ABSTRACT
Objective
To evaluate the cost-effectiveness of the current strategy combining universal vaccination with hepatitis B immunoglobulin (HBIG) treatment for infants of hepatitis B surface antigen (HBsAg) positive mothers compared with universal vaccination with hepatitis B vaccine only.
Methods
A decision tree model with a Markov process was constructed and used to simulate the lifetime of the birth cohort in Zhejiang Province during 2016. The current strategy was compared against universal vaccination with respect to costs and health effects. Costs were assessed from the health care system perspective. Health effects were measured by the number of hepatitis B virus (HBV) related diseases and deaths avoided and quality-adjusted life-years (QALYs) gained. The incremental cost‑effectiveness ratio (ICER) is calculated and compared to standard willingness-to-pay thresholds. A one-way sensitivity analysis and a probabilistic sensitivity analysis (PSA) were performed to assess parameter uncertainties.
Results
Over the cohort’s lifetime, 182 acute symptomatic infections, 2215 chronic infections, 872 cases of cirrhosis, 595 cases of hepatocellular carcinoma (HCC) and 1,350 HBV-related deaths among the cohort of 624,000 infants would be further avoided by the current strategy compared to universal vaccination. Universal vaccination was dominated by the current strategy that produced not only higher total QALYs, but also had lower costs. The results remained robust over a wide range of assumptions.
Conclusions
The current strategy was cost saving compared to universal vaccination, and continuing the current strategy is recommended to further decrease the burden of hepatitis B.
Introduction
The hepatitis B virus (HBV) is a well-known risk factor for liver diseases, including chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC)Citation1. In China, 78 million people are currently estimated to carry the hepatitis B surface antigen (HBsAg), amounting to almost a third of the chronic infections worldwide. Every year, approximately 300,000 Chinese people die from HBV-related liver cirrhosis and HCC, accounting for 37%–50% of worldwide mortality.Citation2 China has made great achievements in hepatitis B immunization. The hepatitis B vaccine (HepB) was first recommended for inclusion in the routine vaccination schedule for infants in 1992 and was subsequently integrated into the National Expanded Program of Immunization in 2002, contributing to the birth-dose HepB coverage of 70.7% in 1992 increase to 95.0% in 2014;Citation3 the prevalence of HBsAg positivity in persons aged ≤5 years dropped nearly 30 times from 9.67% in 1992 to 0.32% in 2014.Citation4,Citation5 Currently, mother-to-child transmission (MTCT) has been a major mode of HBV transmission in China. Hepatitis B immunoglobulin (HBIG) in combination with HepB vaccination could generally further reduce MTCT to as low as 5%.Citation6 In 2011, China launched a program for preventing mother-to-child transmission (PMTCT) of hepatitis B and has since augmented universal vaccination with maternal screening and HBIG treatment. This program provides infants born to HBsAg-positive women with HepB plus HBIG within 24 h after birth, followed by completion of the HepB series, and infants born to HBsAg-negative women receive routine HepB series only.
Zhejiang is a relatively developed province in eastern China. However, the HBV disease burden in Zhejiang once was as high as many other places in China with high endemicity of HBV infection via MTCT.Citation7 Since 1992, the HepB coverage rates for the birth dose and 3-dose schedule in Zhejiang Province have both remained above 90%, and the prevalence of HBsAg decreased from 2.16% in 2006 to 1.05% in 2014 in the age group of 1–29 years.Citation8 Great improvements have been seen in HBV infection control in Zhejiang Province, which might be considered a representative sample for research.
Economic analyses of universal infant hepatitis B vaccination have been carried out by several studies in China and it has been shown to be cost-effective.Citation9–Citation11 However, most previous studies focused on the economic analysis of vaccines alone and did not include combinations of maternal screening test and the use of HBIG to augment a universal vaccination. Few economic studies have examined the current strategy of augmenting universal hepatitis B vaccination with immunoglobin treatment. The purpose of this study was to compare the current strategy combining universal vaccination with HBIG treatment for infants of carrier mothers versus no screening and with hepatitis B vaccine only. The results from this study will provide important and useful evidence and technical support for health policymakers to select the optimal HBV prevention strategy for eradicating the HBV infection.
Methods
Comparator strategies
In this economic evaluation, we compared the current strategy versus universal vaccination. The details of each strategy are as follows:
Universal vaccination: regardless of the HBsAg status of mothers, infants receive the initial dose of HepB within 24 h of birth, followed by the 2nd dose and the 3rd dose at 1 month and 6 months. Together, these doses constitute the primary 3-dose hepatitis B vaccination series (HepB3). No pregnant women are screened for hepatitis B surface antigen (HBsAg); no infants receive HBIG.
