ABSTRACT
This report describes efforts to understand the immune mechanism of action that led to a complete response in a patient with progressive, refractory, metastatic melanoma after treatment with a therapeutic vaccine consisting of autologous dendritic cells (DC) loaded with autologous tumor antigens (ATA) derived from cells that were self-renewing in cell culture. Her histocompatibility type proved to be HLA B27 with extensive mutations in the HLA-A locus. Exomic analysis of proliferating tumor cells revealed more than 2800 non-synonymous mutations compared to her leukocytes. Histology of resected tumor lesions showed no evidence of an existing or suppressed immune response. In in vitro mixed cell cultures, DC loaded with ATA induced increased IL-22 expression, and a four-fold increase in CD8 + T lymphocytes. Cryopreserved blood samples obtained at week-0, 1 week before the first of three-weekly vaccine injections, and at week-4, 1 week after the third dose, were analyzed by protein array and compared for 110 different serum markers. At baseline, she had marked elevations of amyloid A, IL-12p40, IL21, IL-22, IL-10, IL-16, GROa, TNF-alpha, IL-3, and IL-2, and a lesser elevation of IL-15. One week after 3 weekly vaccinations she had a further 80% increase in amyloid A, a further 66% increase in IL-22, a 92% decrease in IL12p40, a 45% decrease in TGF-β and 26% decrease in IL-10. The data suggested that by 3 weeks after the first DCV injection, vaccine-induced changes in this particular patient were most consistent with enhanced innate and Th1 immune responses rather than Th2 or Th17.
Introduction
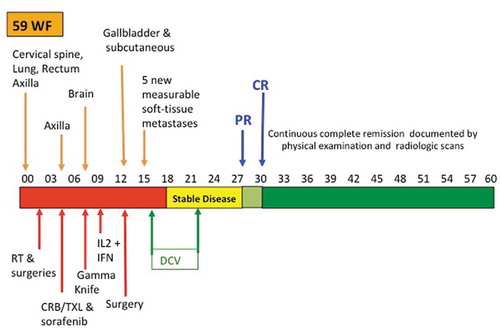
As previously reported, a 59-year-old woman with recurrent, refractory, metastatic melanoma experienced a delayed complete response after treatment with her patient-specific dendritic cell vaccine (DCV), which consisted of autologous dendritic cells that had been incubated with tumor cells from a short-term autologous tumor cell line, with each dose admixed in granulocyte-macrophage colony-stimulating factor shortly before each injection.Citation1 This complete response was still ongoing 5 years after initiating the eight DCV injections that were administered at weeks 1, 2, 3, 8, 12, 16, 20 and 24.Citation2 Of eight patients with measurable metastatic melanoma when DCV therapy was initiated in this trial, she was the only patient with a delayed but durable objective complete regression of all cancer lesions. The best response of the other seven patients was stable disease, and two of the seven survived beyond 4 years. In an effort to better understand the basis of this complete, durable response, we performed ancillary laboratory studies on her tumor cells, dendritic cells (DC), peripheral blood mononuclear cells (PBMC) and serum samples.
The clinical course of this patient is summarized in . She had come to medical attention because of neurologic symptoms due to epidural cervical spine metastases. The diagnosis of metastatic melanoma from unknown primary was made during a decompression laminectomy during which tumor was excised.Citation1 As shown in , during the following year she underwent multiple surgeries, external beam irradiation, chemotherapy, treatment with an anti-RAS-vascular endothelial growth factor signal transduction inhibitor, gamma knife irradiation for brain metastases, and immunotherapy with interleukin-2 (IL-2) and alpha interferon. She had rapid disease recurrence and progression after each treatment. During the year after diagnosis, she had metastases to the axilla, brain, bowel, gallbladder, lung, subcutaneous and other soft tissue sites. Fifteen months after original diagnosis, and 2 months after surgery for gallbladder metastases, she enrolled in an open-label, randomized phase II clinical trial (NCT00436930).Citation2,Citation3 She was stratified as having measurable distant metastatic disease per RECIST based on five new rapidly growing, measurable, soft-tissue metastases, and was randomized to the DCV arm. She received three weekly s.c. injections of cryopreserved DCV that were thawed and admixed in 500 µg of granulocyte-macrophage colony-stimulating factor (GM-CSF), followed by monthly injections at weeks 8, 12, 16, 20, and 24. She received no other treatment just before or after starting DCV. Her lesions initially stabilized, eventually decreased by more than 50% one-year after starting treatment, and were completely gone three months later. At the time of original publication, she had been in continuous remission for over 2 years.Citation1 She received no additional cancer treatment, but remained progression-free 5 years after starting treatment.Citation2
Methods
Patient samples
PBMC were obtained via leukapheresis and enriched into lymphocyte and monocyte fractions using the Elutra® Cell Separation System (CaridianBCT, Lakewood, Colorado). Cells were cryopreserved at −80°C until analyzed. Samples of proliferating tumor cells were cryopreserved from the short-term cell cultures used in manufacturing her patient-specific vaccine. Cell lines were established from an axillary metastasis resected 4 months after surgical resection of her cervical spine metastasis, and a chest wall soft tissue metastasis excised 1 year after her original surgery. Clotted blood for serum and heparinized blood samples were obtained at week-0 (baseline), just before the first vaccine injection, and at week-4, 1 week after the third weekly DCV injection.
Histologic assessment of tumor
Tissue blocks and slides stained with hematoxylin and eosin were available from the original spine surgery and gallbladder surgery. These were reviewed by a board-certified pathologist who also sent tissue to an outside reference laboratory for monoclonal antibody detection of programmed death ligand-1 (PD-L1) and various T cell subset markers. There was no lymphocyte infiltration of the initial tumor and no expression of PDL-1. In most areas of the gallbladder metastasis, which had arisen after treatment with IL-2, there was no infiltration of lymphocytes. However, one small area showed some infiltration of T lymphocytes that proved to be predominantly CD8+ cells with no regulatory T cells based on expression of CD4+, CD25+, and FoxP3+ . PD-L1 expression was again low, overall, but in one area 2% of cells stained positively for PD-L-1.
Genomic analysis and HLA typing
Tumor cells from her two short-term melanoma cell lines and autologous lymphocytes were analyzed for HLA type and tumor-specific mutations by whole exomic sequencing analyses. Exomic regions were captured in solution using the Agilent SureSelect 50 Mb kit according to the manufacturer’s instructions (Agilent, Santa Clara, CA). Paired-end sequencing, resulting in 100 bases from each end of each fragment, was performed using a HiSeq 2500 Genome Analyzer (Illumina, San Diego, CA). Sequence data were mapped to the reference human genome sequence, and sequence alterations were determined by comparison of over 50 million bases of tumor and her normal lymphocyte DNA. Over 100x depth of coverage was obtained for each sample; a high fraction of the bases were from the captured coding regions. Raw sequence tags were aligned to the human genome reference sequence (hg19) using the Burrows–Wheeler algorithm. Custom in-house python scripts were used to annotate each single nucleotide polymorphism (SNP) to the corresponding gene functional units in RefGene database, including nucleotide and amino acid changes. SNP validation and comparison (with dbSNP database, 1000 Genomes Project database, publicly available exome databases (ESP), ENCODE, ClinVar, GWAS, PVFD, BGI-GaP, and YH) were then performed. Similarly, InDel calling and annotation (annotate each InDel to the corresponding gene functional units in RefGene database, including nucleotide and amino acid changes, etc.) were performed. Known polymorphisms recorded in dbSNP were removed from the analysis. Potential somatic mutations were filtered and visually inspected using the University of California Santa Cruz genome browser (Systomic Health LLC, Los Angeles, CA.)
