ABSTRACT
Coxsackievirus A10 (CV-A10) has recently emerged as a major pathogen of hand, foot, and mouth disease in children worldwide. Currently no effective treatments are available; development of anti-CV-A10 vaccine is a most cost-effective way for CV-A10 prevention. Robust assay to measure neutralizing antibody (NtAb) titres elicited by vaccination would greatly prompt anti-CV-A10 vaccine development. Compare to the traditional neutralization assay based on inhibition of cytopathic effects (herein after referred to as cNT) which is time-consuming and labor-intensive, in this study we developed an efficient high-throughput neutralization antibody assay based on CV-A10 pseudoviruses (herein after referred to as pNT). In the pNT, anti-CV-A10 NtAb titre was negatively corresponded with the relative luminescent unit (RLU) produced by luciferase reporter gene incorporated in pseudovirus genome. As described in this study, the NtAb against CV-A10 could be detected within 10–16 h, anti- CV-A10 NtAb in 67 human serum samples were measured in parallel with pNT and cNT assays, a good correlation (r = 0.83,p < .0001) and good agreement(97%) were shown between cNT and pNT, indicating that the pNT provides a rapid and convenient procedure for measuring NtAb production against anti-CV-A10 NtAb measurement.
Introduction
Coxsackievirus A10 (CV-A10) is a virus in the genus Enterovirus of the family Picornaviridae, and has become a major cause of hand, foot and mouth disease (HFMD).Citation1-Citation4 In addition to the typical clinical symptoms of HFMD, CV-A10 infections can lead to other serious complications such as onychomadesis, hypercapnia, convulsions, central nervous system disorders, and even death.Citation5-Citation7 Although CV-A10 has reemerged as a considerable global public health threat, there are still no effective drugs to treat these infections.
As a prevention strategy, there has been substantial research conducted on the development of a CV-A10 vaccine in recent years.Citation8-Citation10 During the development phase, the effectiveness of a vaccine formulation is usually tested by evaluating its ability to induce an immune response via measuring the production of neutralizing antibody (NtAb) as the key indicator of protective immunity.Citation11-Citation14 The neutralization assay based on inhibition of cytopathic effects (cNT) is the standard method recommended by the World Health Organization for measuring NtAb;Citation15 however, this method is time-consuming by virtue of procedures that can take up to 5–7 days and is also associated with a health risk for researchers handling live viruses. Therefore, a new efficient, safe, and high-throughput neutralization assay is required.
Here, we developed a new assay for CV-A10 NtAb in serum using a pseudovirus, which is an efficient tool for virus research and antiviral drug screening. To date, high-throughput NtAb detection based on pseudoviruses (pNT) has been reported and successfully applied for Poliovirus, Rift Valley fever virus, Human papilloma virus, and HIV, demonstrating high sensitivity, objectivity, and biological safety.Citation16-Citation19 Our research group has already constructed numerous types of pseudoviruses relevant to HFMD, including pseudoviruses of enterovirus 71 (EV-A71), CV-A16, CV-B3, CV-B5, and CV-A6, which were used in the development of a pNT assay.Citation20-Citation24 Here, we report the successful construction of a CV-A10 pseudovirus, and development of a CV-A10 pNT to detect the titre of anti-CV-A10 NtAb. The method was validated using 67 clinical serum samples, and the performance and safety were compared to those of the traditional cNT method.
Materials and methods
CV-A10 single-round infection system
Construction of the CV-A10 capsid expression vector pcDNA-P1
The CV-A10 virus strain CV-A10-2 (Genbank accession No. KY012321) was kindly provided by Professor Ningshao Xia. Total RNA was extracted from the supernatant of the CV-A10 virus culture using Mag MAX 96 Viral RNA Isolation Kit (Thermo Fisher, Waltham, MA USA). The CV-A10 capsid gene was amplified from the total RNA, and the enhanced green fluorescent protein (EGFP) reporter gene was inserted upstream of the CV-A10 capsid gene with a 2A protease self-cleavage site (AITTL). The polymerase chain reaction (PCR) product was then subcloned into the pcDNA6.0 vector to produce pcDNA-P1. The EGFP reporter gene was used for monitoring the transfection efficiency and expression level of the structural gene. All of the primers used for amplification are listed in .