Current strategy: universal vaccination plus maternal screening for HBsAg. Infants born to HBsAg-positive women receive the first dose of HepB and HBIG within 24 h after birth, followed by completion of the HepB series. Infants born to HBsAg-negative mothers receive the first dose of HepB within 24 h of birth and followed by completion of the HepB series; none receive HBIG.
Model
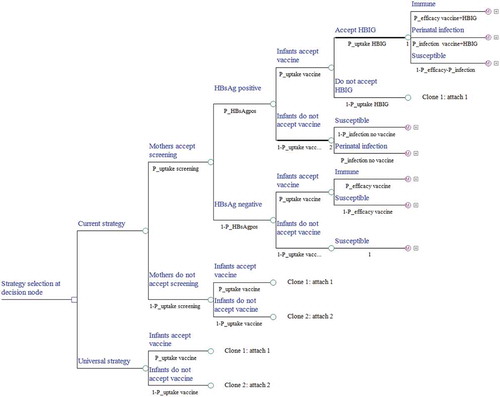
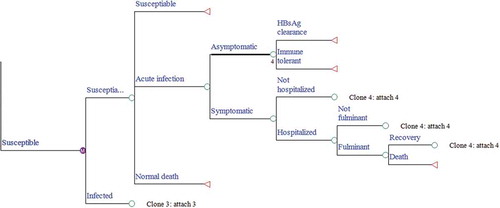
A decision tree was developed to represent the intervention characteristics of the current strategy, and Markov models combined with the decision tree were built to represent the protection state or the natural history of chronic HBV infection. The model is a single cohort analysis based on a closed population (no births) static model, in which both the number of cohort that change over time and herd immunity were ignored. Two preventive strategies against HBV infection were illustrated in our decision tree model (). We identified ten mutually exclusive health states that indicate the progression of HBV morbidity from the susceptible state: susceptible to HBV, immune tolerance, HBeAg-positive chronic hepatitis B (CHB), inactive state, HBeAg-negative CHB, HBsAg clearance, compensated cirrhosis (CC), decompensated cirrhosis (DC), HCC, and death. The Markov process with annual cycles terminates either when all newborns expire or reach the life expectancy age of 81 (–).Citation12 The parameters used in the model were obtained from field surveys, program files, published literature, and unpublished data from the Zhejiang Provincial CDC. The model was created in TreeAge Pro 2016 (TreeAge Software, Inc., Williamstown, MA).
Figure 1. Decision tree model. A square node represents the decision node, a circular node represents a chance node and represents a Markov model. At the end of each branch of the decision tree there is one of three types of Markov models either corresponding to ⅰ. infants under immune ⅱ. infants have got perinatal infection or ⅲ. infants are susceptible. Under each line is a probability represented by P_; HBIG, hepatitis B immunoglobulin. If the tree is cloned, it means that the tree is copied, and a corresponding number will appear under the node of the tree.
Intervention coverage and epidemiological and efficacy parameters
According to the PMTCT project report, the HBsAg screening rate among pregnant women was 99.0%, and 99% of the infants born to HBsAg-positive mothers were administered HBIG. According to the China Information Management System for Immunization Programming (CIMSIP), the reported HepB3 coverage was 99.0% among all infants. The efficacy of immunization and the risk of perinatal infection varied according to maternal HBsAg status, vaccine and HBIG administration. For the infants of HBsAg-positive mothers administered HBIG plus the HepB3 vaccine, the efficacy could be 96.0%, compared to 90.6% for the HepB3 vaccine only. The rate of perinatal infection was 2.9% from HBsAg-positive mothers under immunoprophylaxis with HBIG and HepB3, compared to 5.9% under HepB3 only. The HBsAg-positive rate among pregnant women was 8.0%, which was derived from a meta-analysis by our team, including 10 studies conducted in Zhejiang Province. We made some assumptions that all individuals who are vaccinated would adhere to the full 3-dose vaccination series and that vaccine protection lasts for a lifetime. Detailed information is listed in .
Table 1. Parameter values including ranges used in the sensitivity analyses.
The annual transition probabilities of health states in the Markov model were primarily obtained from the published literature, mainly from Chinese or Asian populations. A wide range was given to each of these parameters to cover the majority of reported data. For those parameters considered to be age-dependent, we adjusted the age-specific base-case values simultaneously by ±50% in the sensitivity analyses. All-cause mortality was taken from Zhejiang Provincial Bureau of Statistics. Specific values of the transition probabilities are listed in .
Table 2. Transition probabilities for each cycle of the Markov model.
Cost estimations and health outcome measures
Costs in this study were assessed from a health care system perspective, including vaccination program costs and direct medical costs of HBV-related diseases.Citation9 The vaccination program costs comprised the cost of the vaccine and the implementation cost. The implementation cost included the following five parameters: Administration, regular maintenance, low-value consumables and materials, immunization digital system maintenance and other relative items. The cost of fixed assets and cold chain equipment purchase were excluded.Citation64 The direct medical costs included the outpatient expenditure and inpatient expenditure. Effectiveness was quantified by quality-adjusted life years (QALYs), as well as new cases of HBV-related morbidity and disease-specific mortality.