To predict neo-antigens from this set of mutations, the affinities of the non-synonymous mutations were analyzed for the patient’s HLA system. Neo-antigenicity score was calculated by integrating the mutation sequence, patient variants, neo-affinity values, databases and models of immune response for all MHC Class I and Class II data points. Neo-affinity scores were based on HLA binding affinity IC 50 < 150 nM (15,16). Additionally, the cellular localization, membrane or intracellular of neo-antigens in her melanoma cells were predicted.
Serum markers
Blood samples were obtained at baseline and at week-4 (3 weeks after the first injection) because that was felt to be sufficient time to demonstrate evidence of antibody and cell-mediated immunity in response to DCV. Cryopreserved serum samples (200 µl) from week-0 and week-4 were analyzed by Raybiotech, Inc. (Norcross, GA) for human cytokine protein array for 110 different proteins (Quantibody®), using a validated, quantitative, multiplex enzyme-linked immunosorbent assay (ELISA). This included multiple cytokines, growth factors, proteases, soluble receptors, and other proteins that are associated with immune response, inflammation, and angiogenesis as previously detailed.Citation2 The baseline values were characterized as normal or above normal based on comparison to the mean values obtained from three healthy individuals. The percent change was then calculated based on the values obtained at week-4 compared to week-0, either as a percent-decrease relative to values that were elevated at baseline, or percent-increase compared to the values that were normal at baseline. Changes of greater than 20% increase or 20% decrease were considered noteworthy.
Enzyme-linked immunospot assays (ELISPOT)
DC production of IL-12 and IL-23 was measured by enzyme-linked immunospot assays (ELISPOTs). Autologous monocytes were cultured with GM-CSF (5 μg/ml) and IL-4 (100 ng/ml) in AIMV® media (Thermo Fisher Scientific, Waltham, MA) for 5 days at 37°C and 5% CO2 to generate immature DCs. The irradiated autologous tumor cells (TCs) were added to the immature DC cultures at 1:3 (TCs:DCs) ratio on day-6 and incubated at 37°C and 5% CO2 for three additional days before loading on the ELISPOT plates. Three different ELISPOT assays were performed on her tumor cell/antigen-primed DCV sample with IL-12 p70 ELISPOT kit (BD Biosciences, San Jose, CA), IL-12/23 p40 ELISPOT kit (R&D Systems Inc., Minneapolis, MN), and IL-12 p19 development module (R&D Systems Inc., Minneapolis, MN). Assays were performed per manufacturer protocols with 200,000 cells seeded in each well on the 96-well ELISPOT plates and incubated for 48 h prior to developing the plates to detect immunospots. Additional test conditions included wells containing no cells, immature DCs, mature DCs treated with IFNγ and lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO), DCV co-cultured with autologous PBMC, and DCV co-cultured with autologous PBMCs in the presence of the costimulatory molecule CD40L (500 ng/ml, Thermo Fisher Scientific, Waltham, MA) and anti-human CD28/CD49d antibodies (500 ng/ml, BD Biosciences, San Jose, CA). Each individual experimental group had six replicates on the ELISPOT plates. The immunospots on the ELISPOT plates were imaged and counted by Cellular Technology Limited (C.T.L.) scanning and analysis service and reported as average number of spots per assay well.
Mixed leukocyte reactions
Autologous mixed leukocyte reactions (MLRs) were performed to assay T-cell responses to DC that had been antigen-loaded by phagocytosis during co-culture with irradiated tumor cells (TC). Autologous PBMC were co-cultured with autologous TC or autologous DCV at PBMC:DCV and PBMC:TC ratios of 3:1 (6 million PBMC to 2 million antigen-loaded DC, or 6 million PBMC to 2 million TC) on a 6-well ultra-low attachment plate. The co-cultures were incubated at 37°C and 5% CO2 for 6 days. On day-6, the PBMCs were re-challenged with another 2 million TC or 2 million antigen-loaded DC, respectively. The co-cultures were analyzed by flow cytometry on day 12. Control MLR groups included PBMC alone, non-antigen-loaded DC, PBMCs treated with phorbol-12-myristate-13-acetate (PMA, 50 ng/ml, Sigma-Aldrich, St. Louis, MO) and ionomycin (1 μg/ml, Sigma-Aldrich, St. Louis, MO), PBMCs co-cultured with antigen-loaded DC in the presence of costimulatory molecule CD40L (500 ng/ml, Thermo Fisher Scientific, Waltham, MA) and anti-human CD28/CD49d antibodies (500 ng/ml, BD Biosciences, San Jose, CA), and PBMCs co-cultured with antigen-loaded DC in the presence of anti-IL-12 antibody (LEAF, 100 μg/ml, BioLegend, San Diego, CA).
Flow cytometry and antibodies
T-cell responses in the MLR were characterized using flow cytometry. Cells were stained with anti-human CD4-PerCP antibody (R&D Systems Inc., Minneapolis, MN), anti-Human CD8-Alexa Fluor 488 antibody (Biolegend, San Diego, CA). The flow cytometry data were acquired by FACS Calibur (BD Biosciences, San Jose, CA) and analyzed with FlowJo 8.8.6 software (FlowJo, LLC. Ashland, OR). ELISPOT and flow cytometry data were expressed as the mean ± SEM.
Results
Histologic assessment of tumor
There was no lymphocyte infiltration of the initial paraspinal tumor lesion and no expression of PDL-1. In most areas of the gallbladder metastasis, which had arisen after treatment with IL-2, there was no infiltration of lymphocytes. However, one small area showed some infiltration of T lymphocytes that proved to be predominantly CD8+ cells with no regulatory T cells based on expression of CD4+, CD25+, and FoxP3+. PDL-1 expression was again low, overall, but in one area 2% of cells stained positively for PDL-1.
HLA-typing and exomic analyses
Her HLA-type was HLA-B*27, and her cancer cells contained mutations in the HLA-A locus of allele 1 that were not present in her PBMC []. HLA-B*27 is a class I surface antigen encoded on chromosome 6 by the B locus of the major histocompatibility complex that is associated with a variety of autoimmune disorders including, psoriatic arthritis, reactive arthritis (Reiter’s syndrome), inflammatory bowel disease, ulcerative colitis, uveitis, iritis, and especially ankylosing spondylitis.Citation4
Table 1. Results of HLA-typing and for patient’s normal and malignant cells.a
summarizes the types of mutations for each cell line. They contained 3968 and 4376 total variants, respectively; thus, there were 408 (10.3%) more mutations in the sample obtained eight months later after intervening chemotherapy. Of the total 4944 different mutations, 3,324 (66.6%) were present in both tumor cell samples. The proportions of mutations unique to each sample were 631/3968 (15.9%) and 1037/4376 (23.7%). The vast majority of mutations were missense variants, and the proportion that were non-synonymous was slightly higher in the second sample. About one-third of the variants were synonymous, that is, they were also contained in her lymphocytes, and two-thirds, or about 2800 variants were non-synonymous. Exomic analysis revealed a probability of 69 neoantigens at B*2705 with 13 peptides having high-affinity scores (<50 nM) suggesting they could be neoantigens that her immune system could recognize. However, her tumor cells also contained mutations in the HLA-A locus, A*01:22 N,A*01:107, which could have impaired the ability for her CTL recognize certain foreign antigens in the context of her tumor cells.
Table 2. Summary of genomic analysis for the short-term cell lines.
shows the sites of mutations, which were the same for both cell lines. As would be expected, the mutations affected pathways involved in the regulation of transcription, tumor suppression, cell proliferation, angiogenesis, and apoptosis. She did not have BRAF mutations, nor did she have mutations in P53. The mutation in KITLG suggests that she possibly could have gotten some benefit from anti-CD117 therapy with a product such as imatinib.Citation5
Table 3. Mutated sites by numbers of mutations present in cells from autologous tumor cell line.
