ABSTRACT
Pregnant women and infants are at high risk for severe influenza and many countries, including France, recommend annual influenza immunization during pregnancy. We aimed to estimate influenza vaccination and refusal rates and assess associated factors among pregnant women during the 2015–16 season in France. We used data from a national representative sample of women who gave birth in March 2016 and were interviewed before hospital discharge (N = 11,752). In the multivariable analysis, robust Poisson regression models were used to study associations with maternal characteristics and prenatal care characteristics. Influenza vaccine coverage among pregnant women was 7.4% (95% confidence interval [CI]: 6.9–7.9). Only 24.9% (95% CI: 24.2–25.7) of women said that they received a care provider proposal for vaccination and 70.4% (95% CI: 68.7–72.0) of these declined it. Vaccine uptake was associated with low parity (prevalence ratio [PR] = 2.1; 95% CI: 1.4–3.2 for parity 0 vs ≥ 3), high educational level (PR = 2.5; 95% CI: 2.0–3.2), healthcare occupation during pregnancy (PR = 1.8; 95% CI: 1.5–2.1) and preexisting conditions at risk for influenza (PR = 1.7; 95% CI: 1.3–2.2). Women were more frequently vaccinated when their main care provider was a general practitioner. Multiparae women and those with medium or low educational level were significantly more likely than others to decline influenza vaccine after a provider proposal. Influenza vaccine coverage is very low in France, mainly because of infrequent care provider proposals and also frequent women’s refusals. Effective interventions should be designed to promote vaccination among medical professionals and reduce vaccine hesitancy among pregnant women.
Introduction
Pregnant women and their newborns are at high risk for severe influenza-related complications.Citation1-Citation4 Influenza infection during pregnancy is associated with adverse obstetric and fetal outcomes, such as preterm birth or fetal death.Citation5,Citation6 In addition, pregnant women are at increased risk of hospitalization for complications as compared with non-pregnant women, especially those with underlying conditions.Citation7,Citation8
Vaccination against influenza can prevent harm and benefit both women and newborns by reducing the risk of influenza-associated complications and hospitalizations.Citation9-Citation11 It could also prevent influenza illness in infants under age 6 months because of the transplacental antibody transmission provided by maternal immunization.Citation12-Citation16 No evidence of adverse pregnancy outcomes after maternal influenza vaccination was reported from systematic reviews and vaccine safety studies, although available safety data are limited, especially for vaccination during the first trimester of pregnancy.Citation17-Citation20
Since 2012, the World Health Organization (WHO) recommendations have identified pregnant women as a priority group for national influenza immunization programs,Citation21 and almost all European countries recommend vaccination against influenza during pregnancy. The same year, French health authorities recommended seasonal influenza vaccination for all women who are pregnant during the influenza season, regardless of the trimester of pregnancy.Citation22 In France, the national health insurance reimburses the cost of seasonal influenza vaccine for high-risk groups identified in national recommendations, including pregnant women.Citation23 Each year, the national public health agency (Santé publique France) monitors the seasonal influenza epidemic and vaccination coverage. However, influenza vaccine coverage among pregnant women is poorly known at the national level.Citation24
Several studies have found vaccination uptake during pregnancy associated with maternal characteristics and the attitude of both women and healthcare providers playing a major role.Citation25-Citation29 However, most of these studies focused on the 2009 influenza A (H1N1) pandemic and were performed in North America.Citation30 Available data on seasonal influenza vaccine coverage are scarce in Europe,Citation31 including France.Citation32,Citation33
We aimed to estimate the seasonal influenza vaccine coverage among pregnant women in mainland France for the 2015–16 season and assess factors associated with vaccine uptake and refusal by using data from the National Perinatal Survey performed in 2016.