The cost of the vaccine and HBIG were determined to be 3.1 Chinese yuan (CNY) and 120 CNY per dose, respectively, according to the current government contract price. The implementation cost was 19.55 CNY per dose, which was obtained from a field survey that covered 1 provincial CDC, 3 municipal CDCs, 9 county CDCs, 27 township hospitals and 91 village clinics.Citation64
We performed a field survey in 8 hospitals from 3 cities in Zhejiang Province to assess the disease burden and QALYs of HBV-related diseases. The 3-level version of EQ-5D was used to determine the QALYs of the target population. Face-to-face interviews with patients were conducted to complete the questionnaire. A total of 626 outpatients and 523 inpatients with different types of HBV-related diseases were enrolled. Costs and the QALYs were analyzed following the analysis framework published by Zhang SLCitation34 and Jia YX.Citation37 The base-case values of these direct costs were simultaneously adjusted by ± 25%, and the 95% CI estimates of QALYs were used in the sensitivity analyses. The QALYs of asymptomatic carriers, immune-tolerant patients and inactive carriers were obtained from the published literature. All costs are from 2016. Costs and QALYs predicted in future years were discounted to the values of 2016 at an annual discount rate of 3%.
Measurement of cost-effectiveness
The expected costs and effectiveness were compared between the two strategies, and the incremental cost-effectiveness ratio (ICER) was calculated based on QALYs. The ICER is the difference in costs between the reference strategy and the comparative strategy divided by the difference in their QALYs.Citation65 According to the WHO recommendation, an intervention is often deemed to be very cost-effective if the ICER is less than the annual per capita gross domestic product (GDP), which was 84,916 CNY in Zhejiang Province in 2016. New cases of HBV-sequelae over the lifetime of the 2016 birth cohort were derived from the Markov cohort analysis.
Sensitivity analyses
A series of one-way deterministic sensitivity analyses that varied the parameters individually over plausible ranges were performed to test the robustness of our findings and to identify key uncertainties. In addition, a probabilistic sensitivity analysis based on Monte Carlo simulations with 10,000 iterations was performed to evaluate the impact of the overall combined uncertainty of all the model parameters on the ICER. .The parameter of all costs of investigation were assigned log-normal distribution found by drawing a distribution histogram. Some parameters were assigned a beta distribution estimated from the mean and 95% CI presented in reference, others were assigned triangular distributions because the distributions of parameters were unclear due to limited literature-based estimates.
Results
Base-case analysis
We estimated the number of infants with HBV infection and their lifetime complications under each strategy (). The universal vaccination had a greater number of infants who developed HBV-related diseases and deaths. Compared with universal vaccination, 182 acute symptomatic infections, 2,215 chronic infections, 872 cases of cirrhosis, 595 cases of HCC and 1,350 HBV-related deaths would be further avoided under the current strategy among the cohort of 624,000 infants and would result in an additional 17,435 QALYs saved. The cost of providing maternal screening and HBIG to infants born to HBsAg-positive mothers was lower compared to the lifetime cost incurred by patients who acquired CHB at birth. The current strategy dominated universal vaccination since it not only produced higher total QALYs but also resulted in lower costs. The results are summarized in and .
Table 3. New cases of HBV-related diseases and deaths in the birth cohort.
Table 4. Per capital costs, QALYs, and the ICERs of two strategies.
Sensitivity analyses
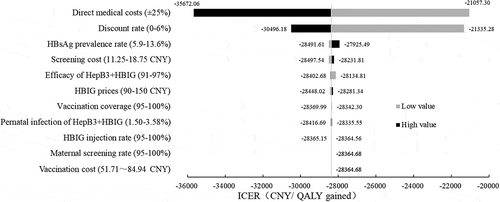
Key parameters were evaluated using a series of one-way sensitivity analyses, and the ICERs were presented in a tornado diagram (). The results showed that the direct medical costs associated with HBV-related diseases were among the most important parameters that could impact the ICER. However, the ICER remained well cost-saving regardless of changes in key parameter values, which demonstrated that none of the single parameters could change the conclusion that the current strategy was cost saving.