Table 1. Primes for the construction and identification of CVA10 pseudovirus.
Preparation of the pseudovirus
The CV-A10 pseudovirus was produced by co-transfection with pcDNA-P1, EV-A71 replicon, and pcDNA3.0A-T7 polymerase, which were constructed in our previous studies.Citation20,Citation23 In brief, the three plasmids were mixed at a 1:1:1 ratio and transfected into 80% confluent HEK-293T cells [American Type Culture Collection (ATCC), Manassas, VA, USA] in a 10-cm dish with jetPRIME® (Polyplus). The cells were cultured in an incubator at 37°C and 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; Gibco). The supernatant was harvested 48 h post-transfection with three rounds of freeze-thaw cycles.
RT-PCR
The CV-A10 pseudovirus was first identified by detecting the Luc gene. Supernatants from the triple-transfected HEK293T cells were collected and treated with Mag MAX 96 Viral RNA Isolation Kit (Thermo Fisher), and the RNA of CV-A10 pseudovirus was extracted with Mag MAX Express Magnetic Particle Processor (Invitrogen). RT-PCR was performed using PrimeScript™ One Step RT-PCR Kit Ver.2 (TaKaRa, Dalian, China) and the primer pair Luc-F and Luc-R ().
Immunoblotting
The pseudovirus was also detected by immunoblotting for VP1 protein. Purified virus stocks were mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, boiled, and separated on 12% polyacrylamide gels. The proteins were transferred onto polyvinylidene fluoride membranes for immunoblotting. The membranes were probed with a mouse anti-VP1 polyclonal antibody diluted 1:1000 in PBS with 0.05%Tween-20 and 5% skim milk, followed by a corresponding horseradish peroxidase-labeled secondary antibody (Transgen Biotech, China) diluted 1:1000. The membranes were developed by chemiluminescence using SuperSignal Chemiluminescent Substrates (Thermo Fisher, USA).
Infectivity and specificity of the pseudovirus
The infectivity and specificity of the obtained CV-A10 pseudoviruses were further verified using a panel of mouse antisera against CV-A10 and other enteroviruses, including mouse anti-EV-A71 strain FY523 (Genbank accession No. EU703812), mouse anti-CV-A16 strain G10 (Genbank accession No. U05876), mouse anti-CV-A6 strain TW-2007-0014 (Genbank accession No. KR706309), mouse anti-CV-B3 strain 112 (Genbank accession No. KP036480), and mouse anti-EV-D68 strain BCH895A (Genbank accession No. KF726085.1). The reference serum obtained from a BALB/c mouse inoculated with inactivated CV-A10 virus was quantified with the cNT based on inhibition of the cytopathic effect, and the titre was determined to be 1:203 according to the Reed-Muench method.Citation25
Quantification of CV-A10 pseudovirus with real-time PCR
The viral titre was quantified by measuring the genome copy equivalents with real-time PCR on the SYBR Premix ExTaq II system (Takara).
Fifty microlitres of the CV-A10 pseudovirus supernatant was treated with Mag MAX 96 Viral RNA Isolation Kit (Thermo Fisher) and the RNA of the CV-A10 pseudovirus was extracted with Mag MAX Express Magnetic Particle Processor (Invitrogen). The cDNA of CV-A10 pseudovirus was then synthesized using the PrimeScript RT reagent Kit (Takara), and virus genome copy equivalents were quantified by real-time PCR with SYBR Premix ExTaq II (Takara) using the primers qLuc-F and qLuc-R () targeting the luciferase reporter gene.