Methods
Survey methods and participants
The National Perinatal Surveys have been conducted periodically since 1995 and aim to monitor perinatal health, providing data on a wide range of topics. They cover all live births and stillbirths with gestational age at least 22 weeks or birth weight at least 500 g during one week in March in all public and private maternity units. Data are obtained from two sources: (1) an interview with mothers in the postpartum ward before discharge to collect information on maternal characteristics and prenatal care and (2) medical records for complications of pregnancy, delivery, and the newborn’s health status. More details about method, data collection and women’s characteristics are described elsewhere.Citation34
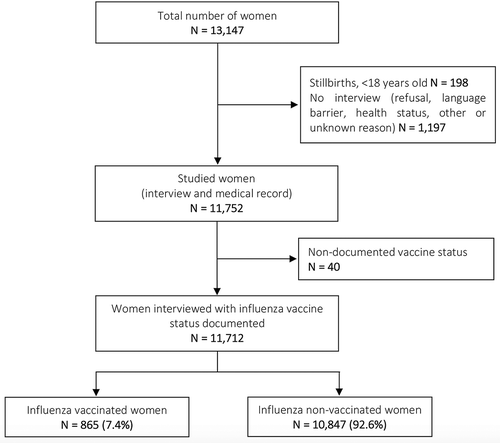
Ethical approval was granted by the French Data Protection Authority (CNIL, authorization 915197) and the INSERM ethics committee (authorization IRB00003888, no. 14–191). In mainland France, 13,147 women delivered during the studied week (March 14–20, 2016). Women were not contacted if they were < 18 years old or had a stillbirth (N = 198). In addition, 1,197 women were not interviewed mainly because of refusal (N = 548), a language barrier (N = 245) and poor health status (N = 144). Finally, 11,752 women were interviewed and influenza vaccine uptake was known for most of them (99.7%, N = 11,712) ().
Studied variables and statistical analysis
Women in mainland France were asked whether they had been vaccinated against seasonal influenza during the influenza vaccine campaign. If the answer was yes, they were asked the qualification of the prescriber (obstetrician-gynecologist [ob-gyn], midwife, general practitioner [GP]) and if no, they were asked why, among the following reasons: “nobody offered it”, “it was offered but I refused”, and “other reason” (please specify). We distinguished several categories of determinants of vaccine uptake: maternal socio-demographic and medical characteristics, and prenatal care. The socio-demographic characteristics included age, parity, family status (cohabiting/single), educational level, country of birth and complementary health insurance. Two broad categories of insurance are used in France for additional costs that are not covered by the national health insurance (e.g., additional cost of medical visits): public insurance for low-income people and private insurance. We also classified women according to their occupation and belonging to priority groups for vaccination: healthcare workers (e.g., doctors, midwives, nurses and nurses’ aides), women in contact with children (e.g., teachers in primary school and daycare staff), women with other occupations and women who were not working during pregnancy.
The medical characteristics collected were the following: (1) preexisting conditions at risk for severe influenza infection (e.g., diabetes, chronic respiratory or kidney disease, immunocompromised state, severe cardiovascular disease, chronic liver disease, and body mass index ≥ 40 kg/m2), (2) other preexisting maternal conditions requiring specialized surveillance by an ob-gyn according to the national medical guidelines,Citation35 and (3) complications during pregnancy requiring hospitalization. The prenatal care characteristics collected were the number of visits and the qualification of the main care provider. Number of prenatal visits were classified according to the recommended calendar of visits and the length of gestation,Citation35 as low (<110% of the minimum expected number), average (110-119%), high (120-149%) and very high (150% or more). Women with no complications can choose care by an ob-gyn, midwife or GP. Complications requiring care by an ob-gyn or hospitalization, care by an ob-gyn or midwife and a high number of visits were considered indicators of numerous contacts with care givers and/or opportunities to follow recommendations for pregnant women.
In our analysis, we first estimated the influenza vaccine coverage during the 2015–16 influenza season and studied factors associated with vaccine uptake. Second, we described the vaccine uptake by qualification of the prescriber (ob-gyn, midwife, GP) and qualification of the main care provider. Finally, we studied factors associated with vaccine refusal. Because we had to choose the groupings for some women’s responses, we repeated the analysis of vaccine refusal by changing these choices to verify the stability of the results.
Univariate and multivariable analyses were conducted by using robust Poisson regression with a robust error variance.Citation36 All statistical analyses were performed with Stata/IC v15.0 (StataCorp LP, College Station, TX). P < .05 was considered statistically significant, and prevalence ratios (PRs) are displayed with their 95% confidence intervals (95% CIs). Missing data were low for maternal and prenatal care characteristics (<1.5%). Women with missing data were excluded in multivariable models.