Serum markers
shows the relationship of the patient’s serum marker levels to the average of the three controls, at week-0 and week 4, and the percent changes in serum markers between week-0 and week-4. Relative to control values, some of the patient’s serum levels were markedly elevated, some were markedly depressed and some were similar. There was also a great variation in the percent changes of levels between week-0 and week-4 with some markedly increased, some markedly decreased, and some with little or no changes.
Table 4. Serum marker levels in relation to normal control values at week-0 baseline week-0 (1 week before first vaccine injection) and week-4 (1 week after third weekly vaccine injection) and percent change.
ranks the individual markers for those whose levels were more than twice that of controls at the week-0 baseline, for those whose levels were less than one-third of controls at the week-0 baseline, and by the greatest percentage increases or decreases between week-0 and week-4 after the first three DCV injections. Because of the wide variation in values, arbitrarily only those with a greater than 20% change were included. In addition to the markers shown in and , serum levels of immunoglobulins and vascular endothelial growth factors were also measured. VEGF, VEGF-C, VEGF-D and VEGFR1, 2 and 3, were all substantially elevated at baseline. All but VEGFR1 were decreased at week-4, especially VEGFR3, which was 3.17 times higher than control at baseline, but decreased by 74% after three DCV injections into the normal range.
Table 5. Rankings of serum markers by baseline levels relative to control values, and greatest percent changes after three vaccine injections.
At baseline, this patient had no detectable IgE, low IgA and IgD, normal IgM, low IgG2, but IgG1, IgG3, and IgG4 at baseline were 8.62, 2.20 and 2.37 times the control value. After three injections they were, respectively, 6.59, 1.25, and 2.52 times the control value. IgG1 usually accounts for about 67% of immunoglobulins in humans and IgG2 makes up about 25%. IgG1 and IgG3 subtypes generally are associated with more effective antibody-dependent cell-mediated toxicity because of their Fc receptors. Both have half-lives of about 3 weeks. GM-CSF levels were in the normal range and were unchanged 4 weeks later despite the injections of 500 μg GM-CSF with each weekly vaccination, which is probably a reflection of its short half-life.
Markers elevated at baseline
The marker that was most elevated relative to control was serum amyloid A (SAA), an acute phase reactant synthesized by hepatocytes, that rises up to 1000-fold in an acute inflammatory state, and remains elevated during chronic inflammatory states.Citation6 The next highest at baseline was IL-12p40, a component of both IL-12 (IL-12p70) and IL-23 that is a chemo-attractant for macrophages that promotes the migration of antigen-loaded DC.Citation7 IL-12p40 combines with IL-12p35 to produce IL-12p70, also known as T-cell stimulating factor, which is a crucial cytokine produced by DC as well as macrophages and neutrophils. DC-derived IL-12p70 stimulates IFN-γ in CD4 + T cells, which promotes a Th1 response. It also provides a negative feedback loop by competitively binding to the IL12 receptor. Both the IL-12p40 monomer and its homodimer construct can competitively inhibit binding of IL-12p70 to its receptor, which can be immunosuppressive.Citation8,Citation9
IL-21 and IL-22 were both elevated more than 14-fold at baseline compared to controls. IL-21 is a Th1 cytokine expressed by Th2, Th17, and NKT cells, that enhances the anti-tumor effects of NK cells of the innate immune response, and CTL that result from a Th1 response.Citation10,Citation11 IL-22, which was originally named IL-10-related T cell-derived inducible factor (IL-TIF), is a member of the IL-10 superfamily that is produced by Th1, Th17, and NKT cells; it acts on epithelial and stromal cells, but not hematopoietic cells.Citation12–Citation15 Monocyte chemotactic protein-3 (MCP-3), also known as CCL7, is produced by macrophages and some tumor cells; it attracts DC, NK cells, T cells, and eosinophils.Citation16 IL-10 is an anti-inflammatory cytokine produced primarily by macrophages, Th2 lymphocytes, and regulatory T cells, that suppresses Th1 responses.Citation17 However, prolonged IL-10 blocking of IL-10 receptors has positive anti-cancer immune effects.Citation18
Growth-regulated alpha protein (GROa), originally described as melanoma growth stimulatory factor and also known as CXCL1, is structurally related to IL-8, functions as a neutrophil chemoattractant, and is secreted by neutrophils, macrophages, epithelial cells, and melanoma cells.Citation19 IL-16, formerly known as lymphocyte chemo-attractant factor (LCF) is a pro-inflammatory cytokine that attracts CD4+ lymphocytes, monocytes, and eosinophils.Citation20 Tissue metalloproteinase inhibitor-1 (TIMP-1) is an inhibitor of matrix metalloproteinases that play an important role in the tumor microenvironment; increased expression of TIMP-1 has been associated with a worse prognosis in melanoma patients.Citation21 Tumor necrosis factor-alpha (TNF-α), also known as cachectin, is an immune regulator secreted by macrophages, NK cells, neutrophils, eosinophils, mast cells, and CD4 + T lymphocytes.Citation22 It has both pro-inflammatory and anti-inflammatory effects depending on the environment. Intracellular adhesion molecule −1 (ICAM-1, CD54), is a member of the immunoglobulin superfamily that includes T cell receptors and antibodies.Citation23 It is expressed continuously at low levels on macrophages, lymphocytes, and endothelial cells, and increases in response to IL-1 and TNF-α. The binding of ICAM-1 to the lymphocyte functional antigen (LFA) integrin is crucial for leukocyte extravasation from blood to inflammatory sites.
IL-5 and IL-13 are cytokines produced by Th2 cells. IL-5 is often associated with eosinophilia and immunoglobulin secretion, especially IgA, and has been targeted with antibodies for the treatment of some allergic disorders. It is not uncommon for IL-5 be to elevated along with IL-3 and GM-CSF.Citation24,Citation25 IL13 is often associated with elevations of IL-4 are common in allergic disorders.Citation15
The CD40-ligand (CD40L, CD154) is a member of the TNF family that is expressed on activated CD4+ cells.Citation26 The interaction between CD40L and CD40 on DC is important in the production of IL-12p70, and is critical for DC activation and antigen presentation to CD4+ cells.Citation27 CD40L is also expressed by B cells, NK cells, and monocytes and is important for B cell memory and immunoglobulin production. IL-3, originally called multi-CSF, is a pluripotent hematopoietic colony-stimulating factor that regulates the production of blood cells, and differentiation of granulocytes and macrophages, but also is produced by CD4+ cells as part of an immune response.Citation28 IL-2, originally identified as T cell growth factor, is secreted by CD4+ and CD8 + T lymphocytes, NK cells, DC, and macrophages and is one of the most important cytokines in the initiation and perpetuation of a Th1 immune response.Citation29,Citation30 It sustains anti-tumor activity of NK cells and CTL.
