ABSTRACT
Human papillomavirus(HPV) infection is a necessary factor for the development of cervical cancer. The HPV vaccine is currently available, but there is still a lack of large-scale research on the distribution and risk factors of HPV. The aim of this study is to investigate the genotype distribution and risk factors of HPV infection in Yangqu which is located in North China. This study enrolled 10086 women aged <65 years from Yangqu County. HPV genotypes were identified via standard HPV DNA testing. The overall prevalence of HPV infection was 8.92%. The prevalence of high-risk HPV types was 8.80%, and it was 0.38% for low-risk HPV types. Single genotype infection accounted for 67.91% in HPV-positive cases. The most common HPV genotypes were HPV-16, −52, and −58. HPV-18 was only the 11th most common type in HPV-positive cases. Women ≥50 years of age had the highest prevalence rate of HPV, and women <30 years had the lowest prevalence rate. The distribution of HPV genotypes also varied among the three age groups: <30, 30–49, and ≥50 years. The risk factors that contributed to the rate of HPV infection included low educational level, low income, smoking, age at first sexual encounter <23 years old, and number of births ≥3 times. This large routine clinical practice report of HPV prevalence and genotype distribution revealed the characteristics of HPV infection-type distributions in Shanxi Province, which should be considered in formulating comprehensive prevention strategies including vaccination for cervical cancer in China.
Introduction
Cervical cancer is one of the major malignant tumors threatening women all over the world. According to statistics, there are 527,600 cases of cervical cancer in the world, including 265,700 cases of death caused by cervical cancer.1 Human papillomavirus (HPV) causes many cancers including oro-pharyngeal, vulvar, vaginal, anal, and penile and so on, but the cervical cancer is the “classic” cancer of HPV. Persistent infection of high-risk human papillomavirus (HR-HPV) is a necessary factor for the occurrence of cervical cancer, but its characteristic is that the infection rate and type are different in different regions.Citation2,Citation3 At present, there is no report of HPV infection in this region in Shanxi Province.
In the post-vaccine era, how to formulate different vaccine intervention measures to prevent and control cervical cancer effectively according to the characteristics of different regions is the focus of scholars. China has approved bivalent (2vHPV preventing infection from HPV16 and 18 genotypes), quadrivalent (4vHPV from HPV6, 11, 16, and 18 genotypes) and 9-valent (9vHPV from HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 genotypes) vaccines,with 9vHPV being the successor to the others. Existing studies have shown that the distribution of HPV genotypes varies with different regions. In the global scope, the top 10 are HPV16, 18, 58, 52, 51, 31, 56, 33, 45 and 53 in order. The top 5 places by region are: Africa HPV16, 18, 58, 52, 51;Central/South America HPV16, 18, 61, 71, 58;North America HPV16, 53, 52, 18, 39; Asia HPV16, 52, 58, 18, 56;Europe HPV16, 18, 31, 33, 58. But the Asian population reported here includes fewer Chinese women. China has vast territory and abundant resources, different populations also have different characteristics of HPV infection. Shanxi has a large region and a wide area, but the study on the characteristics of HPV infection in this region is limited, especially the study on the regional factors of HPV infection in China or our province is very few at present. This study intends to clarify the characteristics of HPV distribution in this region and target the key infected population according to analyze the prevalence of HPV, the distribution characteristics of HPV genotypes and the risk factors affecting HR-HPV infection in Yangqu area of Shanxi Province, so as to provide scientific basis for preventing HR-HPV infection through population behavior and vaccine intervention and achieving cervical cancer prevention gateway forward.
Methods
Study population
This survey was based on data obtained from the screening program and the baseline data of an ongoing prospective cohort study (Shanxi CIN Cohort). The rational, design and methods of the Shanxi CIN Cohort Study have been detailed elsewhere.Citation4,Citation5 Briefly, we organized a free cervical cancer screening program and detected HPV infection for 10086 eligible women permanently residing in Yangqu County of Taiyuan city between September and December of 2014. All participants provided their signed informed consent prior to enrollment. The inclusion criteria were as follows: (1) sexually active woman;(2) aged <65 years;(3)of the Han ethnic group;(4) with ≥1 year of continuous residence in Yangqu County and (5)willingness to participate in the screening program. The exclusion criteria were as follows: pregnancy, history of other malignant tumors and uterine surgery resection or cervical lesion treatment. We investigated a total of 10086 women with a demographic characteristics-related questionnaire and screened them by taking biological specimens from the cervix (see below) in conjunction with gynecologic examinations. Our study was approved by the ethics committee of the Second Hospital, Shanxi Medical University ().