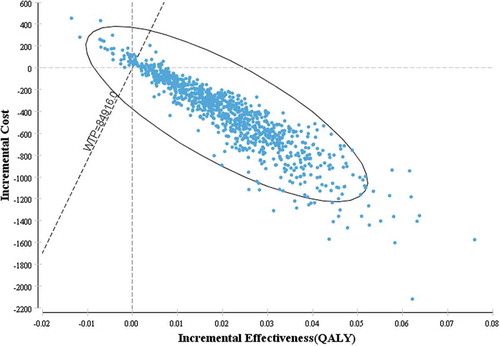
The results of the probabilistic sensitivity analysis are presented as the incremental cost-effectiveness scatter plot in . For 1,000 Monte-Carlo simulations, the incremental cost-effectiveness scatter plot showed that 96% of the incremental cost-effectiveness ratios fell below at a willingness-to-pay threshold of 84,916 CNY, indicating a 96% likelihood of being cost-effective compared to universal vaccination. In addition, 92.8% of the incremental cost-effectiveness ratios accumulated in the fourth quadrant of the cost-effectiveness plane, indicating that the current strategy dominated universal vaccination with increased QALYs and with reduced costs in most case.
Figure 3. Markov model of perinatal infection and progression. A person in any state may die due to the other causes, but a person with decompensated cirrhosis or hepatocellular carcinoma may also die due to HBV infection.
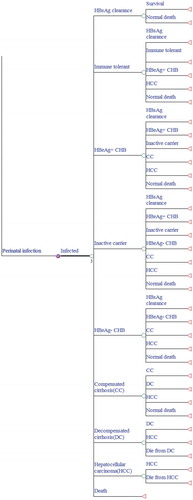
Figure 4. Markov model of hepatitis B virus (HBV) infection and progression. A susceptible individual would follow the HBV infection rate by age (see for the detail formula).
Discussion
China has experienced a significant reduction in perinatal HBV transmission rates and childhood HBV infections since implementing a universal vaccination strategy. Despite this trend, there are still a large number of HBsAg-positive pregnant women, and perinatal transmission has become the predominant mode of HBV transmission. The combination of HBIG and HepB has been confirmed as a more effective preventive intervention, which was reported to further reduce the perinatal breakthrough infections.Citation66,Citation67 Our study demonstrated that augmenting universal hepatitis B vaccination with maternal screening and supplemental use of HBIG for high risk infants continues to be cost saving compared to universal vaccination.
The current strategy was more beneficial with respect to QALYs gained and was less costly, as savings from less disease treatment outweigh the costs of HBsAg testing for pregnant women and HBIG administration to infants. Our results were consistent with and are supported by results from earlier studies. A study conducted in Taiwan found that the combination of maternal screening for HBsAg, HBIG administration to all neonates born to HBsAg carrier mothers was cost-effective at all levels of prevalence.Citation68 A US study showed that the universal vaccination with screening plus HBIG administration remains cost-effective compared to the universal strategy even with a prevalence as low as 0.2% among pregnant women. However, as prevalence decreased, the current strategy became less cost-effective, since the prevalence among pregnant women dropped from 7% to 0.2%, ICER increased from $2,886/QALY saved to $15,552/QALY saved.Citation69 Strategies involving prenatal maternal screening were also shown to be cost-effective compared to vaccination HepB alone in resource-constrained settings.Citation70 These evaluations indicated that augment universal vaccination with immunoglobin treatment, providing HepB plus HBIG to infants born to HBsAg-positive mothers could be cost-effective, even cost saving. Although there was a large variation in the prevalence of HBsAg in pregnant women in these studies (from 0.6% in the US to 7% in Thailand), the robustness of outcomes was not susceptible to the epidemiological status of HBsAg in each country.
Importantly, the current strategy was consistently a cost-saving option in the one-way sensitivity analysis. The direct medical costs of HBV-related diseases had the greatest impact on the results of the one-way sensitivity analysis. The economic effect of the strategy becomes more obvious when the costs increase, which may be related to treatment costs accounting for the majority of the total cost of the strategy. Although increased uptake of interventions is needed to maximize health gains, in our study, uptake of screening and HBIG administration were not large drivers of cost-effectiveness mainly because low rates reduce both the impact and the costs, which scale together. These losses and frailties are also seen in a similar economic evaluation study.Citation71
Our study had both advantages and limitations. One of its major advantages was that the critical parameter inputs in our model, such as the direct medical costs and vaccination costs, were obtained from field surveys conducted in Zhejiang Province, which are more reliable and transparent than sources from the literature. Second, the HepB3 coverage rate, screening rate and HBIG injection rate, which were critical for this model, were obtained from the national surveillance system or the official report on the PMTCT project, which are more reliable and comprehensive than sources used in previous studies.