Markers that were low at baseline
These were defined by levels that were less than 33% of the control value. IL-23 is an inflammatory cytokine that is essential for sustaining Th17 cells, and is associated with increased angiogenesis and reduced CD8+ tumor infiltration.Citation31 IL-23 results from the combining of IL-12p40 with IL-23p19. Programmed death molecule-1 (PD-1) is induced on lymphocytes by IFN-γ and is associated with down-regulation of a Th1 response.Citation32,Citation33 Galectin-3 is a member of the beta galactoside-bindng protein family and is involved in macrophage activation, chemo-attraction, angiogenesis, inflammation, cell-cell adhesion, cancer metastasis, cell-matrix interactions, and apoptosis.Citation34 In some studies, elevations of galectin-3 were associated with poor prognosis in cancer patients.Citation35
IL-18 is a proinflammatory cytokine in the IL-1 superfamily that is produced by macrophages.Citation36 It induces NK cells and T cells to release IFN-γ that can lead to immunosuppression via PD-1.Citation37 IL-17 is a proinflammatory cytokine produced by Th17 cells, especially in response to IL-23.Citation38,Citation39 Secretion of IL-17 by a subset of CD4+ cells led to defining the Th17 response. It has been associated with autoimmunity and anti-cancer effects.Citation40 IFN-γ, also known as immune interferon and type II interferon, was named interferon because it can inhibit viral replication. It is produced by NK and NKT cells, as part of the innate immune response, and by CD4+ cells and CTL as part of a Th1 response, and causes cells to increase both class I and class II histocompatibility antigens.Citation41,Citation42 Her levels of IL-17, IL18, IL23, and IFN-γ were all extremely low at baseline and were unchanged after three DCV vaccinations. Levels of Galectin-3 and PD-1 were also extremely low, increased by about 30% after three DCV injections, but still remained quite low.
Markers that increased by more than 20%
There were four markers that were elevated at baseline, but still increased by more than 20% after three DCV injections: SAA, IL-22, GROa, and CD40L. Even though her SAA level was already 277-fold higher than the control level at baseline, it increased by another 81% after three DCV injections. Similarly, her IL-22 level was already 14.2-fold higher than the control levels at baseline, and increased by another 66%. In contrast to IL-22, IL-21, the level of which was also more than 14 times control at baseline, changed minimally. Her GROa level was 5.3-fold higher than the control level at baseline, but increased by another 29%. Her level of CD40L was 2.8-fold higher than control levels at baseline, but increased another 24%.
As noted earlier, levels of Galectin-3 and PD-1 were extremely low at baseline, increased by about 30% after three DCV injections, but still remained quite low. The other markers that increased by more than 20%, but were neither markedly increased nor decreased relative to control levels at baseline, were IP-10, TARC, gp130, and the acute phase reactant c-reactive protein (CRP).
The greatest increases by far were the more than three-fold increases in IP-10 and TARC. Thymus and activation-regulated chemokine (TARC) is a chemo-attractant for T lymphocytes that has been implicated in many allergic conditions.Citation43,Citation44 TARC is induced by GM-CSF, 500 µg of which were injected at the time of each of the three weekly vaccinations. IFN-γ-induced protein 10 (IP-10), also known as CXCL10, is a chemo-attractant for macrophages, T lymphocytes, NK cells, and DC.Citation45,Citation46 It is secreted by a variety of cell types in response to IFN-γ; therefore it is considered a component of the Th1 response. Gp130, also known as CD130 or IL-6β, is a receptor shared by IL-6, IL-11, and IL-27 that can promote inflammation via IL-6 and IL-11, or suppress inflammation via IL-27.Citation47 Dysregulated gp130 signaling is believed to promote cancer.
Markers that decreased by more than 20%
As shown in , there were 13 markers that decreased by more than 20%. Of the 16 markers that were elevated at baseline, five subsequently decreased by more than 20%: IL-12p40, ICAM-1, TNF-α, IL-10, and IL-2. The largest decrease of any marker was the 92% decline in IL-12p40, although it still remained elevated. Two markers typically associated with immunosuppression, IL-10, which was more than seven-fold elevated at baseline, and TGF-β that was 45% above control values, decreased by 26% and 45%, respectively.Citation48 Transforming growth factor-beta (TGF- β) is produced by regulatory T cells and suppresses macrophages, DC, B cells and other T cells including the secretion of IFN-γ, TNF-α, and various interleukins including IL-2.Citation49,Citation50
Other markers that declined by more than 20%, but were not particularly elevated at baseline, included beta-2-microglobulin (B2M), Eotaxin, IL-1a, IL-6, IL-8, IL-15, and IL-12p70. B2M is a component of the MHC class 1 molecule for antigen presentation. B2M is considered a negative regulator of the immune system, and elevated levels are associated with a worse clinical outcome in patients with myeloma and lymphoma.Citation51 Eotaxin is a chemo-attractant for eosinophils, basophils, and Th2 cells.Citation52 It can be induced by IL-13 and is associated with Th2 responses and some allergic conditions. IL-1a, originally called lymphocyte-activating factor (LAF), is a pro-inflammatory molecule primarily produced by epithelial cells and most activated cells of the innate immune system.Citation53 IL-6 is a pro-inflammatory cytokine secreted by macrophages during the innate immune response and can bind to gp 130. IL-6 induces acute phase reactants such as CRP and SAA, but also has inhibitory effects on IL-1 and TNF-α.Citation54 IL-6 is associated with many autoimmune disorders, and levels are often elevated in cancer patients.Citation55 IL-6 is a therapeutic target for rheumatoid arthritis and other autoimmune diseases.Citation56 IL-8, also known as CXCL8, is a chemokine produced by macrophages and other cells active in the innate immune response, that is an especially strong chemo-attractant for neutrophils.Citation57 IL-15 is a pro-inflammatory cytokine that is critical for NK cell differentiation, and has structural similarity to IL-2.Citation58 It is expressed by macrophages, monocytes, and DC, is stimulated by GM-CSF and viral infection, and supports an anti-cancer immune response.Citation59
IL-12 is a crucial cytokine produced by DC to stimulate NK and CTL.Citation60,Citation61 The active form of IL-12 is IL12-p70, which is made up of IL-12p40 and IL-12p35, while IL-23 is made up of IL-12p40 and IL-23p19.Citation62 It is also known as T cell-stimulating factor because of its effects on naïve and memory T cells and enhancement of NK and CTL.Citation63 IL-12p70 increases the production of IFN-γ and a Th1 response that includes induction of the chemo-attractant IP-10 (CXCL10), which has anti-angiogenic effects.Citation64,Citation65 There are interesting associations between the IL-12B gene and serum levels of IL23 and IL-12p40 levels in patients with ankylosing spondylitis, an autoimmune disease which is associated with the HLA B27 type of this patient.Citation66
Dendritic cell expression of cytokines by ELISPOT
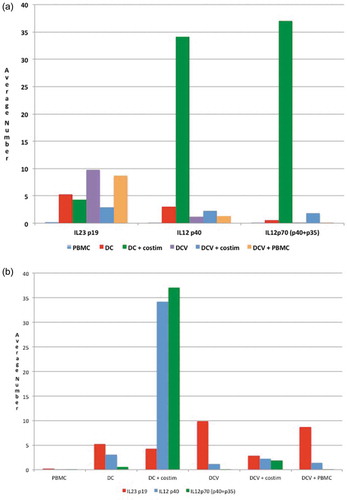
shows the ELISA expression of IL23p19, IL-12p40, and IL12p70 for various cell types. IL-12p70 consists of IL-12p40 + IL12-p35, while IL-23 consists of IL-12p40 and IL-23p19.Citation62 In these experiments, DCV cells alone or with PBMC had an increased expression of IL23p19 compared to PBMC and unloaded DC. Interestingly, the levels decreased when DCV was incubated with co-stimulatory molecules. Phagocytosis of damaged or dying cells induces the upregulation of IL-12 related molecules.Citation67 However, her highest levels of IL-12p40 and IL-12p70 expression were in unloaded DC (no incubation with autologous tumor cells) after they had been co-stimulated with CD40L and anti-human CD28/CD49d. Paradoxically, her antigen-loaded DC (DCV) had low levels of IL-12p40 and IL12-p70 and these levels did not increase in association with CD40L and anti-human CD28/CD49d co-stimulation.