Data collection
Demographic information
In-person interviews were conducted by trained and qualified investigators face-to-face using a unified, structured demographic characteristics-related questionnaire. The demographic information included age, number of education years, yearly income, use of tobacco, passive smoking, reproductive patterns (including menopause, gravidity, number of birth, use of tubal ligation for contraception and age at first sexual intercourse), and history of gynecological events.
Biological specimens
It was forbidden to apply vaginal douching or to have sexual intercourse 48 hours prior to the gynecological examination for each subject. After the cervix was exposed, the HPV testing brush was fully placed into the cervical canal and gently rotated 3–5 times. The exfoliated cervical epithelial cells then adhere to the flat sides of the bristles.The tip of the cervix brush is then placed into a vial containing transport medium. Then, the sample was stored at −20°C and was processed for HPV genotyping within 2 weeks.
Clinical laboratory tests
HPV genotyping by HybriMax was performed using an HPV GenoArray Test Kit (HybriBio Ltd, Chaozhou, China) with the residual Pap test specimens. This assay can identify 21 types of HPV, including 15 high-risk types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68) and six low-risk types (6, 11, 42, 43, 44 and CP8304) by the flow-through hybridization technique using a TC-96/G/H6 HPV DNA Amplification Analyzer and an HMM-2 fast nucleic acid molecule hybridization instrument (HybriBio Ltd).
Statistical analysis
We performed a double-pass entry of all the data using EpiData 3.1 software (Jens M Lauritsen, Odense, Syddan Mark, Denmark). The descriptive statistics were used to describe the frequency and proportion and the mean and standard deviation (SD) of the demographic characteristics.
For comparisons among different age groups, the categorical variables were compared by using the Pearson χ2 test, and a post hoc analysis was examined using Bonferroni’s method. The factors related to HR-HPV infection were analyzed using the nonconditional logistic regression model for multivariate analysis, using a stepwise screening method. All the reported P values were made on the basis of two-sided tests with a significance level of 0.05. The statistical analyses were performed using SPSS 19.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA).
Results
Distribution characteristics of HR-HPV infections
Overall HPV prevalence
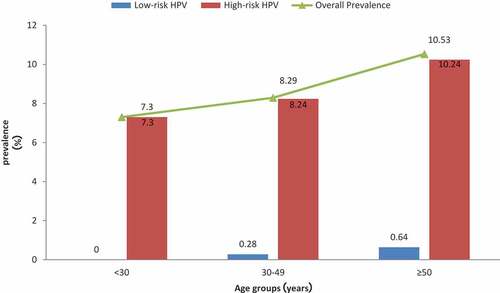
The overall detectable HPV infection rate was 8.92% (900/10086) in this screening sample, with 8.80% (888/10086) positive for one or more HR-HPV and 0.38% (38/10086) positive for one or more LR-HPV.
In all these 10086 subjects, the highest overall prevalence of HPV was found in women ≥50 years old (328/3114, 10.53%), while the lowest HPV prevalence was found in women <30 years old (44/603, 7.30%). The overall HPV prevalence in women 30–49 years was 8.29% (528/6369). The difference was statistically significant among the different age groups (). The rates for different HR-HPV types were also altered among the three age groups. The same results occurred with overall prevalence; the HR-HPV prevalence was the highest in women ≥50 years (10.24%), and the lowest rate was found in women <30 years of age (7.30%). At the same time, the LR-HPV positive rate was 0%, 0.28% and 0.64% in the three age groups (<30 years, 30–49 years, and ≥50 years, respectively).
Table 1. Comparison of HPV infection rates among different age groups (%).