Optimization of the CV-A10 pNT assay
Before carrying out the pNT assay, the optimal susceptible cell line, cell density, incubation time, and pseudovirus concentration were determined. First, RD cells, HeLa cells, and Vero cells (ATCC; 5 × 104 per well) were seeded in 96-well microtiter plates, and cultured in DMEM with 10% FBS at 37°C and 5% CO2 with 50 μl CV-A10 pseudoviruses. The susceptible cells were seeded at various densities (1 × 104, 2 × 104, 5 × 104, 1 × 105, 2 × 105, and 5 × 105 cells per well) in 96-well microtiter plates in culture medium and incubated with 50 μl CV-A10 pseudoviruses.
Luciferase activities in the cells were measured at various time points to determine the optimal incubation time for the pNT (4, 8, 12, 16, 20, and 24 h). To determine the optimal viral inoculum dose for the assay, 2-fold serial dilutions with a total of 12 concentrations of CV-A10 pseudovirus were incubated with the susceptible cells, and the luciferase activities in the cells were measured at the optimal time points.
Neutralization assay of CV-A10 pseudovirus
A total of 67 serum samples were randomly chosen from a clinical trial evaluating the efficacy of EV-A71 vaccine (Clinical trial No. NCT01508247). Independent Ethics Committee approvals were obtained from the Ethics Committee of the Jiangsu Provincial Centre for Disease Prevention and Control as well as the Centre for Disease Control and Prevention of the Guangxi Zhuang Autonomous Region for the trial and this experiment.
The serum samples were inactivated in a water bath at 56°C for 30 min. The serum samples (50 μl/well) were then serially diluted 2-fold and mixed with the same volume of CV-A10 pseudovirus (50 μl/well) in a 96-well plate, and incubated at 37°C for 2 h. Subsequently, RD cells (100 μl/well) were added to each well to obtain a 200-μl final reaction system. To adjust the titre of the serum samples, a set of standard concentration serum with continuous 2-fold serial dilution was set in each well of the 96-well plate. The plates were incubated at 37°C and 5% CO2 for 8 h. The supernatant was discarded, and the cells were dissolved with 50 μl lysis buffer (Promega) for two freeze-thaw cycles. The luciferase activity was determined according to fluorescence, expressed in relative light units (RLU), and all operations were strictly carried out in accordance with the user manual of the Luciferase Analysis System (Berthold). The viral inhibition ratio was calculated according to the following formula: [1 – (RLUserum plasma – RLUbackground)/(RLUvirus control – RLUbackground)] × 100. The NtAb titre was defined as the reciprocal of the dilution at which 50% of the pseudovirus was neutralized. The NtAb titre was then adjusted according to increasing multiples of the titre of the reference serum determined by the pNT.
Agreement and correlation analysis between the pNT and cNT
The NtAb concentrations measured by the pNT method were compared against those obtained with the conventional cNT method based on the receiver operating characteristic (ROC) curve. The cutoff value was chosen as the point at which the pNT produced the best overall balance for agreement, sensitivity, and specificity with the cNT. In addition, regression and Bland-Altman analyzes using double-positive samples were performed to evaluate the correlation between the assays. Bland-Altman analysis is typically used to compare measurement techniques against a reference value, and the Bland-Altman graph plots the difference between two techniques against their averages.Citation26
Statistical analysis
The experiments were conducted in duplicate to reduce errors, and sample data are presented as the mean ± standard deviation. GraphPad Prism software was used to perform the linear regression, Spearman correlation analysis, and Bland-Altman comparison analysis. The ROC curve was constructed and analyzed with Medcalc.
Results
Construction of the CV-A10 pseudovirus for single-round infection
After 48-h co-transfection of HEK293T cells with the CV-A10 capsid expression vector ()), EV-A71 replicon, and T7 RNA polymerase, RT-PCR showed a product of 1.6 kb corresponding to the expected fragment size (), lanes 3,4). The formation of CV-A10 pseudovirus was also confirmed by immunoblotting, which revealed a specific band at 33 kDa (), lane 2) corresponding to the expected size of viral VP1 protein.