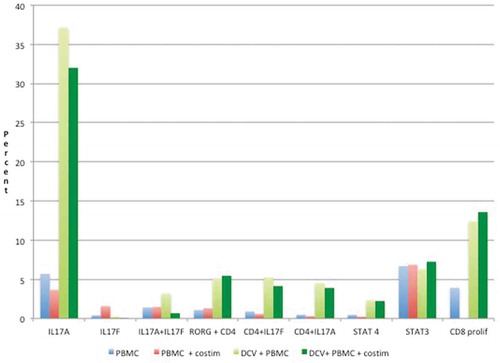
shows the expression of various cell markers as measured by immune-fluorescence and flow cytometry. Relative to cultures of PBMC, DCV cultures were associated with very high levels of IL-17 (IL-17A), and increases in CD8+ cells. DCV had somewhat higher co-expression of CD4+ with RORG, IL17F, or STAT4, and none of these were further increased by co-stimulation with CD40L and anti-human CD28/CD49d. STAT3 expression was similar for both PBMC and DCV regardless of co-stimulation. In T cell proliferation assays, there was a fourfold increase in CD8+ cells in response to co-incubation with antigen-loaded DC, and this was not further enhanced by the addition of CD40 ligand or anti-human CD28/CD49d.
Discussion
Certain cytokines are associated with the innate immune response that features NK cells, macrophages, neutrophils, eosinophils, and mast cells, and each of the CD4+ helper T-cell responses including Th1, which results in antigen-specific cytotoxic T cells, Th2, which results in antigen-specific antibodies produced by B cell clones, and Th17 which can produce pro-inflammatory or anti-inflammatory effects depending on the tumor microenvironment.Citation68 Blood levels and changes in various cytokines and chemokines may provide insight into a vaccine-induced immune response, although they may not reflect what is taking place within tumor sites. At baseline, her circulating cytokines indicated that she had an ongoing inflammatory response, including aspects associated with both innate and adaptive immune response, which is not surprising in a cancer patient, and may also reflect underlying immune effects due to her B27 allotype. Elevated inflammatory markers consistent with an innate immune response included SAA, MCP-3, GROa, TIMP-1, TNF-α, IL-6, IL-15, and IL-16. She also had evidence of ongoing adaptive immune responses at baseline. Elevated markers consistent with an ongoing Th1 response at baseline included CD40 ligand, IL-2, and TNF-α, but IFN-γ levels were low. Also present at baseline were elevations of IL-10 and TGF-β, which increase in response to adaptive immune responses and are associated with their immunosuppression. Elevated markers at baseline consistent with an ongoing Th2 response included elevated IL-5, IL-13, IL-3, IgG1, IgG3, and IgG4, but IL-4 levels were low. Elevated markers at baseline consistent with an ongoing Th17 response included IL-21, IL-22, and IL-6 but levels of IL-17 and IL-23 were very low.
Whether administered with preventive or therapeutic goals, vaccines are injected with the intent to induce a multifaceted immune response that is initiated by dendritic cells, and implemented by CD4+ helper T cells. These antigen-specific adaptive responses include: (1) Th1, that results in CTL through clonal expansion driven by their T cell receptors, (2) Th2 that results in antibodies, immunoglobulins produced by B cells driven by their antigen-specific B-cell receptors, and (3) Th17 responses that modify ongoing inflammatory responses at local tissue sites.Citation68 For the patient-specific vaccines used to treat this patient, DC were loaded with antigen ex vivo, rather than simply injecting antigen and relying on in vivo DC uptake. In terms of antigen presentation by DC, as a general rule, Th1 responses are associated with antigen presentation by MHC class I molecules, while Th2 responses, including Th17, are associated with antigen presentation by MHC class II molecules.Citation69,Citation70 However, cross-presentation of phagocytosed antigens is also known to occur,Citation71,Citation72 and Th2 and Th17 helper T cells can facilitate Th1 responses.Citation73 In vitro studies showed that her antigen-loaded DC were capable of enhancing CD8+ responses, and eliciting IL-17 expression, which is typical of a Th17 response. However, in vivo the changes in her cytokines and other markers after three DCV injections were consistent with an increased innate inflammatory response and additional Th1 response, with a decrease in markers associated with a Th2 response. Other than a very high IL-22 at baseline that increased even further, there was no evidence for an enhanced Th17 response.
Some of the major changes following vaccination suggested induction of an additional innate immune response with increased inflammation (increased TARC, gp130, and even greater increase in the already elevated SAA). Other major changes following vaccination suggested a Th1 response (increased IP-10, CD-40L, IL-22, and PD-1). Even though IFN-γ levels were low and did not increase after three DCV injections, there were elevations of markers that are induced by this hallmark cytokine of a Th1 response, such as IP-10, PD-1, and CD40-L. After three DCV injections, there were no changes suggesting an increase in the Th2 response, i.e., no increase in IL-4, IL-5, IL-6, IL13, and decreases in IgG1 and IgG3 immunoglobulin levels. The declines in the suppressive markers IL-10 and TGF-β after vaccination suggest that there was a shift in the balance of immunosuppression and immune stimulation that had a favorable effect in terms of tumor control. Therefore, the serologic week-4 data suggest that for her the primary changes induced by her patient-specific vaccine were an enhanced innate immune response and Th1 response more than Th2 or Th17.
Incubation of her PBMC with antigen-loaded DCV in vitro resulted in a fourfold increase in CD8+ cells, which suggests that a Th1 tumor-antigen-specific response could be induced by her antigen-loaded DC. CTL are the most important effector cell resulting from a Th1 response. Unfortunately, there were insufficient lymphocytes to determine whether the co-incubation had increased the cytotoxic potential of these CD8+ lymphocytes specifically against her tumor cells, or increased antigen recognition based on IFN-γ expression in lymphocytes after co-culture with her tumor cells.
Incubation of her PBMC with antigen-loaded DC in vitro greatly increased the expression of IL17 on her mononuclear cells. IL-17 expression and secretion are the hallmark of Th17 cells, although other cell types can secrete IL-17 as well. There has been increasing interest in the immunologic role of Th17 lymphocytes, both in cancer and autoimmune disorders.Citation39,Citation40,Citation74 Th17 cells appear to be important for long-term immunologic memory.Citation75 It has been suggested that Th17 cells may be part of an effective anti-cancer immune response since high levels of tumor-infiltrating Th17 lymphocytes are associated with better survival in patients with advanced ovarian cancer.Citation76 Th17 cells and IL-17 stimulate Th-1 chemokines (CXCL9 and CXCL10) that recruit effector T cells and NK cells into the tumor microenvironment.Citation76 Such chemokines are associated with a robust effector T cell phenotype in melanoma samples.Citation77 It has been suggested that strategies to increase Th17 cells may be beneficial in cancer immunotherapy,Citation78 although in some tumors they seem to be associated with immunosuppression,Citation39,Citation40 Interestingly, in a B16 melanoma model, CD4+ Th17 adoptive cell therapy was highly effective, and actually more effective than CD4+ Th1 cells.Citation79 Despite these changes in vitro, in vivo, she had very low levels of both IL-17 and IL-23 at baseline, and the levels did not increase after three weekly DCV injections, so it is not clear whether a Th17 response in vivo contributed to her tumor regression.
