemental Material
Download MS Word (147 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Bardach A, Cafferata ML, Klein K, Cormick G, Gibbons L, Ruvinsky S. Incidence and use of resources for chickenpox and herpes zoster in Latin America and the Caribbean–a systematic review and meta-analysis. Pediatr Infect Dis J. 2012 Dec;31(12):1263–68. doi:10.1097/INF.0b013e31826ff3a5.
- Heininger U, Seward JF. Varicella. Lancet. 2006 Oct 14;368(9544):1365–76. doi:10.1016/S0140-6736(06)69561-5.
- Sadzot-Delvaux C, Rentier B, Wutzler P, Asano Y, Suga S, Yoshikawa T, Plotkin SA. Varicella vaccination in Japan, South Korea, and Europe. J Infect Dis. 2008 Mar 1;197(Suppl 2):S185–190. doi:10.1086/522163.
- Davis MM, Patel MS, Gebremariam A. Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics. 2004 Sep;114(3):786–92. doi:10.1542/peds.2004-0012.
- Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med. 2005 Feb 3;352(5):450–58. doi:10.1056/NEJMoa042271.
- Lopez AS, Zhang J, Brown C, Bialek S. Varicella-related hospitalizations in the United States, 2000–2006: the 1-dose varicella vaccination era. Pediatrics. 2011 Feb;127(2):238–45. doi:10.1542/peds.2010-0962.
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. JAMA. 2005 Aug 17;294(7):797–802. doi:10.1001/jama.294.7.797.
- Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics. 2011 Aug;128(2):214–20. doi:10.1542/peds.2010-3385.
- Kwong JC, Tanuseputro P, Zagorski B, Moineddin R, Chan KJ. Impact of varicella vaccination on health care outcomes in Ontario, Canada: effect of a publicly funded program? Vaccine. 2008 Nov 5;26(47):6006–12.
- Avila-Aguero ML, Beltran S, Castillo JBD, Castillo Díaz ME, Chaparro LE, Deseda C, Debbag R, Espinal C, Falleiros-Arlant LH, González Mata AJ, et al. Varicella epidemiology in Latin America and the Caribbean. Expert Rev Vaccines. 2018 Feb;17(2):175–83. doi:10.1080/14760584.2018.1418327.
- Cabrera GDA, Muños MW, Gómez ACM. Comportamiento epidemiológico de la varicela en Mexico: 18 años de estudios y estimaciones para los proximos cinco años. Revista de Enfermedades Infecciosas en Pediatría. 2009;22:77–82.
- Centers for Disease Control & Prevention. Binational immunization resource tool for children from birth through 18 years. 2018 Jan [accessed 2018 Aug 1]. https://www.cdc.gov/vaccines/schedules/downloads/child/binational-schedule-pr.pdf.
- SLIPE. Prevención de Varicela en América Latina y el Caribe. Varicella Task Force – Documento de Posición de la SLIPE. [accessed 2018 Aug 1]. https://www.the-ahf.org/sites/default/files/FINAL%20-%20Varicella%20Position%20Paper%20June%2020%202016.pdf.
- Thiry N, Beutels P, Van Damme P, Van Doorslaer E. Economic evaluations of varicella vaccination programmes: a review of the literature. Pharmacoeconomics. 2003;21(1):13–38. doi:10.2165/00019053-200321010-00002.
- Hammerschmidt T, Bisanz H, Wutzler P. Universal mass vaccination against varicella in Germany using an MMRV combination vaccine with a two-dose schedule: an economic analysis. Vaccine. 2007 Oct 16;25(42):7307–12. doi:10.1016/j.vaccine.2007.08.017.
- Rozenbaum MH, van Hoek AJ, Vegter S, Postma MJ. Cost-effectiveness of varicella vaccination programs: an update of the literature. Expert Rev Vaccines. 2008 Aug;7(6):753–82. doi:10.1586/14760584.7.6.753.
- Soarez PC, Novaes HM, Sartori AM. Impact of methodology on the results of economic evaluations of varicella vaccination programs: is it important for decision-making? Cad Saude Publica. 2009;25(Suppl 3):S401–414. doi:10.1590/s0102-311x2009001500006.