Figure 1. Characterization of CV-A10 pseudovirus. (a) Development of the CV-A10 capsid expression construct, including the P1 gene and EGFP gene, with an EV-A71 2A protease cleavage site (AITTL) inserted upstream of the P1 sequence. (b) Electrophoretic profile of the PCR products of CV-A10 pseudovirus. The reporter gene Luc was detected in the supernatant of the HEK293T cell culture medium co-transfected with three plasmids. Lane 1: 1-kb ladder DNA marker; Lane 2: PCR products of the plasmid expressing Luc; Lanes 3 and 4: PCR products of the supernatant. (c) Immunoblot analysis for CV-A10 pseudovirus. VP1 protein was detected both in the CV-A10 pseudovirus and wild-type virus using mouse anti-VP1 mAb. Lane 1: protein marker; Lane 2: anti-VP1 mAb reacted with pseudovirus; Lane 3: anti-VP1 mAb reacted with wild-type virus. (d) CV-A10 pseudovirus neutralized by anti-CV-A10 serum based on detection of luciferase activity. (e) Specificity of CV-A10 pseudovirus tested against mouse anti EV-A71, CV-A16, CV-A6, CV-B3, and EV-D68 serum with the same amount of a diluted CV-A10 pseudovirus particle suspension (50 μl).
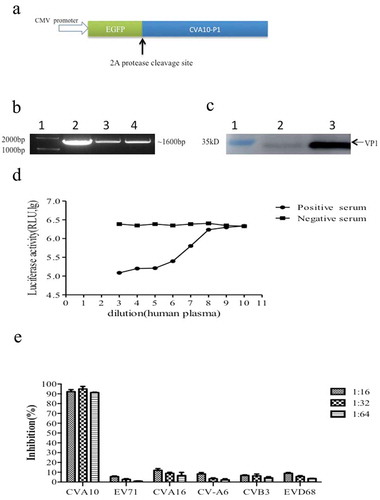
Figure 2. Optimization of the CV-A10 pseudovirus luciferase assay. (a) Infectivity of CV-A10 pseudovirus in different susceptible cell lines. (b) Determination of optimal RD cell density. (c) Reaction time optimization. (d) CV-A10 pseudovirus infection linearity. Serially diluted CV-A10 pseudovirus was mixed with RD cells, and luciferase activity was measured at 10 h post-0infection. Points beyond the linear range were excluded.
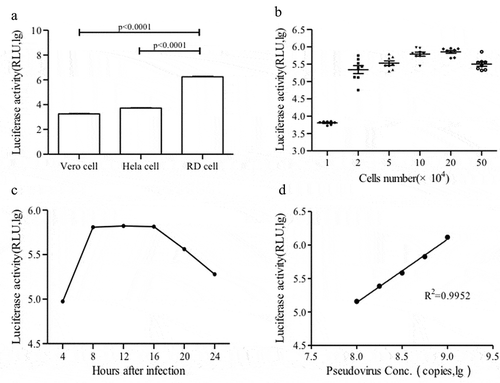
Infection of CV-A10 pseudovirus could be inhibited by mouse anti-CV-A10 serum in a dose-dependent manner; thus, an RLU at a 1:1024 dilution of positive serum was equivalent to a negative serum sample ()). The specificity of CV-A10 pseudovirus was further confirmed using a panel of mouse antisera against other enteroviruses (EV-A71, CV-A16, CV-A6, CV-B3 and EV-D68). CV-A10 pseudovirus could be neutralized by antisera against CV-A10 at a dilution of 1:161:32 and 1:64, but could not be neutralized with antisera against the other enterovirus ()).
Taken together, these results showed that CV-A10 could be efficiently pseudotyped with EV-A71 replicon RNA and CV-A10 capsid protein, providing a valuable tool for anti-CV-A10 NtAbs detection. Therefore, we next aimed to develop an in vitro neutralization assay based on the CV-A10 pseudovirus.