Results
Inluenza vaccine coverage and determinants
Influenza vaccine coverage among pregnant women was 7.4% (n = 865/11712; 95% CI: 6.9–7.9). shows the maternal characteristics associated with influenza vaccine uptake. Coverage was higher for women with age 30–34 years, low parity, preexisting conditions at risk for flu, and high level of education, birth in France, a healthcare occupation, and private complementary health insurance. Pregnant women were more frequently vaccinated when their main care provider was a GP than other care provider. In the multivariable model, influenza vaccination was mainly associated with maternal age (≥ 30 years old), low parity (< 3 children), preexisting conditions at risk for severe influenza infection, high educational level (college and postgraduate) and maternal occupation related to healthcare (all p < .001). The adjusted PR was 2.5 (95% CI: 2.0–3.2) with a postgraduate education versus middle school level or lower education, 2.1 (95% CI: 1.4–3.2) for nulliparity and 1.7 (95% CI: 1.2–2.6) with 1 or 2 previous children compared to other women.
Table 1. Influenza vaccine uptake and associated factors among women in mainland France, 2015–2016 season
For the first 6 months of pregnancy, the main care providers were an ob-gyn (n = 574, 68%), midwife (n = 170, 20%) and GP (n = 72, 9%) (). The qualification of the influenza vaccine prescriber was often similar to that of the main care provider. However, in each group of care providers, GPs prescribed the influenza vaccine very often.
Table 2. Distribution of prescribers by main care provider during the first six months of pregnancya.
Non-vaccinated women and factors associated with influenza vaccine refusal
Among the 10,847 non-vaccinated women, 8,172 reported receiving no provider offer, 1,861 refused to be vaccinated after a proposal, 195 reported other reasons for not being vaccinated and 619 did not specify why they did not get vaccinated. The other reasons were beliefs that the vaccine is not necessary or not a priority (n = 57/195), lack of knowledge and lack of awareness about influenza and/or the vaccine (n = 44/195) and medical exemptions (n = 17/195). We hypothesized that women who did not specify why they were not vaccinated did not receive a provider offer and that women who were not vaccinated because of other reasons had an offer but were not in favor of the vaccination and refused it. Therefore, the overall rate of vaccine offers was 24.9% (95% CI: 24.2–25.7).
Vaccine refusal was estimated to be a fraction of the total estimation of women receiving a provider offer, grouped as vaccinated women, women who refused vaccination after a proposal and those reporting other reasons. Thus, 70.4% (n = 2056/2921; 95% CI: 68.7–72.0) of women with an offer refused vaccination. presents the vaccine refusal data according to maternal characteristics. Refusal was frequent among women aged < 25 years, who were multiparae, did not have a post-graduate education and who were not working during pregnancy. In the multivariable analysis including socio-demographic and medical characteristics, number of prenatal visits and qualification of the main care provider, only multiparity and less than postgraduate education were associated with vaccine refusal (both p < .001).
Table 3. Vaccine refusal rates according to maternal characteristics in mainland France, 2015–2016 season
We then considered that the proposal of vaccination was unknown for women who did not give the reasons for lack of vaccination and that the reasons for non-vaccination other than non-proposal and refusal were not close to refusal and could not be included in this category. Under these hypotheses, the overall rate of vaccine offer was 25.0% and the refusal rate was 68.3%. The associations of refusal with the studied determinants were very similar to those found in (results not shown).
Discussion
During the 2015–2016 influenza vaccine campaign, the influenza vaccine coverage among pregnant women in France was low, but only about a quarter of pregnant women received a care-provider offer of vaccination (24.9%), and most declined vaccination after receiving the proposal (70.4%). These results highlight the critical role of care providers in the vaccine uptake and prominent hesitancy surrounding influenza vaccine acceptance during pregnancy, especially in women with high parity and low educational level.
The main strength of our study is the use of a large national sample of births. The overall sample (all births during 1 week in March) is representative of annual births in France.Citation34 The proportion of women who agreed to be interviewed was very high. Furthermore, the study design was optimal to assess the vaccine coverage because all women were exposed during pregnancy to both the national influenza vaccination campaign (from mid-October to late January) and the wave of seasonal influenza epidemic (from early February to late April 2016).Citation37 Finally, the NPS provides a wide range of data on maternal sociodemographic characteristics, chronic diseases and complications, and characteristics of prenatal care that could be used to assess the determinants of vaccine uptake.
Nevertheless, our results are subject to several limitations. First, because the NPS covers many topics, we could not focus on attitudes, knowledge and perceptions of pregnant women toward influenza vaccination and explore further misconceptions or barriers to vaccination acceptance. Secondly, women who did not participate may be slightly different from the overall sample; for instance, we found underrepresentation of non-French women (18.7% in our study vs 22.2% in the vital statistics),Citation38 probably because of language difficulties. Finally, some women may have forgotten the vaccination; however, the under-reporting is probably low because vaccination requires special procedures for most women (e.g., vaccine picked up from a pharmacy and administration by a doctor or nurse).