- Unim B, Saulle R, Boccalini S, Taddei C, Ceccherini V, Boccia A, Bonanni P, La Torre G. Economic evaluation of varicella vaccination: results of a systematic review. Hum Vaccin Immunother. 2013 Sep;9(9):1932–42. doi:10.4161/hv.25228.
- Carvalho N, Jit M, Cox S, Yoong J, Hutubessy RCW. Capturing budget impact considerations within economic evaluations: a systematic review of economic evaluations of rotavirus vaccine in low- and middle-income countries and a proposed assessment framework. Pharmacoeconomics. 2018 Jan;36(1):79–90. doi:10.1007/s40273-017-0569-2.
- Loze PM, Nasciben LB, Sartori AMC, Itria A, Novaes HMD, de Soarez PC. Vaccines are different: a systematic review of budget impact analyses of vaccines. Vaccine. 2017 May 15;35(21):2781–93. doi:10.1016/j.vaccine.2017.03.088.
- Schuette MC, Hethcote HW. Modeling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull Math Biol. 1999 Nov;61(6):1031–64.
- Brisson M, Melkonyan G, Drolet M, De Serres G, Thibeault R, De Wals P. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine. 2010 Apr 26;28(19):3385–97. doi:10.1016/j.vaccine.2010.02.079.
- van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine. 2011 Mar 16;29(13):2411–20. doi:10.1016/j.vaccine.2011.01.037.
- Wolfson LJ, Daniels VJ, Pillsbury M, Kurugöl Z, Yardimci C, Kyle J, Dinleyici EC, Verjans GMGM. Cost-effectiveness analysis of universal varicella vaccination in Turkey using a dynamic transmission model. PLoS One. 2019;14(8):e0220921. doi:10.1371/journal.pone.0220921.
- Documento metodológico: proyecciones de la población de México 2010–2050. México: Consejo Nacional de Población; 2012. https://www.gob.mx/cms/uploads/attachment/file/63977/Documento_Metodologico_Proyecciones_Mexico_2010_2050.pdf.
- Conde-Glez C, Lazcano-Ponce E, Rojas R, DeAntonio R, Romano-Mazzotti L, Cervantes Y, Ortega-Barria E. Seroprevalences of varicella-zoster virus, herpes simplex virus and cytomegalovirus in a cross-sectional study in Mexico. Vaccine. 2013 Oct 17;31(44):5067–74. doi:10.1016/j.vaccine.2013.08.077.
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, Kim LL, Lupinacci L, Hartzel J, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004 Feb;23(2):132–37. doi:10.1097/01.inf.0000109287.97518.67.
- Ouwens MJ, Littlewood KJ, Sauboin C, Téhard B, Denis F, Boëlle P-Y, Alain S. The impact of 2-dose routine measles, mumps, rubella, and varicella vaccination in France on the epidemiology of varicella and zoster using a dynamic model with an empirical contact matrix. Clin Ther. 2015 Apr 1;37(4):816–829 e810. doi:10.1016/j.clinthera.2014.12.017.
- Holl K, Sauboin C, Amodio E, Bonanni P, Gabutti G. Coverage, efficacy or dosing interval: which factor predominantly influences the impact of routine childhood vaccination for the prevention of varicella? A model-based study for Italy. BMC Public Health. 2016 Oct 21;16(1):1103. doi:10.1186/s12889-016-3738-x.
- Oh SH, Choi EH, Shin SH, Kim Y-K, Chang JK, Choi KM, Hur JK, Kim K-H, Kim JY, Chung EH, et al. Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol. 2014 May;21(5):762–68. doi:10.1128/CVI.00645-13.
- Lee YH, Choe YJ, Cho SI, Kang CR, Bang JH, Oh MD, Lee JK. Effectiveness of varicella vaccination program in preventing laboratory-confirmed cases in children in Seoul, Korea. J Korean Med Sci. 2016 Dec;31(12):1897–901. doi:10.3346/jkms.2016.31.12.1897.
- Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, Orlewska E, Penna P, Rodriguez Barrios J-M, Shau W-Y. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Health. 2014 Jan–Feb;17(1):5–14. doi:10.1016/j.jval.2013.08.2291.