HPV genotype distribution
shows the distribution of HR-HPV subtypes in all the tested women. The most commonly detected HR-HPV type were HPV-16, HPV-52, and HPV-58. Meanwhile, when considering the prevalence of these genotypes in the different age groups (<30 years, 30–49 years, and ≥50 years), the HR-HPV genotype distributions were different as well (). The five most common HR-HPV genotypes in women who were 30–49 years old were HPV-16, −52, −58, −56, and −66. The five most common HR-HPV types in women ≥50 years, in decreasing order, were HPV-16, −52, HPV-58, −53, and −66. The two most common HR-HPV types were the same (HPV-16 and −52) in these two older age groups, while the same two HPV types were also the most common in younger women <30 years old; HPV-16 was still the most common genotypes in the younger group. The next most common HPV genotypes in women <30 years were HPV-53, −68, and −33. The three least frequently detected HR-HPV genotypes were HPV-35, −45, and −59 ().
Table 2. HPV prevalence and genotype distribution.
Distribution of single and multiple HR-HPV infections
When considering the characteristics of co-infection with multiple HR-HPV genotypes, we found that single HR-HPV infections were the most common in the local population, with an overall prevalence of 5.98%, which accounted for 67.91% of all the HR-HPV infectious women. The overall prevalence of multiple HR-HPV types of infection was 2.83%, which accounted for 32.09% of HR-HPV infectious cases, and with the increase of age, the percentage of multiple infections in the same age group gradually increased, which were 22.73%, 31.62% and 34.17%, respectively. Two HPV genotypes (double infection) were the most common in multiple HR-HPV infection distributions, with an overall rate of 1.99% (). Three HPV genotypes (triple infection) and four or more detectable HR-HPV genotypes were the next most common, with an overall prevalence of 0.58% and 0.26%, respectively.
Table 3. Distribution of single and multiple HR-HPV infections (n = 888).
Analysis of the potential risk factors for HR-HPV infection
Associated factors with HR-HPV infection in the univariate analysis between the study group (HR-HPV positive) and the control group were as follows: age, education level, annual income, smoking history, spouse smoking history, menopause status, age at first intercourse, gravidity, number of birth, use of tubal ligation for contraception, and gynecologic surgery history ().
Table 4. Univariate analysis of the potential risk factors for HR-HPV infection (n = 10086).
The multivariate analysis suggested that education level, annual income, smoking history, age at first intercourse, and number of birth were independently associated with HR-HPV infection (). The regression equation was logit P = −1.103–0.333 × 2–0.233 × 3 + 0.813 X4 – 0.179 × 7 + 0.205 X9. Education level and annual income were protective factors for HR-HPV infection. For each 1-grade increase in education level, the risk of HR-HPV infection was reduced by 28.4%. For each 1-grade increase in annual income, the risk of HR-HPV infection was reduced by 20.8%. The HR-HPV infection risk in female smokers was 2.254 times greater than that of nonsmokers. Women whose age at first intercourse was less than 23 years old had the highest risk of HR-HPV infection. The risk in women whose age at first intercourse was more than 23 years old was 83.6% of that in women whose age at first intercourse was less than 23 years old. The HR-HPV infection risk in women with number of birth ≥3 times was 1.228 times greater than that of women with number of birth <3 times. Accordingly, the multivariate analysis showed that low educational level, low income, smoking, age at first intercourse <23 years old, and number of birth ≥3 times were potential risk factors for infection with HR-HPV ().
Table 5. Results of multivariate analysis of the potential risk factors for HR-HPV infection (n = 10086).