Her IL-22 levels were very high at baseline but increased by another 66% after vaccination. IL-22 can be elevated as part of innate or adaptive immune responses. IL-23 is a major inducer of IL-22, but her IL-23 levels were very low at baseline and at week-4. In humans in a normal state of health, it is estimated that of IL-22-producing CD4 + T cells in peripheral blood, about 50% produce IL-22 alone (Th22 cells), 33% co-express IFNγ (Th1 cells) and 15% also produce IL-17 (Th17 cells).Citation12 Like IL-17, depending on the setting, IL-22 can be pathogenic or protective, i.e., pro-inflammatory or anti-inflammatory.Citation13,Citation14 CD4+ helper cells that produce IL-22 often also produce IL-17 and/or IFNγ. IL-22 has pro-inflammatory effects, especially in combination with IL-17, and is often elevated during inflammation. IL-22 is often elevated along with IL-1β, IL-6, and IL-23 in autoimmune disorders including inflammatory bowel disease, rheumatoid arthritis, psoriasis, systemic lupus erythematosis, and atopic dermatitis, and in association with various infections.Citation15 However, these four markers were not correlated in her case: IL-22 was markedly elevated and increased another 66% after three DCV injections, while IL-1β was somewhat elevated at baseline and increased by 16%; IL-6 was somewhat elevated at baseline and decreased, and IL-23 was markedly low at both week-0 and week-4. The marked elevation of IL-22 could be interpreted as part of a Th17 response, but it is regulated differently than other Th17-associated cytokines, and is also secreted by other lymphoid cells. In this patient, there were no increases in IL17, IL-21, or IL-23 as might be expected in a Th17 response. IL-22 upregulates gene expression of SAA,Citation15 which was extremely high at baseline and increased even more after three DCV injections.
The largest percentage decrease between week-0 and week-4 was in IL12-p40, the subunit of IL-12. As noted earlier, IL-12p40 is a component of both IL12-p70 and IL-23, so one possible explanation for this would have been increase in either or both of those markers. However, after vaccination, her IL-12p70 and IL-23 levels both decreased. However, it is possible this was a rapid effect that could only have been detected in blood samples collected during the first 3 weeks of injections.
In terms of modifying her underlying immune response, it is noteworthy that she had declines in the immunosuppressive factors IL-10 and TGF-β. She also had declines in various VEGF-related proteins, although at least three remained quite elevated. As far as putative melanoma-associated tumor markers, she did not have elevated levels of S100b, LDH, or neuron-specific enolase at baseline.
B2M is a component of MHC class 1 antigen presentation by antigen-presenting cells. B2M was elevated at baseline, but decreased after three vaccinations. B2M is often considered a prognostic marker for poor outcome in patients with malignancy, so the decrease observed may be evidence of an early anti-tumor effect.
HLA-typing and genomic studies showed that the patient was HLA-B*27 with a high mutational tumor burden. HLA-B*27 is a class I surface antigen encoded on chromosome 6 by the B locus of the major histocompatibility complex that is associated with a variety of autoimmune disorders including, psoriatic arthritis, reactive arthritis (Reiter’s syndrome), inflammatory bowel disease, ulcerative colitis, uveitis, iritis, and especially ankylosing spondylitis.Citation4 The patient’s medical records did not indicate that she had symptoms or signs of any of these maladies. Patients with ankylosing spondylitis have elevations of Th1 and Th2 cytokines,Citation80 and even though she did not carry this diagnosis, it is possible that immune effects associated with her HLA-B*27 allotype contributed to the baseline elevations of some of these cytokines. Based on her non-synonymous mutations, there was a high probability that she had at least 13 neoantigens that her immune system could recognize. However, her tumor cells also had mutations in the HLA-A locus, which could have impaired the ability for her CTL to recognize certain foreign antigens in the context of her tumor cells.
The mutations identified in her genomic analysis included the types of mutations one would expect for any patient with advanced cancer, including mutations related to cell proliferation, cell cycle control, apoptosis, cell adhesion, metastasis, angiogenesis and avoidance of immune recognition.Citation81,Citation82 However, she did not have mutations that typically result in targetable altered protein expression in melanoma patients, such as mutations in BRAF, NRAS, and KIT.Citation83 She had none of the mutations most frequently associated with different origins of melanoma, such as BRAF, CDKN2A, NRAS and TP53 in cutaneous melanoma, BRAF, NRAS and NF1 in acral melanoma and SF3B1 in mucosal melanoma.Citation83
Despite repeated recurrences and failure to respond to systemic therapies available at the time, this patient appears to have been a good candidate for a vaccine approach because she had large numbers of non-synonymous mutations that resulted in potential antigenic targets, and because she had no evidence of an existing immune response to her cancer based on absent T cell infiltration and lack of PD-L1 expression on her tumor tissue, and a baseline serum PD-1 level of only 75 pg/ml. It is possible that such a patient is more likely to benefit from a therapeutic vaccine than from treatment with anti-PD-1 or anti-PD-L1 antibodies that rely on the existence of an effective anti-tumor immune response that has been suppressed by PD-1/PD-L1 interaction.Citation84
By using her own tumor cells as the source of antigen, her autologous vaccine potentially included any antigens to which her immune system was capable of making a response. Because the tumor cells were cultured under conditions that favor self-renewing tumor-initiating cells, the vaccine may have included antigens that were only expressed on her cancer stem cells or progenitor cells, which may at least partially explain why it took so long for a clinical response to become apparent. In terms of phenotypic expression, 73% of her melanoma cells expressed CD146, a marker associated with both melanoma cells and capillary pericytes,Citation85 and 26% expressed nerve growth factor CD271, a melanoma stem cell marker.Citation86 We also know that in the presence of serum there is often differentiation of cells in culture, and that such cells express many common melanoma differentiation antigens including S100, Melan-A, MART-1, MAGE, and HMB45.
A limitation of this analysis is the lack of serologic data during the 3 weeks of vaccine administration. It is possible that significant changes may have occurred earlier during treatment and were no longer evident by week-4. Another limitation is the lack of serial biopsies that might have shown changes in her tumors, which initially stabilized, and eventually regressed. Nevertheless, the changes at week-4 are of note because they are consistent with immune modulation with an increase in innate and Th1 immunity. The other limitation is the lack of experiments to show that PBMC co-cultured with ATA-loaded DC actually increased tumor cell killing in vitro compared to the cytotoxic effects of PBMC that had not been stimulated by ATA-loaded DC. Another limitation in the data is that fold-changes over control and percent changes compared to baseline are only relative rather than absolute numbers. Furthermore, what is a biologically relevant percentage increase for one marker might not be biologically meaningful for a different marker. The rank-order presented is only relative and does not reflect the biological relevance of the markers. It is possible that biologically significant changes were reflected in a less than 20% change, and these are omitted using the arbitrary 20% cut off in the data. For these reasons, it is possible that one might make different interpretations of her immune response from the same data. We were unable to identify other case reports of complete tumor responses following vaccination in patients with metastatic melanoma that included the same sort of detailed genomic and proteomic analysis as detailed in this report. Therefore, we were unable to compare the data acquired in this report to show similarities or differences to other such patients.
Summary
In this particular patient, administration of the patient-specific DCV loaded with ATA from self-renewing tumor cells resulted in relatively rapid disease control (stable disease) and eventual durable complete remission of measurable progressive metastatic melanoma. The basis for this anti-tumor effect appears to be DC-induced effects that resulted in increased innate and Th1 responses, but there was no evidence of a Th17 response, and no further increase in an active Th2 response. It is possible that the vaccination induced new immune responses to ATA and also overcame the suppression of underlying anti-ATA immune responses. She may have been more likely to derive benefit from her DCV because she had a tumor that lacked infiltration with lymphocytes in the setting of a high mutational burden.
Authors’ contributions
Conception and design: R Dillman, G Nistor, A Poole.
Development of methodology: G Nistor, A Poole.
Acquisition of data: (provided animals, acquired and managed patients, provided facilities, etc.): R Dillman.
Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): G Nistor.
Writing, review, and/or revision of the manuscript: R Dillman, G Nistor, A Poole.