This study was subject to several limitations. A static rather than a dynamic model was used to simulate the HBV infection rate, static models can only simulate fixed populations, in which the force of infection remains constant and herd-immunity is ignored. Thus, the observed incidence over one year was used to estimate the number of cases as the cohort ages by one year in one discrete markov cycle. We found that a birth cohort that were offered universal infant vaccination with Hep B vaccine and HBIG had a lower incidence of HBV infection compared with a cohort offered universal vaccine only. A dynamic model might be considered more realistic in simulating the transmission of HBV and in capturing the impact of herd immunity.Citation72 However, in endemic areas such as China, which have a high prevalence of HBV carriers, a static model such as the current one is probably sufficient to simulate the predominantly perinatal transmission, even if it may underestimate the impact of vaccination.Citation9 The validity of models and the results rely largely on the availability and reliability of the model parameter inputs, which varied substantially in the literature. However, the main findings remained stable when parameters and assumptions were varied in the sensitive analyses. A probabilistic sensitivity analysis was conduct by applied triangular distributions in the models, while beta and gamma distributions were more recommended. However, a previous study that applied triangular distributions had approved that comparing with studies used other parameter distributions arriving at the same conclusion, and it would not affected the accuracy and reliability of parameters.Citation42 In addition, antiviral prophylaxis can reduce high HBV-DNA loads in pregnant women and reduce the probability of perinatal HBV transmission, which has been reported.Citation65,Citation69 However, due to specific economic and medical system issues, antiviral drugs have not been widely used by Chinese patients, and thus we did not take these drugs into account in our model. Further research may take antiviral prophylaxis for pregnant women into consideration.
In summary, the present study provides important insights into the value of HepB immunization and an understanding of economic evidence. Our results suggest that that maternal screening for HBsAg and HBIG treatment for infants of carrier mothers could be cost saving addition to universal vaccination, and could further reduce HBV-related morbidity and mortality. These results support a recommendation to China’s health policy makers for the continued allocation of central and regional funding for HBV vaccination combined with HBIG for high-risk infants.
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests and that there has been no significant financial support for this work that could have influenced its outcome.
Ethics
The ethics committee of Zhejiang Provincial CDC approved this study. The purpose of the study was explained to the patients, and written informed consent was obtained before the interview.
Acknowledgments
We gratefully acknowledge the efforts made by the health workers from the CDC in 3 cities for their excellent collaboration in conducting this survey.
Additional information
Funding
References
- McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. doi:10.1002/hep.v49.5s.
- Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017;11:490–501. doi:10.1007/s11684-017-0598-4.
- Cui FQ, Zhuang H. Hepatitis B control in China:progress,challenges and strategies. Chin J Viral Dis. 2016;02:81–87.
- Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. 2012;16:e82–8. doi:10.1016/j.ijid.2011.10.009.
- Xu AQ, Zhang L. Immunization strategy of hepatitis B vaccine among adults in China: evidence based-medicine and consideration(in Chinese). Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:473–77. doi:10.3760/cma.j.issn.0253-9624.2016.06.001.
- Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328–36. doi:10.1136/bmj.38719.435833.7C.
- He H, Zhou Y, Xie S. Assessment of the duplicate notifiable reporting of hepatitis B infection in Zhejiang province, China, 2005–2015. Vaccine. 2017;35:4702–06. doi:10.1016/j.vaccine.2017.07.039.
- Zhou Y, He H, Deng X, Yan R, Tang X, Xie S, Yao J. Significant reduction in notification and seroprevalence rates of hepatitis B virus infection among the population of Zhejiang Province, China, aged between 1 and 29years from 2006 to 2014. Vaccine. 2017;35:4355–61. doi:10.1016/j.vaccine.2017.06.078.
- Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine. 2013;31:1864–69. doi:10.1016/j.vaccine.2013.01.020.
- Yin J, Ji Z, Liang P, Wu Q, Cui F, Wang F, Liang X, Zhuang G. The doses of 10 mug should replace the doses of 5 mug in newborn hepatitis B vaccination in China: A cost-effectiveness analysis. Vaccine. 2015;33:3731–38. doi:10.1016/j.vaccine.2015.05.082.
- Chen Y-S, Zheng H, Liu Y-M, Wang F-Z, Wu Z-H, Miao N, Sun X-J, Zhang G-M, Cui F-Q, Liang X-F, et al. Economic evaluation on infant hepatitis B vaccination combined with immunoglobulin in China, 2013. Hum Vaccin Immunother. 2016;12:1838–46. doi:10.1080/21645515.2016.1141845.
- Zhou M, Li Y, Wang H, Zeng X, Wang L, Liu S, Liu YN, Liang XF. Analysis on life expectancy and healthy life expectancy in China, 1990–2015. Chin J Epidemiol. 2016;37:1439–43. doi:10.3760/cma.j.issn.0254-6450.2016.11.001.
- Zhou Y, He H, Deng X, Yan R, Tang X, Xie S. Comparative analysis on actual cost and reasonable cost of expanded program on immunization in Zhejiang. Chin Health Econ. 2018;37:86–89.
- Xin CG. Observation of immune effect of hepatitis B vaccine on newborn babies in linyi city. Occup Health. 2011;27:2540–42.