Despite the optimal period of pregnancy covered by our sample, the vaccination coverage was very low in France as compared with that described in other population-based studies – 54% in the United States,Citation39 45% in Flanders (Belgium),Citation40 42% in EnglandCitation41 and 23% in GermanyCitation42 – although methods of data collection varied in these studies.
Our results suggest that both care providers and women play a major role in the situation in France. Their respective roles might be slightly biased because we did not directly ask every woman whether her care providers offered vaccination and whether she was against vaccination. We may have slightly underestimated the proportion of women receiving a care-provider offer of vaccination, because some women may have forgotten the proposal. In addition, the care provider offers may be slightly underestimated, and consequently refusal may be slightly underestimated if providers sometimes refrain from recommending or offering vaccination when they already know the adverse opinion of pregnant women regarding influenza vaccination. However, our results were similar when we excluded women who did not know why they were not vaccinated. The rates of provider offers and women’s refusal are very different from those found in other countries and cannot be explained by our questionnaire alone; for instance, in the 2016–17 season in the United States, about three quarters of women who were offered vaccination were vaccinated.Citation39
In France, the very low vaccine coverage among pregnant women and low attention paid to vaccination by both care providers and women could be explained in part by the fact that national recommendations were relatively recent (3 years at the time of the survey), whereas longer lead times are needed to observe changes in immunization coverage.Citation25,Citation43 Furthermore, unlike older people and other at-risk groups of severe influenza infection, pregnant women do not receive a voucher for vaccination by the national health insurance, and in 2015–2016 they were not specifically targeted by national media campaigns. However, vaccine coverage for the 2015–2016 influenza season was also low among other at-risk groups: 51% for people ≥ 65 years old and 39% for those < 65 years old with preexisting conditions at risk for severe influenzaCitation44 as compared with the national target of at least 75% for both groups. These low coverage rates result from controversies surrounding the pandemic 2009 influenza A (H1N1) national vaccination campaign for which vaccination coverage was low among people ≥ 65 years old (10.4%) and pregnant women (29.3%).Citation24,Citation33 More generally, the French population, including healthcare workers, has a strong negative attitude to vaccines, with strong skepticism about their safety and effectiveness, especially the influenza vaccine.Citation45-Citation47 This attitude may be reinforced during pregnancy in that mothers are very concerned for their newborn’s safety regarding medication,Citation30,Citation48 and could be unaware of the severity of influenza infection and the risks associated with influenza for them and their newborn,Citation28 may be because of the rareness of severe infections in both groups.Citation49
Our results on the determinants of vaccination and vaccine refusal show that some women have better vaccination coverage and a higher rate of acceptance than other women, mainly nulliparae women, women with a very high education level, women with preexisting conditions at risk for influenza complications and healthcare workers. Other studies of vaccination coverage found some of these maternal characteristicsCitation28,Citation33,Citation39 and they are well-known determinants of other maternal preventive behavior. For instance, it is known that nulliparous women pay more attention to prevention during pregnancy than women with higher parity.Citation50,Citation51 In addition, multiparae women who could benefit from the cumulative effect of experience and information gained by repeated contacts with care providers often did not receive a vaccine recommendation during previous pregnancies because guidelines for influenza vaccination were recent. However, vaccine coverage never exceeded 15%, including in women with significant co-morbidities or pregnancy complications, which suggests that any campaign or public policy in France should reach the overall population of pregnant women.