Optimization of the pNT for anti-CV-A10 NtAb quantification
The relative infectivity of CV-A10 pseudovirus was compared in three common cell lines. The luciferase activity in RD cells was significantly higher than that in HeLa cells and Vero cells upon CV-A10 pseudovirus infection (p < .0001); thus, RD cells were chosen for further assay development ()). The optimal number of input cells was then determined based on the maximum fluorescent reading in RLU. The luciferase activity peaked at a density of 2 × 105 cells/well ()), which was used for the subsequent assays. The kinetic pattern of CV-A10 pseudovirus infectivity was determined by measuring the luciferase activity of infected RD cells over a time course ()). The luciferase activity reached a plateau at about 8 h post-infection and decreased at 16 h. Therefore, 10 h was chosen as the optimized incubation time. Further, the CV-A10 pseudovirus infection system showed a good linear response (r2 > 0.99) between luciferase activity and virus input over a narrow range (9.0 × 108 to 9.0 × 109 genome equivalents per well) ()); therefore, 9.0 × 109 copies/50 μl was used in the subsequent assays.
Comparison of the pNT and cNT for anti-CV-A10 NtAb quantification
The NtAb titres obtained from the cNT were defined as the reciprocal of the highest dilution at which over 50% of the wells showed complete inhibition as a cytopathic effect; samples with a titre ≥ 8 were considered positive, and other samples were considered negative.
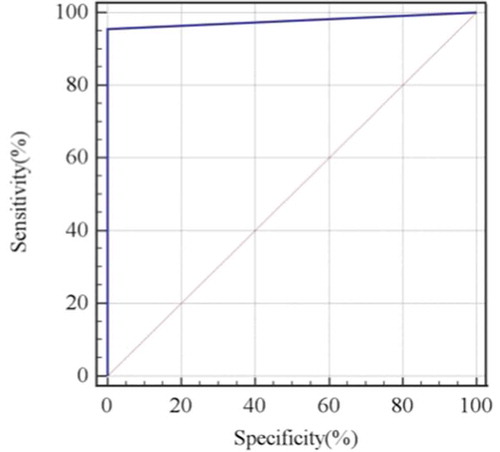
The ROC curve was applied to estimate the cutoff value of the pNT. When the cutoff value of the pNT was set to 8, as the level producing the best balance of overall agreement, sensitivity, and specificity with respect to the reference cNT, the highest level of agreement was obtained with the cNT, with an area under the ROC curve value of 0.979 and a Youden’s index of 0.957 ().
Figure 3. ROC curve analysis to estimate the degree of agreement between the cNT and pNT and to determine the cutoff value of the CV-A10 pseudovirus pNT. The hollow dot represents the cut off of CV-A10 pseudovirus.
Overall, 47 of the 67 serum samples were positive in the cNT, and 45 of these cNT-positive samples were also positive in the pNT (double-positive serum samples). The sensitivity and specificity of the pNT were 95.7% [95% confidence interval (CI): 85.5–99.5%] and 100% (95% CI: 83.2–100%), with a positive and negative predictive value of 100% (95% CI: 92.1–100%) and 90.9% (95% CI: 70.8–998.9%), respectively ().
Table 2. Calculated sensitivity, specificity, positive and negative predictive values for the pNT in comparison with the cNT.
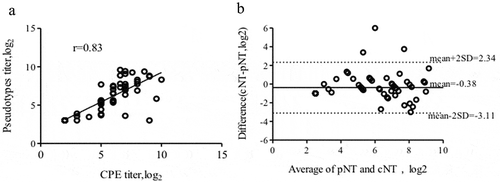
The titres of these 45 double-positive serum samples were compared with Spearman correlation analysis showing strong correlation between the results from these two assays (r = 0.83, P < .0001) ()). The Bland-Altman comparison method further showed that these two methods were highly consistent. The average logarithmic difference for the quantitative results of the two methods was −0.38, and the standard deviation was 1.39 with only three values falling outside ±2 standard deviations (–3.11 to 2.34log10) ()). These results indicated that the new method has a high degree of agreement with the current gold-standard assay.