Discussion
General background and age characteristics of HPV infected women
This population-based study showed that the overall HPV infection rate of women in Yangqu, Shanxi province, China was 8.92%, and the total positive rate was slightly lower than the worldwide HPV infection rate of 10.4% (95% CI 10.2–10.7). The rate was close to the corresponding estimated Asian region data reported by de Sanjos SCitation6 of 8% (7.5–8.4). However, the rate was lower than that of Africa (22.1%), Central America and Mexico (20.4%), and North America (11.3%) and was close to the infection rate of Europe (8.1%).Citation7–Citation9
This study showed that all the different age groups with HPV had a higher infection rate and were mainly consistent with a high risk of infection. The HR-HPV infection peak in the high risk age group was similar to the result in Qiao YL’s study,Citation10 but there was no peak that appeared in the younger group. The reason for this result may be as follows. First, the result may attribute to selection bias. Because our study was a free screening that looked deeply into local areas, the 10086 screening participants were concentrated with childbearing ages and older women. Women under 20 years old accounted for only 0.05% (5/10086) of the study population, and women under 25 years old accounted for only 1.50% (151/10086). It has been reported that, because of a frequent sex life and an unsensitized immune system, approximately 16–24 year old women are always susceptible to HPV infection.Citation11,Citation12 If a high-risk HPV infection is persistent, it is more likely to cause cervical malignant lesions.Citation13,Citation14 Also, too many sexual partners, a premature sex life, sexual behavior disorders, too many pregnancies, smoking and bad health habits were the main reasons for HPV infection.Citation4,Citation15–Citation22 Maybe because of this selection bias, which was a small constituent ratio for women less than 25 years old, there was no peak in 16 to 24 year-old young women. Second, due to decreased ovarian function, estrogen levels, and immunological function, as well as the resistance to harmful factors in the world and the ability to clear HPV, women more than 50 years old have more opportunities to acquire an HPV infection.Citation23–Citation26 At the same time, older women are exposed to risk factors for a longer time, resulting in an increased persistent HPV infection rate. Thus, age is an important factor for HPV infection, and women more than 50 years old are the focus of the local prevention and treatment of cervical cancer. It is of great importance for the prevention and treatment of cervical precancerous lesions and cervical cancer to improve these women’s self-healthcare consciousness and to strengthen their screening. In addition, to understand the characteristics of HPV infection in different age groups also contributes to effective selection of the target population for the application of a preventive vaccine.
Distribution of HPV genotypes
HR-HPV-persistent infection is necessary for cervical cancer and precancerous lesions.Citation27 HPV genotype distributions have regional differences, and the types associated with cervical cancer are not the same.Citation28–Citation30 Although the detection rate of HPV16 varies according to different countries and areas, it always has a relatively high rate.Citation31,Citation32 In our study, we used an HPV GenoArray Test Kit (HybriBio Ltd, Chaozhou, China) that had good interassay agreement, with the reference standard Roche Linear ArrayCitation33 and hybrid capture IICitation34 for the detection of most HPV genotypes. Except for the two types not detected (42 and 44), the detection rate of HPV16 was still the highest, accounting for 38.33% of the total positive infection (345/900), followed by 52, 58, 53, 66, 56, 51, 33, 31, 68, 18, 39, 35, 59, CP8304, 43, 6, 11, and 45. The top three high-risk HPV types were 16, 52, and 58, which were the same as those reported in several domestic studies.Citation29,Citation35,Citation36 In this study, 7 high-risk types (HPV16, 18, 31, 33, 45, 52, and 58) and 2 low-risk types (HPV 6 and 11), covered by nine valent HPV vaccines, accounted for 76.9% of all HPV infections, which means that we can prevent 76.9% of HPV infections through vaccination.
Characteristics of single and multiple infections of HPV
In this study, every age group had a higher infection rate of HPV than normal people,Citation37 especially for high-risk HPV infections. The positive infections in this type were mainly single infections. Studies have shown that most of cervical endothelial injuries are caused by single HR-HPV-type infection,Citation36 while the other types of co-infection have little effect because of the dormant infection status for other types of HPV when one primary type of HPV proliferates.Citation38,Citation39 However, other studies have indicated that Infection with multiple HPV types may increase the risk of abnormal cell proliferation and cervical lesions in women.Citation40 Further studies need to be done to clarify it.
Risk factors for HR-HPV infection
Education level and annual income
Education level and annual income were protective factors of HR-HPV infection. The education level of primary school or below had the highest risk of HR-HPV infection. According to different education years (primary school or below, junior middle school, senior middle school or above), the likelihood of HR-HPV infection was reduced by 28.4% for each additional level when other factors were the same. Women whose annual income was less than 1 million were most likely to be infected with HR-HPV, and the possibility of HR-HPV infection was reduced by 20.8% for each additional level. Possibly, those who had a high level of education and good economic foundation always had a better health condition, as well as better health consciousness and health habits, than those who had a low education level and poor economic foundation. Because HPV spreads through feculent sexual life,Citation39,Citation41 women with a poor economic foundation may be more susceptible to HR-HPV.
Age at first intercourse and number of birth
Women with an age at first intercourse <23 years old and number of birth ≥3 times had the highest risk of HR-HPV infection. The results of this study showed that the risk of HR-HPV infection in the first intercourse age ≥23 years group was only 83.6% of that in the first intercourse age <23 years group. The risk of HR-HPV infection in the number of birth ≥3 times group was 1.228 times greater than that of the number of birth <3 times group. These results are consistent with previous studies,Citation42 showing that the age at first sexual intercourse and number of birth were risk factors. The possible reason for this result might be that long lengths of exposure to risk factors for women with earlier sexual behavior and more number of birth led to more serious cervical injury. Our study indicated that women whose age at first intercourse was less than 23 years, and whose number of birth was more than 3 times, should be classified as a key population for the prevention of HR-HPV infection.