Other factors in our survey provide information on the potential role of the health system. First, we found that vaccine coverage was not associated with insurance coverage, which suggests that financial barriers do not contribute significantly to differences in vaccination rates. Second, we found that GPs have a role in the vaccine uptake and management of seasonal influenza: the vaccine coverage was higher when GPs were the main care provider and GPs frequently prescribed the vaccine, even if they were not the main care provider for this pregnancy. However, less than 10% of the women said that their main care provider was a GP. Our results underline the role of family physicians in vaccine coverage but also the low adherence of the main care providers to recommendations, but these professionals examine the women at least every month during pregnancy, and they could have a strong influence on vaccine uptake, discussing and providing relevant information and clear recommendations, as it was pointed out in many studies.Citation25,Citation28,Citation30,Citation52
Seasonal influenza vaccination among pregnant women illustrates the difficulties in implementing new public health policies, especially routine immunization programs. Several systematic reviews have identified strategies and interventions that may help to improve vaccine coverage during pregnancy.Citation29,Citation53 The most effective strategies would be (1) communication approaches targeting pregnant women, emphasizing scientific knowledge on the effectiveness and safety of vaccination for both the mother and baby, (2) education and training of healthcare providers to help ensure that they have the best knowledge to apply recommendations and the best information tools to promote them to pregnant women, (3) extended access to vaccination by enhancing procurement and vaccine storage in care-provider offices and maternity units and (4) implementation of alerts about vaccination on electronic medical records. In this perspective, a multicomponent intervention implemented in a maternity unit in Paris, including information given to pregnant women during prenatal visits, on-site vaccination, and active involvement of antenatal care providers, resulted in a significant increase in immunization coverage.Citation54
Conclusion
Three years after the release of national recommendations, seasonal influenza vaccine uptake among pregnant women was very low in mainland France. Significant efforts are needed to promote influenza immunization during pregnancy by improving the dissemination of information on its benefits but also implementing effective interventions, targeting both pregnant women and healthcare providers. In this perspective, research is necessary to better understand barriers to vaccination, especially factors influencing vaccine acceptance during pregnancy.
Disclosure of potential conflicts of interests
The authors declare that they have no competing interests.
Acknowledgments
The authors thank the department heads who agreed to perform the survey in their unit, the investigators and all the women who agreed to participate.
Additional information
Funding
References
- Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–102. doi:10.1093/oxfordjournals.aje.a009587.
- Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012 Sep;207(3):S3–8. doi:10.1016/j.ajog.2012.06.068.
- Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, Likos AM, Posey DL, Klimov A, Lindstrom SE, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353(24):2559–67. doi:10.1056/NEJMoa051721.
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–58. doi:10.1016/S0140-6736(09)61304-0.
- Skowronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine. 2009;27:4754–70. doi:10.1016/j.vaccine.2009.03.079.
- Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008 Jan;8(1):44–52. doi:10.1016/S1473-3099(07)70311-0.
- Lindsay L, Jackson LA, Savitz DA, Weber DJ, Koch GG, Kong L, Guess HA. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol. 2006;163(9):838–48. doi:10.1093/aje/kwj095.
- Dodds L, McNeil SA, Fell DB, Allen VM, Coombs A, Scott J, MacDonald N. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176(4):463–68. doi:10.1503/cmaj.061435.
- Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355–61. doi:10.1086/652986.
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–64. doi:10.1056/NEJMoa0708630.
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018;2(CD001269). doi:10.1002/14651858.CD001269.pub6.
- Steinhoff MC, Omer SB. Influenza immunization in pregnancy : antibody responses in mothers and infants. N Engl J Med. 2010;362(17):1644–46. doi:10.1056/NEJMc0912599.
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, Adrian PV, van Niekerk N, Treurnicht F, Ortiz JR, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371(10):918–31. doi:10.1056/NEJMoa1401480.
- Tapia MD, Sow SO, Tamboura B, Tégueté I, Pasetti MF, Kodio M, Onwuchekwa U, Tennant SM, Blackwelder WC, Coulibaly F, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16(9):1026–35. doi:10.1016/S1473-3099(16)30054-8.
- Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, Stewart L, Mullany LC, Chu HY, LeClerq SC, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17(9):981–89. doi:10.1016/S1473-3099(17)30252-9.
- Thompson MG, Kwong JC, Regan AK, Katz MA, Drews SJ, Azziz-Baumgartner E, Klein NP, Chung H, Effler PV, Feldman BS, et al. Influenza vaccine effectiveness in preventing influenza-associated hospitalizations during pregnancy: a multi-country retrospective test negative design study, 2010–2016. Clin Infect Dis. 2019 Apr 24;68(9):1444–53. doi:10.1093/cid/ciy737.
- Tamma PD, Ault KA, Del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009 Dec;201(6):547–52. doi:10.1016/j.ajog.2009.09.034.
- Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr. 2014;164(5). doi:10.1016/j.jpeds.2014.01.037.
- Loubet P, Kerneis S, Anselem O, Tsatsaris V, Goffinet F, Launay O. Should expectant mothers be vaccinated against flu? A safety review. Expert Opin Drug Saf. 2014;13(12):1709–20. doi:10.1517/14740338.2014.977252.