Figure 4. Correlation between the results of the CV-A10 pNT and cNT test. (a) Titres of anti-CV-A10 NtAb in human serum samples detected by the pNT and cNT analyzed by Spearman correlation (r = 0.83, P < .0001). (b) Titres of anti-CV-A10 NTAbs in human serum with the pNT and cNT compared by the Bland-Altman method.
Discussion
HFMD is a common viral illness that mainly affects children, which causes a mild febrile illness, maculopapular rash, and even severe neurological and systemic complications.Citation27 Although EV-A71 vaccines have proven to be effective to prevent a severe HFMD epidemic caused by EV-A71,Citation28 multivalent HFMD vaccines against the major HFMD-associated enteroviruses (EV-A71,CV-A16, CV-A6, and CV-A10) are desirable for improved HFMD prevention and control.Citation29 The high-throughput quantification of NtAb elicited by multivalent vaccines will significantly contribute to their development and evaluation.
In recent years, CV-A10 has gradually become one of the major pathogens associated with HFMD.Citation30 Therefore, evaluation of the NTAbs produced against CV-A10 in a population is essential for understanding the status of herd immunity and can facilitate the development of vaccines and antiviral reagents. The standard cNT method for this task is labor-intensive, subjective, and time-consuming. As an alternative, Liu et al.Citation31 developed an enzyme-linked immune absorbent spot-based neutralization assay using the monoclonal antibody 3D1 against CV-A10, which effectively shortened the detection period; however, the production of false positive spots may influence interpretation of result.
In this study, a single-round CV-A10 pseudovirus was successfully established, and a fast and quantitative method was developed to measure the CV-A10 neutralizing antibodies in serum. We encapsulated EV-A71 replicon RNA with CV-A10 capsid protein to produce an infectious pseudovirus. We previously found that capsid proteins from CVA16 could package the EV-A71 replicon into infectious CV-A16 pseudovirus, capsid proteins from CV-B5 were used to package CV-B3 replicon RNA into infectious CV-B5 pseudovirus, and capsid proteins from CV-A6 could package EV-A71 replicon RNA into infectious CV-A6 pseudovirus. Therefore, the present work expands the successful application of this trans-encapsidation strategy to produce infectious CV-A10 pseudovirus. A new NtAbs quantification assay was then developed using this CV10 pseudovirus, which showed good correlation with the results obtained with the traditional cNT based on the cytopathic effect. Therefore, the established CV-A10 pNT offers a high-throughput method for anti-CV-A10 NtAb detection as a useful tool for CV-A10 vaccine evaluation and serology surveillance. We had proven the principle of utilizing the pNT assay as a convenient assay to detect anti-CV-A10 NtAb in human serum samples, though the number of serum samples analyzed was limited. Further improvement and validation will be performed in the future before standardizing this pNT assay for efficacy evaluation of CV-A10 vaccine candidates. By the way, development of anti-serum reference standard for CV-A10 would help to optimize this pseudovirus assay.
To date, we have established pseudovirus-based NtAb quantification assays for EV-A71, CV-A16, CV-B3, CV-B5, CV-A6, and CV-A10. Although the EV-A71 vaccine is already licensed for use in China, several challenges remain for multivalent vaccine development. Our pseudovirus-based assays can therefore offer a toolbox for improved efficacy evaluation of HFMD-associated vaccines in the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Bian L, Gao F, Mao Q, Sun S, Wu X, Liu S, Yang X, Liang Z. Hand, foot, and mouth disease associated with coxsackievirus A10: more serious than it seems. Exp Rev Anti-infect Ther. 2019;17(4):233–42. doi:10.1080/14787210.2019.1585242.
- Ji T, Guo Y, Huang W, Shi Y, Xu Y, Tong W, Yao W, Tan Z, Zeng H, Ma J, et al. The emerging sub-genotype C2 of CoxsackievirusA10 associated with hand, foot and mouth disease extensively circulating in mainland of China. Sci Rep. 2018;8:13357. doi:10.1038/s41598-018-31616-x.