Smoking history
Smoking has been recognized as a risk factor for the development of cervical intraepithelial neoplasia,Citation43 but it remains a controversial topic whether smoking increases the risk of HR-HPV infection. This study concluded that smoking is a risk factor for HR-HPV infection, and the risk value was as high as 2.254 times that of nonsmokers, which is consistent with Coker’s study.Citation44 Various measures should be taken to achieve good primary prevention and to work toward a smoke-free society, such as a smoking ban for youth, advocating smoking abstinence for middle-aged people, and advising the elderly to smoke less or use harmless cigarettes.
Many studies have shown that the incidence of cervical cancer is closely related to HPV infection, especially a persistent, repeated HR-HPV infection, but the distribution of HPV genotypes varies according to different countries, regions, geographical environments and races.Citation45 This is the largest routine clinical practice report of HPV prevalence and genotype distribution in Shanxi Province. But it also has some limitations.The main limitation is that there are no pathological results in these cases.Due to the limited funding of the study, we can not conduct free colposcopy examination on these participants. Secondly, we only detected the expression of 21 HPV genotypes,including 15 high-risk types and 6 low-risk HPV genotypes, which may lead to overestimation of the high-risk HPV genotype in the HPV-positive female participants. Further studies are therefore necessary to confirm these associations. Additionaly, given that our study focuses on cervical cancer, it can not provide a basis for vaccine use in men, and performing similar studies for other HPV cancers, especially oro-pharyngeal cancer in man, would further guide vaccine use.
In this study, the HPV infection rate of the female population in the Yangqu area was high, and the infection rate was increased with age in women who were 20–65 years old. HPV-16 was still the most prevalent HR-HPV type in this population of women, followed by HPV-52 and HPV-58. The overall and age-specific prevalence and genotype distribution of HR-HPV are different in this Chinese population compared to reports from western countries. The characteristics of an HPV infection-type distribution should be considered in formulating comprehensive prevention strategies for cervical cancer in China. According to our study, HPV52 and 58 are the main genotype in Yangqu, China, but not the HPV18. So our epidemiological survey can effectively guide local goverment to develop HPV vaccination programs for targeted vaccination in the Yangqu area in particular. As the incidence of cervical cancer in Shanxi Province is nearly 10 times higher than the national average This region is the worst disaster area for cervical cancer. Therefore, this region should be first covered in the promotion of vaccine in China. Furthermore this policy, especially for use of the vaccine in men, will depend on a molecular epidemiology study of this type in oro-pharyngeal, penile and anal cancers of men.The independent risk factors of HR-HPV infection include the following: education of primary school and below, an annual income <1 million, use of tobacco, age at first sexual encounter <23 years old, and number of birth ≥3 times. Those who have these risk factors are key populations for HR-HPV infection prevention in the Yangqu area, and we should first vaccinate this particular group of people who may be infected.
Conclusions
At the present stage, the bivalent HPV vaccine has been approved in the domestic market; the nine valent vaccine has almost completed clinical trials and will enter clinical application in the near future, benefiting the majority of women. This study provides a basis for exploring the characteristics and risk factors of HR-HPV infection in Shanxi, Yangqu so that we can concentrate on this high risk population in cervical cancer screening. This study provides a reference for further studies on therapeutic vaccine strategies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
MH conceived and designed the research; WW, ZW, YW, WZ, DL, HL, and JY conducted the research; JY analyzed the data; JY, ZW, and JW performed statistical analysis; JY wrote the paper; MH had primary responsibility for final content.
Ethics approval
The study was conducted according to the current ethical standards including the WMA Declaration of Helsinki and was submitted to the appropriate regulatory authorities including ethical committees in Yangqu, as requested by local regulations. This study was approved by the ethics committee of the Second Hospital, Shanxi Medical University and the reference number was 2013002.