- Xiong JX, Tang YZ, XIONG XL. Immune effect of two different hepatitis b vaccines. J Med Pest Control. 2013;29:219.
- Zhang YH, Kuang G, Li QQ. Study on immune effect of hepatitis b vaccine. Contemp Med. 2014;28:51.
- Yang SJ, Dong HJ. Meta analysis and systematic review of the effect of hepatitis B immunoglobulin combined with hepatitis B vaccines. Chin J Vaccines Immunization. 2013;19:319–24.
- Zhu GQ. Effect of recombinant hepatitis b vaccine combined with hepatitis b immunoglobulin to block mother-to-child transmission of hepatitis b and related factors. Maternal and Child Health Care China. 2017;32:965–68.
- Yu LP, Chen FF, Wang L, CHEN GJ. Stusy on the effect of combined immunization and passive intervention on mother-to-child transmission of HBV. Chin J Rural Med Pharm. 2014;21:33–34.
- Yao J, Chen YD, Lou B, REN JJ. Evaluation of hepatitis B vaccination with different dose on block ingmother-to-child transmission. Dis Surveillance. 2010;25:981–83.
- Zhang L, Gui XE, Teter C, Zhong H, Pang Z, Ding L, Li F, Zhou Y, Zhang L. Effects of hepatitis B immunization on prevention of mother-to-infant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine. 2014;32:6091–97. doi:10.1016/j.vaccine.2014.08.078.
- Wang F, Zhang G, Zheng H, Miao N, Shen L, Wang F, Dong P, Du F, Chen C, Zhang X, et al. Post-vaccination serologic testing of infants born to hepatitis B surface antigen positive mothers in 4 provinces of China. Vaccine. 2017;35:4229–35. doi:10.1016/j.vaccine.2017.06.019.
- Zhou SY, Bian GL, Ma R, Ni HX, Fang T, Chen XY. A study on immunologic response at different periods with different hepatitis B vaccine in infants. Zhejiang Prev Med. 2015;27:466–68.
- Liu SK, Huang ML, Ge BX, Wang F, HU LL, Hu DB. Observation on immunogenicity of different formulation of hepatitis B vaccine in newborns. Mod Preventive Med. 2014;41:2001–03.
- Zhu FC, Sun KX, Pan HX, Yang ZH, Lu Y, Liang ZL, Liang X-F, Wang F-Z, Zeng Y, Li J, et al. The immunogenicity in healthy infants and efficiency to prevent mother to child transmission of Hepatitis B virus of a 10mug recombinant yeast-derived Hepatitis B vaccine (Hep-KSC). Vaccine. 2016;34:2656–62. doi:10.1016/j.vaccine.2016.04.042.
- Su WJ, Chen SF, Yang CH, Chuang PH, Chang HF, Chang MH. The impact of universal infant hepatitis B immunization on reducing the hepatitis B carrier rate in pregnant women. J Infect Dis. 2019;220(7):1118–26.
- Yan WW, Zhang Y, Lei YF, Wang SL, Li RF. An evaluation of immune persistence of neonates vaccinated with yeast hepatitis b vaccine. Sichuan Med J. 2005;26:1149–50.
- Huang ZY, Zhu JQ, Chen Q, Huang XM, Pi SC, Li DQ. Surveillance on immunization effects of hepatitis B vaccine in 981 infants. Pract Preventive Med. 2008;15:1438–39.
- Wei KP, Zhu FC, Liu JX, Yan L, Lu Y, Zhai XJ, Chang Z-J, Zeng Y, Li J, Zhuang H, et al. The efficacy of two different dosages of hepatitis B immunoglobulin combined with hepatitis B vaccine in preventing mother-to-child transmission of hepatitis B virus: A prospective cohort study. Vaccine. 2018;36:256–63. doi:10.1016/j.vaccine.2017.11.037.
- Lin X, Guo Y, Zhou A, Zhang Y, Cao J, Yang M, Xiao F, Zhang B, Du Y. Immunoprophylaxis failure against vertical transmission of hepatitis B virus in the Chinese population: a hospital-based study and a meta-analysis. Pediatr Infect Dis J. 2014;33:897–903. doi:10.1097/INF.0000000000000315.
- Kang W, Ding Z, Shen L, Zhao Z, Huang G, Zhang J, Xiong Q, Zhang S, Zhang S, Wang F, et al. Risk factors associated with immunoprophylaxis failure against mother to child transmission of hepatitis B virus and hepatitis B vaccination status in Yunnan province, China. Vaccine. 2014;32:3362–66. doi:10.1016/j.vaccine.2014.04.045.
- Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105:94–98. doi:10.1093/oxfordjournals.aje.a112370.
- Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–74. doi:10.1056/NEJM197504102921503.