- Munivenkatappa A, Yadav PD, Nyayanit DA, Majumdar TD, Sangal L, Jain S, Sinha DP, Shrivastava A, Mourya DT. Molecular diversity of Coxsackievirus A10 circulating in the southern and northern region of India [2009–17]. Infect Genet Evol. 2018;66:101–10. doi:10.1016/j.meegid.2018.09.004.
- Yang Q, Ding J, Cao J, Huang Q, Hong C, Yang B. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Wuhan, China from 2012 to 2013: outbreaks of Coxsackieviruses A10. J Med Virol. 2015;87:954–60. doi:10.1002/jmv.v87.6.
- Bracho MA, González-Candelas F, Valero A, Córdoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223–31. doi:10.3201/eid1712.110395.
- Okada H, Wada M, Sato H, Yamaguchi Y, Tanji H, Kurokawa K, Kawanami T, Takahashi T, Kato T. Neuromyelitis optica preceded by hyperckemia and a possible association with coxsackie virus group a10 infection. Int Med. 2013;52:2665–68. doi:10.2169/internalmedicine.52.1042.
- Kumar A, Shukla D, Kumar R, Idris MZ, Jauhari P, Srivastava S, Dhole TN. Molecular identification of enteroviruses associated with aseptic meningitis in children from India. Arch Virol. 2013;158:211–15. doi:10.1007/s00705-012-1476-7.
- Zhang W, Dai W, Zhang C, Zhou Y, Xiong P, Wang S, Ye X, Liu Q, Zhou D, Huang Z. A virus-like particle-based tetravalent vaccine for hand, foot, and mouth disease elicits broad and balanced protective immunity. Emerging Microbes Infect. 2018;7:94. doi:10.1038/s41426-018-0094-1.
- Shen C, Liu Q, Zhou Y, Ku Z, Wang L, Lan K, Ye X, Huang Z. Inactivated Coxsackievirus A10 experimental vaccines protect mice against lethal viral challenge. Vaccine. 2016;34:5005–12. doi:10.1016/j.vaccine.2016.08.033.
- Zhou Y, Zhang C, Liu Q, Gong S, Geng L, Huang Z. A virus-like particle vaccine protects mice against Coxsackievirus A10 lethal infection. Antiviral Res. 2018;152:124–30. doi:10.1016/j.antiviral.2018.02.016.
- Jin P, Li J, Zhang X, Meng F, Zhou Y, Yao X, Gan Z, Zhu F. Validation and evaluation of serological correlates of protection for inactivated enterovirus 71 vaccine in children aged 6–35 months. Hum Vaccines Immunother. 2016;12:916–21. doi:10.1080/21645515.2015.1118595.
- Gao F, Bian L, Hao X, Hu Y, Yao X, Sun S, Chen P, Yang C, Du R, Li J, et al. Seroepidemiology of Coxsackievirus B5 in infants and children in Jiangsu Province, China. Hum Vaccines Immunother. 2017;14:74–80. doi:10.1080/21645515.2017.1384107.
- Sun SY, Gao F, Hu YL, Bian LL, Mao QY, Wu X, Li JX, Zhu FC, Wang JW, Liang ZL. Seroepidemiology of enterovirus d68 infection in infants and children in Jiangsu, China. J Infect. 2018;76:563–69. doi:10.1016/j.jinf.2018.02.003.
- Dai WL, Xiong P, Zhang XY, Liu Z, Chen J, Zhou Y, Ye X, Zhang C. Recombinant virus-like particle presenting a newly identified Coxsackievirus A10 neutralization epitope induces protective immunity in mice. Antiviral Res. 2019;164:139–46. doi:10.1016/j.antiviral.2019.02.016.
- World Health Organization. Manual for the virological investigation of poliomyelitis[M]. Geneva (Switzerland): World Health Organization; 2000.