Supplemental Material
Download MS Word (23.8 KB)Acknowledgments
We are grateful for the contribution of our coworkers involved in conducting the study and writing this article. We thank all the study participants, as well as the investigators and local healthcare workers from Yangqu County Hospital, for their help with the study.
Supplementary material
Supplemental data for this article can be online at http://dx.doi.org/10.1080/21645515.2019.1689743.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262.
- Gutierrez-Xicotencatl L, Salazar-Pina DA, Pedroza-Saavedra A, Chihu-Amparan L, Rodriguez-Ocampo AN, Maldonado-Gama M, Esquivel-Guadarrama FR. Humoral Immune response against human papillomavirus as source of biomarkers for the prediction and detection of cervical cancer. Viral Immunol. 2016;29:83–94. doi:10.1089/vim.2015.0087.
- Senapati R, Nayak B, Kar SK, Dwibedi B. HPV genotypes co-infections associated with cervical carcinoma: special focus on phylogenetically related and non-vaccine targeted genotypes. PLoS One. 2017;12:e0187844. doi:10.1371/journal.pone.0187844.
- Wang Z, Wang J, Fan J, Zhao W, Yang X, Wu L, Li D, Ding L, Wang W, Xu J, et al. Risk factors for cervical intraepithelial neoplasia and cervical cancer in Chinese women: large study in Jiexiu, Shanxi Province, China. J Cancer. 2017;8(6):924–32. doi:10.7150/jca.17416jcav08p0924.
- Zhao W, Hao M, Wang Y, Feng N, Wang Z, Wang W, Wang J, Ding L. Association between folate status and cervical intraepithelial neoplasia. Eur J Clin Nutr. 2016;70(7):837–42. doi:10.1038/ejcn.2016.35ejcn201635.
- de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–59. doi:10.1016/S1473-3099(07)70158-5.
- Castellsague X, Menendez C, Loscertales MP, Kornegay JR, Dos Santos F, Gomez-Olive FX, Lloveras B, Abarca N, Vaz N, Barreto A, et al. Human papillomavirus genotypes in rural Mozambique. Lancet. 2001;358:1429–30. doi:10.1016/S0140-6736(01)06523-0.
- Obiri-Yeboah D, Akakpo PK, Mutocheluh M, Adjei-Danso E, Allornuvor G, Amoako-Sakyi D, Adu-Sarkodie Y, Mayaud P. Epidemiology of cervical human papillomavirus (HPV) infection and squamous intraepithelial lesions (SIL) among a cohort of HIV-infected and uninfected Ghanaian women. BMC Cancer. 2017;17:688. doi:10.1186/s12885-017-3682-x.
- Munoz N, Franco EL, Herrero R, Andrus JK, de Quadros C, Goldie SJ, Bosch FX. Recommendations for cervical cancer prevention in Latin America and the Caribbean. Vaccine. 2008;26(Suppl 11):L96–L107. doi:10.1016/j.vaccine.2008.05.062.
- Zhao Y, Zhao F, Hu S, Chen W, Chen F, Cui J, Liu B, Zhang W, Zhang X, Pan Q, et al. Multi-center cross-sectional study on type-specific human papillomavirus infection among Chinese women. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:1351–56.
- Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20–26 years). Hum Vaccin Immunother. 2015;11:2337–44. doi:10.1080/21645515.2015.1066948.
- Oliveira CR, Rock RM, Shapiro ED, Xu X, Lundsberg L, Zhang LB, Gariepy A, Illuzzi JL, Sheth SS. Missed opportunities for HPV immunization among young adult women. Am J Obstet Gynecol. 2018;218(3):326.
- Bahrami A, Hasanzadeh M, Shahidsales S, Farazestanian M, Hassanian SM, Moetamani Ahmadi M, Maftouh M, Gharib M, Yousefi Z, Kadkhodayan S, et al. Genetic susceptibility in cervical cancer: from bench to bedside. J Cell Physiol. 2018;233:1929–39. doi:10.1002/jcp.v233.3.
- Schiffman M, Wentzensen N. Effective use of human papillomavirus testing for cervical cancer screening requires extended intervals to target persistent infections and precancerous lesions. Prev Med. 2017;105:378–80. doi:10.1016/j.ypmed.2017.09.010.