Administrative, technical, or material support (i.e. reporting or organizing data, constructing data bases): R Dillman, G Nistor.
Study supervision: R Dillman, G Nistor.
Disclosure of potential conflicts of interest
R Dillman, G Nistor, and A Poole are employees of AIVITA Biomedical Inc.
Disclaimer
The contents of this article are solely the responsibility of the authors and do not represent the official view of any entity.
Acknowledgments
We wish to thank Lu Chen for her technical assistance in performing the mixed leukocyte reactions and Janet Stallman, M.D. for the evaluation of archival tumor samples. We wish to acknowledge the assistance of Andrew Cornforth, Ph.D. in the processing and storage of blood samples.
Additional information
Funding
References
- Dillman RO, Nanci AA, Williams ST, Kim RB, Hafer RL, Coleman CL, Wang PC, Duma CM, Chen PV, Selvan SR, et al. Durable complete response of refractory, progressing metastatic melanoma after treatment with a patient-specific vaccine. Cancer Biother Radiopharm. 2010;25(5):553–57. PMID: 20849310. doi:10.1089/cbr.2010.0819.
- Dillman RO, Cornforth AN, McClay EF, Amatruda TT, Depriest C. Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in patients with metastatic melanoma: 5-year follow up and additional analyses. J ImmunoTher Cancer. 2018;6(1):19. PMID: 29510745. doi:10.1186/s40425-018-0330-1.
- Dillman RO, Cornforth AN, Depriest C, McClay EF, Amatruda TT, de Leon C, Ellis RE, Mayorga C, Carbonell D, Cubellis JM. Tumor stem cell antigens as consolidative active specific immunotherapy: a randomized phase II trial of dendritic cells versus tumor cells in patients with metastatic melanoma. J Immunother. 2012;35(8):641–49. doi:10.1097/CJI.0b013e31826f79c8.
- Thomas GP, Brown MA. Genetics and genomics of ankylosing spondylitis. Immunol Rev. 2010;233(1):162–80. PMID: 20192999. doi:10.1111/j.0105-2896.2009.00852.
- Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonazlez R, Weber JS, Gajewski TF, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182–90. PMID: 23775962. doi:10.1200/JCO.2012.47.7863.
- Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol. 2012;32(4):335–48. PMID: 23237509. doi:10.1615/CritRevImmunol.v32.i4.
- Cooper AM, Khader SA. Il-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi:10.1016/j.it.2006.11.002.
- Ling P, Gately MK, Gubler U, Stern AS, Lin P, Hollfelder K, Su C, Pan YC, Hakimi J. Human IL-12p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1994;154(1):116–27. PMID: 7527811.
- Klinke DJ 2nd. Monomer to dimer is an important determinant of IL-12 bioactivity. J Theor Biol. 2006;240(2):323–35. PMID: 16448670. doi:10.1016/j.jtbi.2005.09.022.
- Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175(4):2261–69. PMID: 16081794. doi:10.4049/jimmunol.175.4.2261.
- Davis MR, Zhu Z, Hansen DM, Bai Q, Fang Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015;358(2):107–14. PMID: 25575696. doi:10.1016/j.canlet.2014.12.047.
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–63. PMID: 19578369. doi:10.1038/ni.1767.
- Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252(1):116–32. PMID: 23405899. doi:10.1111/imr.12027.
- Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and patology. Ann Rev Immunol. 2015;33:747–85. PMID: 25706098. doi:10.1146/annurev-immunol-032414-112123.
- Zenewicz LA. IL-22: there is a gap in our knowledge. ImmunoHorizons. 2018;2(6):198–207. PMID: 31022687. doi:10.4049/immunohorizons.1800006.
- Fioretti F, Fradelizi D, Stoppacciaro A, Ramponi S, Ruco L, Minty A, Sozzani S, Garlanda C, Vecchi A, Mantovani A. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protei-3 (MCP-3) gene transfer: perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J Immunol. 1998;161(1):342–46. PMID: 9647242.
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. PMID: 11244051. doi:10.1146/annurev.immunol.19.1.683.
- Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2(3):194–99. PMID: 24778315. doi:10.1158/2326-6066.CIR-13-0214.
- Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72(1):9–18. PMID: 12101257.
- Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J Leukoc Biol. 2000;67(6):757–66. PMID: 10857846. doi:10.1002/jlb.2000.67.issue-6.
- Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci. 2014;71(4):659–72. PMID: 23982756. doi:10.1007/s00018-013-1457-3.
- Mehta AK, Gracias DT, Croft M. TNF activity and T cells. Cytokine. 2018;101:14–18. PMID: 27531077. doi:10.1016/j.cyto.2016.08.003.
- Reina M, Espel E. Role of LFA-1 and ICAM-1 in cancer. Cancers (Basel). Nov 3 2017;9(11):E153. PMID: 29099772. doi: 10.3390/cancers9110153.
- Broughton SE, Dhagat U, Hercus TR, Nero TL, Grimbaldeston MA, Bonder CS, Lopez AF, Parker MW. The GM-CSF/IL-3/IL-5 cytokine receptor family: from ligand recognition to initiation of signaling. Immunol Rev. 2012;250:277–302. PMID: 23046136. doi:10.1111/j.1600-065X.2012.01164.
- Ghandi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–37. PMID: 28277826. doi:10.1080/1744666X.2017.1298443.
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. PMID: 10647992. doi:10.1002/jlb.2000.67.issue-1.
- Elizondo DM, Andargie TE, Kubhar DS, Gugssa A, Lipscomb MW. CD40-CD40L cross-talk drives fascin expression in dendritic cells for efficient antigen presentation to CD4+ T cells. Int Immunol. 2017;29(3):121–31. PMID: 28369442. doi:10.1093/intimm/dxx013.
- Frendl G. IL-3: from colony-stimulating factor to pluripotent immunoregulatory cytokine. Int J Immunopharmacol. 1992;14(3):421–30. PMID: 1618595. doi:10.1016/0192-0561(92)90172-H.
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. PMID: 18062768. doi:10.1146/annurev.immunol.26.021607.090357.
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–90. PMID: 22343569. doi:10.1038/nri3156.
- Croxford AL, Mair F, Becher B. IL23: onecytokine in control of autoimmunity. Eur J Immunol. 2012;42(9):2263–73. PMID: 22949325. doi:10.1002/eji.201242598.
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24. PMID: 17606980. doi:10.1093/intimm/dxm057.
- Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in immunity and diseases. Curr Top Microbiol Immunol. 2017;410:75–97. PMID: 28929192. doi:10.1007/82201767.
- Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta. 2015;1855(2):235–47. PMID: 25819524. doi:10.1016/j.bbcan.2015.03.003.
- Colomb F, Wang W, Simpson D, Zafar M, Beynon R, Rhodes JM, Yu LG. Calectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J Biol Chem. 2017;292(20):8381–89. PMID: 28364041. doi:10.1074/jbc.M117.783431.
- Esmailbeig M, Ghaderi A. Interleukin-18: a regulator of cancer and autoimmune diseases. Eur Cytokine Netw. 2017;28(4):l127–140. PMID: 29478963. doi:10.1684/ecn.2018.0401.
- Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–5393. PMID: 21724589. doi:10.1158/0008-5472.CAN-11-0993.
- Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43(3):402–07. PMID:18701318. doi:10.1016/j.cyto.2008.07.017.
- Zuniga LA, Jain R, Haines C, Cua DJ. Th17 cell development from cradle to grave. Immuno Rev. 2013;252(1):78–88. PMID: 23405896. doi:10.1111/imr.12036.