- Jiang Z, Liu G, Guo-Yang L, Sun M, Xu K, Ying Z, Wang J, Li X, Li C. A simple and safe antibody neutralization assay based on polio pseudoviruses. Hum Vaccines Immunother. 2019;15:349–57. doi:10.1080/21645515.2018.1526553.
- Li Y, Zhao Y, Wang C, Zheng X, Wang H, Gai W, Jin H, Yan F, Qiu B, Gao Y, et al. Packaging of Rift Valley fever virus pseudoviruses and establishment of a neutralization assay method. J Vet Sci. 2018;19:200–06. doi:10.4142/jvs.2018.19.2.200.
- Du P, Brendle S, Milici J, Camacho F, Zurlo J, Christensen N, Meyers C. Comparisons of VLP-based ELISA, neutralization assays with native HPV, and neutralization assays with PSV in detecting HPV antibody responses in HIV-infected women. J AIDS Clin Res. 2015;6:433. doi:10.4172/2155-6113.1000433.
- Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439–52. doi:10.1128/JVI.02108-09.
- Wu X, Mao Q, Yao X, Chen P, Chen X, Shao J, Gao F, Yu X, Zhu F, Li R, et al. Development and evaluation of a pseudovirus-luciferase assay for rapid and quantitative detection of neutralizing antibodies against enterovirus 71. PLoS One. 2013;8:e64116. doi:10.1371/journal.pone.0064116.
- Hao X, Chen P, Mao Q, Shao J, Lin H, Luo Z, Wu X, Liang Z. Establishment of a pseudovirus luciferase assay for the detection of coxsackievirus A16 neutralizing antibody. Chin J Viral Dis. 2016;6:6–11.
- Chen P, Wu X, Mao Q, Gao F, Hao X, Bian L, Zhu F, Li W, Xu M, Liang Z. A rapid and quantitative assay for measuring neutralizing antibodies of Coxsackievirus B3. J Virol Methods. 2016;232:1–7. doi:10.1016/j.jviromet.2016.02.010.
- Chen P, Wu X, Su Y, Hao X, Mao Q, Liang Z. Development of a pseudovirus based assay for measuring neutralizing antibodies against Coxsackievirus B5. J Virol Methods. 2017;246:21–26. doi:10.1016/j.jviromet.2017.04.005.
- Su Y, Chen P, Gao F, Bian L, Sun S, Dong F, Hu Y, Mao Q, Jiang W, Wu X, et al. A surrogate assay for measuring Coxsackievirus A6 neutralizing antibodies. Hum Vaccin Immunother. 2018;14(12):3034–40. doi:10.1080/21645515.2018.1504540.
- Reed LJ, Muensch H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–97.
- Braždžionytė J, Macas A. Bland–altman analysis as an alternative approach for statistical evaluation of agreement between two methods for measuring hemodynamics during acute myocardial infarction. Medicina. 2006;43:3. doi:10.3390/medicina43030025.
- Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AE. Epidemic hand, foot and mouth disease caused by human enterovirus 71, Singapore. Emerg Infect Dis. 2003;9:78–85. doi:10.3201/eid1301.020112.
- Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Rev Vaccines. 2016;15:599–606. doi:10.1586/14760584.2016.1138862.
- Klein M, Chong P. Is a multivalent hand, foot and mouth disease vaccine feasible? Hum Vaccines Immunother. 2015;11:2688–2674. doi:10.1080/21645515.2015.1049780.
- Fu Y, Sun SY, Mao QY, Bian LL, Wu X, Zhu FC, Jiang CL, Gao F, Liang ZL. Seroepidemiology of Coxsackievirus A10 infection in infants and children: a prospective cohort study in Jiangsu, China. J Infect. 2018;77:158–64. doi:10.1016/j.jinf.2018.04.005.
- Liu D, Xu L, Zhu R, Yin Z, Lin Y, Hou W, Li S, He S, Cheng T, Xia N. Development of an efficient neutralization assay for Coxsackievirus A10. Appl Microbiol Biotechnol. 2019;103:1931–38. doi:10.1007/s00253-018-09598-7.