- Wang X, Ji Y, Li J, Dong H, Zhu B, Zhou Y, Wang J, Zhou X, Wang Y, Peppelenbosch MP, et al. Prevalence of human papillomavirus infection in women in the autonomous region of Inner Mongolia: A population-based study of a Chinese ethnic minority. J Med Virol. 2018;90:148–56. doi:10.1002/jmv.v90.1.
- Farsi NJ, Rousseau M-C, Schlecht N, Castonguay G, Allison P, Nguyen-Tan PF, Souliéres D, Coutlée F, Hier M, Madathil S, et al. Aetiological heterogeneity of head and neck squamous cell carcinomas: the role of human papillomavirus infections, smoking and alcohol. Carcinogenesis. 2017;38:1188–95. doi:10.1093/carcin/bgx106.
- Khoo SP, Bhoo-Pathy N, Yap SH, Anwar Shafii MK, Hairizan Nasir N, Belinson J, Subramaniam S, Goh PP, Zeng M, Tan HD, et al. Prevalence and sociodemographic correlates of cervicovaginal human papillomavirus (HPV) carriage in a cross-sectional, multiethnic, community-based female Asian population. Sex Transm Infect. 2018;94(4):277–83.
- Ma S, Stern JE, Feng Q, Hughes JP, Hawes SE, Winer RL. Incidence and risk factors for human papillomavirus infections in young female online daters. J Med Virol. 2017;89:2029–36. doi:10.1002/jmv.v89.11.
- Cao D, Zhang S, Zhang Q, Wei X, Zhao M, Ma Q, Li Y, Wang L, Pei M, Yang T, et al. Prevalence of high-risk human papillomavirus infection among women in Shaanxi province of China: A hospital-based investigation. J Med Virol. 2017;89:1281–86. doi:10.1002/jmv.24748.
- Shi N, Lu Q, Zhang J, Li L, Zhang F, Dong Y, Zhang X, Zhang Z, Gao W. Analysis of risk factors for persistent infection of asymptomatic women with high-risk human papilloma virus. Hum Vaccin Immunother. 2017;13:1–7. doi:10.1080/21645515.2016.1239669.
- Shakya S, Syversen U, Asvold BO, Bofin AM, Aune G, Nordbo SA, Vaidya KM, Karmacharya BM, Afset JE, Tingulstad S. Prevalence of human papillomavirus infection among women in rural Nepal. Acta Obstet Gynecol Scand. 2017;96:29–38. doi:10.1111/aogs.13036.
- Baloch Z, Yasmeen N, Li Y, Ma K, Wu X, Yang SH, Xia X. Prevalence and risk factors for human papillomavirus infection among Chinese ethnic women in southern of Yunnan, China. Braz J Infect Dis. 2017;21:325–32. doi:10.1016/j.bjid.2017.01.009.
- Zhang Q, Cao D, Ma Q, Li N, Cui XQ, Yang XF. Natural outcome of genital tract high-risk human papillomavirus infection and associated factors among 760 women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2015;37:534–40. doi:10.3881/j.issn.1000-503X.2015.05.008.
- Mollers M, Boot Hein J, Vriend Henrike J, King Audrey J, van den Broek Ingrid VF, van Bergen Jan EA, Brink Antoinette ATP, Wolffs Petra FG, Hoebe Christian JPA, Meijer Chris JLM, et al. Prevalence, incidence and persistence of genital HPV infections in a large cohort of sexually active young women in the Netherlands. Vaccine. 2013;31:394–401. doi:10.1016/j.vaccine.2012.10.087.
- Louvanto K, Rintala MA, Syrjanen KJ, Grenman SE, Syrjanen SM. Genotype-specific persistence of genital human papillomavirus (HPV) infections in women followed for 6 years in the Finnish family HPV study. J Infect Dis. 2010;202:436–44. doi:10.1086/653015.
- Ma Q, Hou M, Yang XF. Screening of the genital human papillomavirus infection among 8581 women in the first affiliated hospital of Xi’an Jiaotong University. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2014;36:277–82. doi:10.3881/j.issn.1000-503X.2014.03.009.
- Di Paola M, Sani C, Clemente AM, Iossa A, Perissi E, Castronovo G, Tanturli M, Rivero D, Cozzolino F, Cavalieri D, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. 2017;7:10200. doi:10.1038/s41598-017-09842-6.
- Zhong TY, Zhou JC, Hu R, Fan XN, Xie XY, Liu ZX, Lin M, Chen Y-G, Hu X-M, Wang W-H, et al. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province, China. J Infect Public Health. 2017;10:783–88. doi:10.1016/j.jiph.2017.01.011.