- Llosa NJ, Geis AL, Thiele Orberg E, Housseau F. Interleukin-17 and type 17 helper T cells in cancer management and research 2014. Immunotargets Ther. 2014;10(3):39–54. eCollection 2014 PMID: 27471699. doi:10.2147/ITT.S56529.
- Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13(2):95–109. PMID: 11900986. doi:10.1016/S1359-6101(01)00038-7.
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–89. PMID: 14525967.
- Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272(23):15036–42. PMID: 9169480. doi:10.1074/jbc.272.23.15036.
- Achuthan A, Cook AD, Lee M-C, Saleh R, Khiew H-W, Chang MWN, Louis C, Fleetwood A, Lacey DC, Christensen AD, et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J Clin Invest. 2016;126(9):3453–66. PMID 27525438. doi:10.1172/JCI87828.
- Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monoyctes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177(6):1808–14. PMID 8496693. doi:10.1084/jem.177.6.1809.
- Gattass CR, King LB, Luster AD, Ashwell JD. Constitutive expression of interferon gamma-inducible protein 10 in lymphoid organs and inducible expression in T cells and thymocytes. J Exp Med. 1994;179(4):1373–78. PMID:8145049. doi:10.1084/jem.179.4.1373.
- Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88(6):1145–56. PMID: 20610800. doi:10.1189/jlb.0410217.
- Wiguna AP, Walden P. Role of IL-10 and TGF-β in melanoma. Exp Dermatol. 2015;24(3):209–14. PMID: 25565012. doi:10.1111/exd.12629.
- Jakowlew SB. Transforming growth factor-beta in cancer metastasis. Cancer Metastasis Rev. 2006;25(3):435–57. PMID: 16951986. doi:10.1007/s10555-006-9006-2.
- Haque S, Morris JC. Transforming growth factor-β: a therapeutic target for cancer. Hum Vaccin Immunother. 2017;13(8):1741–50. PMID: 28575585. doi:10.1080/21645515.2017.1327107.
- Xie J, Wang Y, Freeman ME 3rd, Barlogie B, Yi Q. Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood. 2003;101(10):4005–12. PMID: 12531797. doi:10.1182/blood-2002-11-3368.
- Ogilvie P, Paoletti S, Clark-Lewis I, Uguccioni M. Eotaxin-3 is a natural antagonist for CCRT and experts a repulsive effect on human monocytes. Blood. 2003;102(3):789–94. PMID: 12689946. doi:10.1182/blood-2002-09-2773.
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281(1):8–27. PMID: 29247995. doi:10.1111/imr.12621.
- Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL/6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122(4):143–59. PMID: 22029668. doi:10.1042/CS20110340.
- Taher MY, Davies DM, Maher J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc Trans. 2018;46(6):1449–62. PMID: 30467123. doi:10.1042/BST20180136.
- Jones BE, Maerz BJH. IL-6: a cytokine at the crossroads of autoimmunity. Curr Opin Immunol. 2018;55:9–14. PMID: 30248523. doi:10.1016/j.coi.2018.09.002.
- Waugh DJJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14(21):8735–41. PMID: 18980965. doi:10.1158/1078-0432.CCR-07-4843.
- Santana Carrero RM, Beceren-Braun F, Rivas SC, Hegde SM, Gangadharan A, Plote D, Pham G, SM A, Schluns KS. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc Natl Acad Sci USA. 2019;116(2):599–608. PMID:30587590. doi:10.1073/pnas.1814642116.
- Zhang M, Wen B, Anton OM, Yao Z, Dubois S, Ju W, Sato N, DiLillo DJ, Bamford RN, Ravetch JV, et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci. 2018;l115:E10915–E10924. doi:10.1073/pnas.1811615115.
- Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8(1):40–52. PMID: 19275692. doi:10.2174/187152809787582507.
- Lu X. Impact of IL-12 in cancer. Curr Cancer Drug Targets. 2017;17(8):682–97. PMID: 28460617. doi:10.2174/1568009617666170427102729.
- Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112–31. doi:10.1111/imr.2008.226.issue-1.
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. PMID: 19161420. doi:10.1111/j.1600-065X.2008.00700.
- Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. PMID 8760828. doi:10.1002/eji.1830260323.
- Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, Kulig P, Becher B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–46. PMID:25190142. doi:10.1038/cdd.2014.134.
- Ivanova M, Manolova I, Miteva L, Gancheva R, Stoilov R, Stanilova S. Genetic variations in the IL-12B gene in association with IL-23 and IL12p40 serum levels in ankylosing spondylitis. Rheumatol Int. 2019;39(1):111–19. PMID: 30443744. doi:10.1007/s00296-018-4204-0.
- Dixon KO, O’Flynn J, van der Kooij SW, van Kooten C. Phagocytosis of apoptotic or necrotic cells differentially regulates the transcriptional expression of IL-12 family members in dendritic cell. J Leukoc Biol. 2014;96(2):313–24. PMID: 24782489. doi:10.1189/jlb.3A1013-538RR.
- Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunol. 2008;123(3):326–28. PMID: 17983439. doi:10.1111/j.1365-2567.2007.02719.x.
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77. PMID: 22437871. doi:10.1038/nrc3258.
- Mellman I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013;1(3):145–49. PMID: 24777676. doi:10.1158/2326-6066.CIR-13-0102.
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–69. PMID: 22790179. doi:10.1038/nri3254.
- Van Endert P. Intracellular recycling and cross-presentation by MHC class I molecules. Immuno Rev. 2016;272(1):80–96. PMID: 27319344. doi:10.1111/imr.12424.
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell based vaccination. Cancer Res. 2013;73:19–29. PMID: 23087058. doi:10.1158/0008-5472.CAN-12-1127.
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control Th17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986–91. PMID: 19797626. doi:10.1126/science.1172702.
- Wei S, Zhao E, Kryczek I, Zou W. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology. 2012;1(4):516–19. PMID: 22754771. doi:10.4161/onci.19440.
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–49. PMID:19470694. doi:10.1182/blood-2009-03-208249.
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+T-cell recruitment. Cancer Res. 2009;69:3077–85. PMID: 19293190. doi:10.1158/0008-5472.CAN-08-2281.
- Cannon MJ, Goyne SPJ, Chiriva-Internati M. Dendritic cell vaccination against ovarian cancer—tipping the Treg/Th17 balance to therapeutic advantage? Expert Opin Biol Ther. 2011;11(4):441–45. PMID: 21271951. doi:10.1517/14712598.2011.554812.
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th-17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–73. PMID: 18354038. doi:10.1182/blood-2007-11-120998.
- Wang J, Zhao Q, Wang G, Yang C, Xu Y, Li Y, Yang P. Circulating levels of Th1 and Th2 chemokines in patients with ankylosing spondylitis. Cytokine. 2016;81:10–14. PMID: 26827189. doi:10.1016/j.cyto.2016.01.012.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. PMID:2137623. doi:10.1016/j.cell.2011.02.013.
- Turner N, Ware O, Bosenberg M Genetics of metastasis: melanoma and other cancers. Clin Exp Metastasis. 2018 Aug;35(5–6):379–91. PMID: 29722002. doi:10.1007/s10585-018-9893-y.
- Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch A-M, Kakavand H, Alexandrov LB, Burke H, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80. PMID: 28467829. doi:10.1038/nature22071.
- Dillman RO. Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum Vaccin Immunother. 2017;13(3):528–32. PMID: 27808593. doi:10.1080/21645515.2016.1244149.
- Lei X, Guan CW, Song Y, Wang H. The multifaceted role of CD146/MCAM in the promotion of melanoma progression. Cancer Cell Int. 2015;15(1):3. eCollection 2015 PMID:25685061. doi:10.1186/s12935-014-0147-z.
- Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466(7302):133–37. PMID: 20596026. doi:10.1038/nature09161.