- zhang S, Ma Q, Liang S, Xiao H, Zhuang G, Zou Y, Tan H, Liu J, Zhang Y, Zhang L, et al. Annual economic burden of hepatitis B virus‐related diseases among hospitalized patients in twelve cities in China. J Viral Hepat. 2016;23:202–10. doi:10.1111/jvh.2016.23.issue-3.
- Jin H, Wang B, Gao Q, Chao J, Wang S, Tian L, Liu P. Comparison between EQ-5D and SF-6D utility in Rural residents of Jiangsu Province, China. PLoS One. 2012;7:e41550. doi:10.1371/journal.pone.0041550.
- Xiao M, Wang H, Zhang W, Pang XH. Cost-effectiveness analysis of hepatitis B vaccination strategy for high risk adults in Beijing. Chin J Dis Control Prev. 2015;19:730–34.
- Jia YX, Cui FQ, Li L, Zhang DL, Zhang GM, Wang FZ, Gong XH, Zheng H, Wu ZH, Miao N, et al. Comparison between the EQ-5D-5L and the EQ-5D-3L in patients with hepatitis B. Qual Life Res. 2014;23:2355–63. doi:10.1007/s11136-014-0670-3.
- Zhou MG, Li YC, Wang HD, Zeng XY, Wang LJ, Liu SW, Liu YN, Liang XF. Analysis on life expectancy and healthy life expectancy in China, 1990–2015. Chin J Epidemiol. 2016;37:1439–43. doi:10.3760/cma.j.issn.0254-6450.2016.11.001.
- Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28:2356–59. doi:10.1016/j.vaccine.2009.06.035.
- Ji. ZH. The cost-effectivness analysis of hepatitis B immunization strategy in Chinese newborns. Xi’an Xi’an Jiaotong University; 2012.
- Zhang Y, Zhang H, Elizabeth A, Liu XQ. Epidemiology of hepatitis B and associated liver diseases in china. Chin Med Sci Jl. 2012;27:243–48. doi:10.1016/S1001-9294(13)60009-7.
- Jia Y, Li L, Cui F, Zhang D, Zhang G, Wang F, Gong X, Zheng H, Wu Z, Miao N, et al. Cost-effectiveness analysis of a hepatitis B vaccination catch-up program among children in Shandong Province, China. Hum Vaccin Immunother. 2014;10:2983–91. doi:10.4161/hv.29944.
- Huifang H, Chen H. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: an experience from Taiwan with high hepatitis B virus infection and Hepatitis B e Antigen positive prevalence. Vaccine. 2009;27:6770–76. doi:10.1016/j.vaccine.2009.08.082.
- Yang PC. Development of markov models for hepatitis B vaccination or treatment and cost-effectiveness analysis of prophylactic entecavir use for population based-on community in China Guangdong pharmaceutical university. 2016.
- Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310:974–76. doi:10.1001/jama.2013.276701.
- Locarnini S, Hatzakis A, Chen DS, Lok A. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J Hepatol. 2015;62:S76–86. doi:10.1016/j.jhep.2015.01.018.
- Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201.
- Hong SJ, Park HJ, Chu MA, Choi BS, Choe BH. The rate of conversion from immune-tolerant phase to early immune-clearance phase in children with chronic hepatitis B virus infection. Pediatr Gastroenterol Hepatol Nutr. 2014;17:41–46. doi:10.5223/pghn.2014.17.1.41.
- Wong WW, Woo G, Heathcote EJ, Krahn M. Disease burden of chronic hepatitis B among immigrants in Canada. Can J Gastroenterol. 2013;27:137–47. doi:10.1155/2013/924640.
- Lim TH, Gane E, Moyes C, Borman B, Cunningham C. Serological and clinical outcomes of horizontally transmitted chronic hepatitis B infection in New Zealand Maori: results from a 28-year follow-up study. Gut. 2014;64:966–72. doi:10.1136/gutjnl-2013-306247.
- Geier A, Gartung C, Dietrich CG. Hepatitis B e antigen and the risk of hepatocellular Carcinoma. N Engl J Med. 2002;347:1721.
- Chen JG, Lu JH, Zhu YR, Zhu J, Zhang YH. A thirty-one year prospective follow-up program on the HBsAg carrier state and primary liver cancer in Qidong, China. Chin J Epidemiol. 2010;31:721–26.
- Yan HJ. Long-term efficacy of hepatitis b vaccine and HBeAg seroconversion during chroric infection. Shanghai: Fudan University; 2013.
- Chen CR. The predictive factors of spontaneous surface antigen loss in chronic hepatitis B virus carriers. Xiamen: Xiamen University; 2013.
- Barbosa C, Smith EA, Hoerger TJ, Fenlon N, Schillie SF, Bradley C, Murphy TV. Cost-effectiveness analysis of the national perinatal hepatitis B prevention program. Pediatrics. 2014;133:243–53. doi:10.1542/peds.2013-0718.
- Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun C, Liaw Y, Chen C, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2014,19(1):71–73. doi:10.1053/j.gastro.2010.01.042.
- Ren TT, Xu GH, Li CX. A natural history of HBsAg inactive carrier. Chin Hepatol. 2014;71–73.
- Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Wang LY, You S, Iloeje UH, Chen C. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139:474–82. doi:10.1053/j.gastro.2010.04.048.
- Liu J, Lee MH, Batrlautermann R, Jen CL, Iloeje UH, Lu SN, Wang L-Y, You S-L, Hsiao CK, Yang H-I, et al. A predictive scoring system for the seroclearance of HBsAg in HBeAg-seronegative chronic hepatitis B patients with genotype B or C infection. J Hepatol. 2013;58:853–60. doi:10.1016/j.jhep.2012.12.006.
- Kwak MS, Cho EJ, Jang ES, Lee JH, Yu SJ, Kim YJ, Yoon J-H, Lee H-S. Predictors of HBsAg seroclearance in HBeAg-negative chronic hepatitis B patients. Digestion. 2011;84:23–28. doi:10.1159/000333211.
- Kobayashi M, Hosaka T, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Kawamura Y, Kobayashi M, Saitoh S, Arase Y, et al. Seroclearance rate of hepatitis B surface antigen in 2,112 patients with chronic hepatitis in Japan during long-term follow-up. J Gastroenterol. 2014;49:538–46. doi:10.1007/s00535-013-0821-2.
- Tseng TC, Liu CJ, Chen CL, Yang HC, Su TH, Wang CC, Yang W-T, Kuo SFT, Liu C-H, Chen P-J, et al. Risk stratification of hepatocellular carcinoma in hepatitis B virus e antigen-negative carriers by combining viral biomarkers. J Infect Dis. 2013;208:584–93. doi:10.1093/infdis/jit209.
- Thiele M, Gluud LL, Fialla AD, Dahl EK, Krag A. Large variations in risk of hepatocellular carcinoma and mortality in treatment naïve hepatitis B patients: systematic review with meta-analyses. PLoS One. 2014;9:e107177. doi:10.1371/journal.pone.0107177.
- Zeng Y, Luo M, Chen J, He H, Deng X, Xie S, Fang Y. An economic evaluation of the current measles vaccination program: A case study in Zhejiang Province, east China. Vaccine. 2019;37:3071–77. doi:10.1016/j.vaccine.2019.04.057.
- Lee D, Shin HY, Park SM. Cost-effectiveness of antiviral prophylaxis during pregnancy for the prevention of perinatal hepatitis B infection in South Korea. Cost Eff Resour Allocation. 2018;16:2–11. doi:10.1186/s12962-018-0088-9.
- Stevens CE, Toy P, Kamili S, Taylor PE, Tong MJ, Xia GL, Vyas GN. Eradicating hepatitis B virus: the critical role of preventing perinatal transmission. Biologicals. 2017;50:3–19. doi:10.1016/j.biologicals.2017.08.008.
- Wen WH, Lai MW, Chang MH. A review of strategies to prevent mother-to-infant transmission of hepatitis B virus infection. Expert Rev Gastroenterol Hepatol. 2016;10:317–30. doi:10.1586/17474124.2016.1120667.
- Chen SC, Toy M, Yeh JM, Wang JD, Resch S. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobin treatment. Pediatrics. 2013;131:e1135–43. doi:10.1542/peds.2012-1262.
- Fan L, Owusu-Edusei K Jr., Schillie SF, Murphy TV. Cost-effectiveness of active-passive prophylaxis and antiviral prophylaxis during pregnancy to prevent perinatal hepatitis B virus infection. Hepatology. 2016;63:1471–80. doi:10.1002/hep.v63.5.
- Devine A, Harvey R, Min AM, Gilder MET, Paw MK, Kang J, Watts I, Hanboonkunupakarn B, Nosten F, McGready R, et al. Strategies for the prevention of perinatal hepatitis B transmission in a marginalized population on the Thailand-Myanmar border: a cost-effectiveness analysis. BMC Infect Dis. 2017;17:552. doi:10.1186/s12879-017-2660-x.
- Nayagam S, Conteh L, Sicuri E, Shimakawa Y, Suso P, Tamba S, Njie R, Njai H, Lemoine M, Hallett TB, et al. Cost-effectiveness of community-based screening and treatment for chronic hepatitis B in The Gambia: an economic modelling analysis. Lancet Global Health. 2016;4:E568–E78. doi:10.1016/S2214-109X(16)30101-2.
- Kim SY, Goldie SJ. Cost-effectiveness analyses of vaccination programmes: a focused review of modelling approaches. Pharmacoeconomics. 2008;26:191–215. doi:10.2165/00019053-200826030-00004.