- Zeng XX, Yan LX, Huang XX, He CH, Liu WG, Yuan WQ, Qiu Y-P, Liu Z-X. Prevalence and genotype distribution of human papillomavirus among Hakka women in China. Ann Transl Med. 2016;4:276. doi:10.21037/atm.
- Luo ZY, Chen Q, Yang H, Lin M, Chen CY, Yang C, Yang L-Y. The prevalence and genotype of human papillomavirus from patients with genital warts in Eastern Guangdong province. Asian Pac J Cancer Prev. 2015;16:5675–79. doi:10.7314/APJCP.2015.16.14.5675.
- Gadelha SR, Soares-Barreto DMV, Costa GB, Leal VCN, Gomes LGS, Santos UR, Ferreira GCS, Carvalho LD, Soraes-Almeida SMV, Mello MAG, et al. Clinical, laboratory and epidemiological aspects of HPV infection in a low-income population from South Bahia, Brazil. Epidemiol Infect. 2017;145(16): 3398–404.
- Volpini LPB, Boldrini NAT, de Freitas LB, Miranda AE, Spano LC. The high prevalence of HPV and HPV16 European variants in cervical and anal samples of HIV-seropositive women with normal Pap test results. PLoS One. 2017;12:e0176422. doi:10.1371/journal.pone.0176422.
- Low HC, Silver MI, Brown BJ, Leng CY, Blas MM, Gravitt PE, Woo YL. Comparison of Hybribio GenoArray and Roche human papillomavirus (HPV) linear array for HPV genotyping in anal swab samples. J Clin Microbiol. 2015;53:550–56. doi:10.1128/JCM.02274-14.
- Zhang L, Lin Y, Li JK. Concordance in cervical HPV detection between hybrid capture 2 and HPV GenoArray tests. Asian Pac J Cancer Prev. 2014;15:4465–66. doi:10.7314/APJCP.2014.15.11.4465.
- Zeng Z, Yang H, Li Z, He X, Griffith CC, Chen X, Guo X, Zheng B, Wu S, Zhao C, et al. Prevalence and genotype distribution of HPV infection in China: analysis of 51,345 HPV genotyping results from China’s largest CAP certified laboratory. J Cancer. 2016;7:1037–43. doi:10.7150/jca.14971.
- Zhang C, Huang J, Wu Z, Mei X, Shi W. Prevalence and genotype distribution of human papillomavirus among females in the suburb of Shanghai, China. J Med Virol. 2018;90:157–64. doi:10.1002/jmv.24899.
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC information centre on HPV and Cancer (HPV information centre). Human papillomavirus and related diseases in the world. Summary Report 22 January 2019. [Date Accessed]
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi:10.1136/jcp.55.4.244.
- Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110:S4–7.
- Dickson EL, Vogel RI, Geller MA, Downs LS Jr. Cervical cytology and multiple type HPV infection: a study of 8182 women ages 31–65. Gynecol Oncol. 2014;133:405–08. doi:10.1016/j.ygyno.2014.03.552.
- Ayres ARG, Silva GAE, Teixeira MTB, Duque KCD, Machado M, Gamarra CJ, Levi JE. HPV in women assisted by the family health strategy. Rev Saude Publica. 2017;51:92.
- Yetimalar H, Kasap B, Cukurova K, Yildiz A, Keklik A, Soylu F. Cofactors in human papillomavirus infection and cervical carcinogenesis. Arch Gynecol Obstet. 2012;285:805–10. doi:10.1007/s00404-011-2034-3.
- Kapeu AS, Luostarinen T, Jellum E, Dillner J, Hakama M, Koskela P, Lenner P, Love A, Mahlamaki E, Thoresen S, et al. Is smoking an independent risk factor for invasive cervical cancer? A nested case-control study within Nordic biobanks. Am J Epidemiol. 2009;169:480–88. doi:10.1093/aje/kwn354.
- Coker AL, Bond SM, Williams A, Gerasimova T, Pirisi L. Active and passive smoking, high-risk human papillomaviruses and cervical neoplasia. Cancer Detect Prev. 2002;26:121–28. doi:10.1016/S0361-090X(02)00039-9.
- Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi:10.1086/653024.