- Instituto Mexicano Del Seguro Social. IMSS comprό. [accessed 2017 July 21]. http://compras.imss.gob.mx/.
- Portnoy A, Ozawa S, Grewal S, Norman BA, Rajgopal J, Gorham KM, Haidari LA, Brown ST, Lee BY. Costs of vaccine programs across 94 low- and middle-income countries. Vaccine. 2015 May 7;33(Suppl 1):A99–108. doi:10.1016/j.vaccine.2014.12.037.
- Secretaria de gobernacio: Diario Oficial De La Federación. Costos Unitarios por Nivel de Atención Médica. [accessed 2017 July 21.]. http://www.dof.gob.mx/nota_detalle.php?codigo=5476988&fecha=21/03/2017.
- Rampakakis E, Pollock C, Vujacich C, Toniolo Neto J, Ortiz Covarrubias A, Monsanto H, Johnson KD. Economic burden of herpes zoster (“Culebrilla”) in Latin America. Int J Infect Dis. 2017 May;58:22–26. doi:10.1016/j.ijid.2017.02.021.
- CCEMG - EPPI-Centre. CCEMG - EPPI-Centre Cost Converter. 2016 Apr 29 [accessed 2018 May 15]. https://eppi.ioe.ac.uk/costconversion/.
- Vergara-Castaneda A, Escobar-Gutierrez A, Ruiz-Tovar K, Sotelo J, Ordoñez G, Cruz-Rivera MY, Fonseca-Coronado S, Martinez-Guarneros A, Carpio-Pedroza JC, Vaughan G. Epidemiology of varicella in Mexico. J Clin Virol. 2012 Sep;55(1):51–57. doi:10.1016/j.jcv.2012.06.004.
- Mauskopf J, Earnshaw S. A methodological review of US budget-impact models for new drugs. Pharmacoeconomics. 2016 Nov;34(11):1111–31. doi:10.1007/s40273-016-0426-8.
- Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2016 Sep 2;65(34):902–05. doi:10.15585/mmwr.mm6534a4.
- Wolfson LJ, Daniels VJ, Altland A, Black W, Huang W, Ou W. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: updated evidence from observational databases, 1991–2016. Clin Infect Dis. 2019 Apr 24. doi:10.1093/cid/ciz305.
- Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kang Y. Vaccination coverage among children aged 19–35 months - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017 Nov 3;66(43):1171–77. doi:10.15585/mmwr.mm6643a3.
- Hyman JM. Disease transmission models with biased partnership selection. Appl Numer Math. 1997;24(2–3):379–92. doi:10.1016/S0168-9274(97)00034-2.
- Kiss IZ, Green DM, Kao RR. The effect of network mixing patterns on epidemic dynamics and the efficacy of disease contact tracing. J R Soc Interface. 2008 Jul 6;5(24):791–99. doi:10.1098/rsif.2007.1272.
- Secretaría de Salud. Sistema Nacional de Vigilancia Epidemiologíca- Epidemiología. [accessed 2019 Feb 14]. https://www.gob.mx/salud/acciones-y-programas/direccion-general-de-epidemiologia-boletin-epidemiologico.
- Secretaría de Salud. Dirección General de Epidemiología Anuario de Morbilidad 1984–2017. [accessed 2017 Sep 1]. http://www.epidemiologia.salud.gob.mx/anuario/html/anuarios.html.
- Monsanto H, Cashat M, Kyle J, Perez-Bolde C, Pillsbury M, Weiss TJ, Wolfson LJ. The implications of vaccine characteristics and private sector vaccination on varicella: a model-based analysis for Mexico. Paper presented at: ISPOR Latin America; 2017; Sao Paolo, Brazil. doi:10.1016/j.jval.2017.08.3011.
- Bernstein HH, Rothstein EP, Watson BM, Reisinger KS, Blatter MM, Wellman CO, Chartrand SA, Cho I, Ngai A, White CJ. Clinical survey of natural varicella compared with breakthrough varicella after immunization with live attenuated Oka/Merck varicella vaccine. Pediatrics. 1993 Dec;92(6):833–37.