- Moro P, Baumblatt J, Lewis P, Cragan J, Tepper N, Cano M. Surveillance of adverse events after seasonal influenza vaccination in pregnant women and their infants in the vaccine adverse event reporting system, July 2010–may 2016. Drug Saf. 2017;40(2):145–52. doi:10.1007/s40264-016-0482-1.
- World Health Organization. Vaccines against influenza WHO position paper – november 2012. Wkly Epidemiol Rec. 2012;47(87):461–76.
- Haut Conseil de la Santé publique (HCSP). Vaccination contre la grippe saisonnière. Actualisation des recommandations : femmes enceintes et personnes obèses, 2012 (French). [accessed 2019 Nov]. https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=260
- Ministère des Affaires Sociales et de la Santé. Calendrier des vaccinations et recommandations vaccinales, 2019 (French). [accessed 2019 Nov]. https://solidarites-sante.gouv.fr/prevention-en-sante/preserver-sa-sante/vaccination/calendrier-vaccinal
- Vaux S, Van Cauteren D, Guthmann J-P, Le Strat Y, Vaillant V, de Valk H, Lévy-Bruhl D. Influenza vaccination coverage against seasonal and pandemic influenza and their determinants in France: a cross-sectional survey. BMC Public Health. 2011;11(1):30. doi:10.1186/1471-2458-11-30.
- Blanchard-Rohner G, Meier S, Ryser J, Schaller D, Combescure C, Yudin MH, Burton-Jeangros C, de Tejada BM, Siegrist C-A. Acceptability of maternal immunization against influenza: the critical role of obstetricians. J Matern Neonatal Med. 2012;25(9):1800–09. doi:10.3109/14767058.2012.663835.
- Yudin MH, Salaripour M, Sgro MD. Pregnant women’s knowledge of influenza and the use and safety of the influenza vaccine during pregnancy. J Obstet Gynaecol Canada. 2009;31(2):120–25. doi:10.1016/S1701-2163(16)34095-6.
- Mak DB, Regan AK, Joyce S, Gibbs R, Effler PV. Antenatal care provider’s advice is the key determinant of influenza vaccination uptake in pregnant women. Aust New Zeal J Obstet Gynaecol. 2015;55(2):131–37. doi:10.1111/ajo.12292.
- Yuen CYS, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – A systematic review. Vaccine. 2014 Aug;32(36):4602–13. doi:10.1016/j.vaccine.2014.06.067.
- Wong VWY, Lok KYW, Tarrant M. Interventions to increase the uptake of seasonal influenza vaccination among pregnant women: A systematic review. Vaccine. 2016 Jan;34(1):20–32. doi:10.1016/j.vaccine.2015.11.020.
- Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. 2015;33(47):6420–29. doi:10.1016/j.vaccine.2015.08.046.
- Jorgensen P, Mereckiene J, Cotter S, Johansen K, Tsolova S, Brown C. How close are countries of the WHO European region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018 Jan;36(4):442–52. doi:10.1016/j.vaccine.2017.12.019.
- Freund R, Le Ray C, Charlier C, Avenell C, Truster V, Tréluyer JM, Skalli D, Ville Y, Goffinet F, Launay O, et al. Determinants of non-vaccination against pandemic 2009 H1N1 influenza in pregnant women: A prospective cohort study. PLoS One. 2011;6(6):e20900. doi:10.1371/journal.pone.0020900.
- Blondel B, Mahjoub N, Drewniak N, Launay O, Goffinet F. Failure of the vaccination campaign against A(H1N1) influenza in pregnant women in France: results from a national survey. Vaccine. 2012;30(38):5661–65. doi:10.1016/j.vaccine.2012.06.077.
- Blondel B, Coulm B, Bonnet C, Goffinet F, Le Ray C. Trends in perinatal health in metropolitan France from 1995 to 2016: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46(10):701–13. doi:10.1016/j.jogoh.2017.09.002.
- Haute Autorité de Santé (HAS). Suivi et orientation des femmes enceintes en fonction des situations à risque identifiés, 2016 (French). [accessed 2019 Nov]. https://www.has-sante.fr/jcms/c_547976/fr/suiviet-orientation-des-femmes-enceintes-en-fonction-des-situations-a-risque-identifi
- Zou G, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013 Dec 8;22(6):661–70. doi:10.1177/0962280211427759.
- Équipes de surveillance de la grippe. Surveillance de la grippe en France métropolitaine, saison 2015–2016. Bull Epidémiologique Hebd. 2016;32–33:558–63.
- INSEE. Les naissances en 2016. INSEE Résultats, Paris; 2017 (French). [accessed 2019 Nov]. https://www.insee.fr/fr/statistiques/2904779?sommaire=2898646
- Ding H, Black CL, Ball S, Fink RV, Williams WW, Fiebelkorn AP, Lu P-J, Kahn KE, D’Angelo DV, Devlin R, et al. Influenza vaccination coverage among pregnant women — United States, 2016–17 influenza season. MMWR Morb Mortal Wkly Rep. 2017;66(38):1016–22. doi:10.15585/mmwr.mm6638a2.
- Maertens K, Braeckman T, Top G, Van Damme P, Leuridan E. Maternal pertussis and influenza immunization coverage and attitude of health care workers towards these recommendations in Flanders, Belgium. Vaccine. 2016;34(47):5785–91. doi:10.1016/j.vaccine.2016.09.055.
- Public Health England (PHE). Influenza immunisation programme for England: GP patient groups data collection survey season 2015 to 2016, 2016. [accessed 2019 Nov]. https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-gp-patients-in-england-winter-season-2015-to-2016
- Bödeker B, Walter D, Reiter S, Wichmann O. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine. 2014;32(33):4131–39. doi:10.1016/j.vaccine.2014.06.007.
- Le Maréchal M, Agrinier N, Fressard L, Verger P, Pulcini C. Low uptake of meningococcal c vaccination in France: a cross-sectional nationwide survey of general practitioners’ perceptions, attitudes and practices. Pediatr Infect Dis J. 2017 Jul;36(7):e181–8. doi:10.1097/INF.0000000000001553.
- Santé publique France. Couverture vaccinale grippe par saison et dans chaque groupe d’âge (French). [accessed 2019 Nov]. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-grippe-par-groupe-d-age
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- Rey D, Fressard L, Cortaredona S, Bocquier A, Gautier A, Peretti-Watel P, Verger P. Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk–benefit balance. Eurosurveillance. 2018 Apr 26;23(17). doi:10.2807/1560-7917.ES.2018.23.17.17-00816.
- Raude J, Fressard L, Gautier A, Pulcini C, Peretti-Watel P, Verger P. Opening the ‘vaccine hesitancy’ black box: how trust in institutions affects French GPs’ vaccination practices. Expert Rev Vaccines. 2016;15(7):937–48. doi:10.1080/14760584.2016.1184092.
- Maisa A, Milligan S, Quinn A, Boulter D, Johnston J, Treanor C, Bradley DT. Vaccination against pertussis and influenza in pregnancy: a qualitative study of barriers and facilitators. Public Health. 2018 Sep;162:111–17. doi:10.1016/j.puhe.2018.05.025.
- Santé publique France. Bulletin épidémiologique grippe. Point au 4 mai 2016 (French). [accessed 2019 Nov]. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/grippe/documents/bulletin-national/bulletin-epidemiologique-grippe.-point-au-4-mai-2016
- Tort J, Lelong N, Prunet C, Khoshnood B, Blondel B. Maternal and health care determinants of preconceptional use of folic acid supplementation in France: results from the 2010 national perinatal survey. BJOG An Int J Obstet Gynaecol. 2013;120(13):1661–67. doi:10.1111/1471-0528.12414.
- Bonet M, Kaminski M, Blondel B. Differential trends in breastfeeding according to maternal and hospital characteristics: results from the French national perinatal surveys. Acta Paediatr. 2007 Sep;96(9):1290–95. doi:10.1111/j.1651-2227.2007.00410.x.
- Moniz MH, Vitek WS, Akers A, Meyn LA, Beigi RH. Perceptions and acceptance of immunization during pregnancy. J Reprod Med. 2013;58:383–88.
- Bisset KA, Paterson P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: A systematic review. Vaccine. 2018 May;36(20):2751–59. doi:10.1016/j.vaccine.2018.04.013.
- Alessandrini V, Anselem O, Girault A, Mandelbrot L, Luton D, Launay O, Goffinet F. Does the availability of influenza vaccine at prenatal care visits and of immediate vaccination improve vaccination coverage of pregnant women? Angelillo IF, editor. PLoS One. 2019 Aug 1;14(8):e0220705. doi:10.1371/journal.pone.0220705.