ABSTRACT
An Escherichia. coli-produced HPV-16/18 bivalent vaccine has been proved to be well-tolerated and highly efficacious against diseases associated with vaccine HPV types. As a part of the multi-center, randomized, double-blind phase III clinical trial, this lot-to-lot consistency study aimed to assess the safety and immunogenicity consistency of this novel HPV vaccine, which is also one of the objectives of the phase III trial. A total of 3689 healthy women aged 18–45 years were enrolled and randomly assigned 1:1:1 to three lots of the HPV vaccine groups. The primary outcomes were the IgG antibody level at 1 month after the last dose (month 7). In the immunogenicity per-protocol set (PPS), almost all of the participants seroconverted at month 7 and remained seropositive at month 42. For each paired comparison of the three lot groups, the two-sides of 90% CIs of GMC ratios for both IgG and neutralizing antibodies for HPV-16 and HPV-18 at month 7 were within the equivalence interval [0.5, 2]. Lot consistency was also demonstrated at month 42. The majority of recorded solicited reactions were mild or moderate. The incidences of solicited reactions of Lot 2 and Lot 3 were slightly higher than Lot 1. However, the incidences of solicited reactions of ≥ grade 3 and solicited reactions by symptoms were all similar among the three lot groups. None of the SAEs was considered related to vaccination by the investigator. In conclusion, this study demonstrates lot-to-lot consistency of the 3 consecutive lots of the E. coli-produced HPV-16/18 bivalent vaccine.
Introduction
Human papillomavirus (HPV) is recognized as the cause of almost all cervical cancer, a substantial proportion of other anogenital cancers and a subset of oropharyngeal cancers.Citation1,Citation2 It is estimated that 4.5% of the new cancer cases worldwide are attributable to HPV.Citation3 Three prophylactic HPV vaccines are currently available and had been introduced in many countries. In countries that had implemented national HPV vaccination programs with high vaccination coverage rates, the prevalence and incidence of HPV vaccine types related infections, cervical intraepithelial neoplasia grade 2+ (CIN2+), and anogenital warts decreased substantially after national HPV vaccination program introduction.Citation4-Citation7 However, in most middle and lower income countries, the three HPV vaccines are unaffordable due to the high cost, and the limited production capacity affects availability.Citation8
An Escherichia coli (E. coli)-produced bivalent human papillomavirus (type 16 and 18) vaccine has been developed and proved to be well-tolerated and highly efficacious against HPV-16/18-associated high-grade genital lesions and persistent infections in adult women aged 18–45 y in China.Citation9-Citation11 And the immune responses of this candidate HPV vaccine in adolescent girls aged 9–14 y receiving two doses or girls aged 9–17 y receiving three doses were non-inferior to that in adult women in an immunobridging study.Citation12 The safety and immunogenicity consistency in the production of this novel E. coli-produced bivalent HPV vaccine still remains to be assessed.
Although there was no immunological correlate of protection for HPV vaccines, antibody levels had been used to bridge the efficacy in women to adolescents of which gynecologic examination could not be conducted,Citation13-Citation16 and to optimize the immunization scheduleCitation17 or to provide insights into the potential for long-term protection; thus, it is also an appropriate marker for assessing lot consistency. There were several assays developed to evaluate the type-specific HPV antibody levels, among which neutralizing antibodies measured by pseudovirion-based neutralization assay (PBNA) was deemed as the gold standard for analyzing protective antibodies.Citation18,Citation19 However, due to the complex and labor-consuming characteristics of the PBNA assay, it could hardly be used in large clinical trials with hundreds or thousands of samples. Immunoglobin G (IgG) antibodies measured by HPV L1 virus-like particle (VLP)-based enzyme-linked immunosorbent assays (ELISAs) had been proved to be an acceptable surrogate for the neutralizing antibody assay when measuring antibodies induced by vaccination.Citation20
The participants were randomized to receive three consecutive lots of the HPV-16/18 bivalent vaccine or the control vaccine (a commercialized hepatitis E vaccine) in the Phase III efficacy clinical trial. This study was to analyse the safety and immunogenicity consistency of three consecutive lots of vaccine, with HPV-16 and −18 specific IgG antibodies as the primary immunogenicity endpoints and neutralizing antibodies as the secondary immunogenicity endpoints.
Materials and methods
Study design and participants
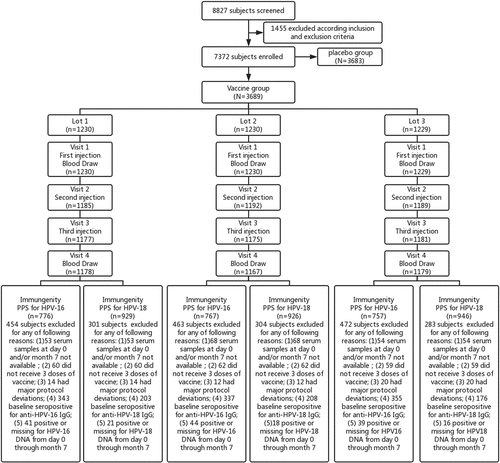
This lot-to-lot consistency study was a part of the multi-center, randomized, double-blind, placebo-controlled phase III clinical trial which was to assess the efficacy of the E. coli-produced HPV-16/18 bivalent vaccine in adult women (NCT01735006). This study was conducted at four study centres including Cancer Hospital Chinese Academy of Medical Sciences (having two sites: Xinmi city in Henan, Yangcheng city in Shanxi), Peking University People’s Hospital (one site: Fengning city in Hebei), Jiangsu Provincial Centre for Disease Control and Prevention (one site: Funing city in Jiangsu Province), and Guangxi Liuzhou Centre for Disease Control and Prevention (one site: Liuzhou city in Guangxi). The study was approved by the Independent Ethics Committees of each center (12-72/606, 2012–48, 2012044, IRB00001594) and conducted in accordance with the Good Clinical Practice and Chinese regulatory requirements.
The aim of this study is to evaluate lot-to-lot consistency of the test HPV bivalent vaccine which is one of the objectives of the phase III clinical trial. Healthy adult women aged 18 to 45 years with intact cervix and without acute cervicitis and acute lower reproductive tract infections were eligible for the inclusion. The main study exclusion criteria were: pregnancy; lactation; history of severe internal diseases; history of sexually transmitted diseases; receipt of any HPV vaccines. Detail eligibility criteria had been previously described.Citation11
Study of vaccines
The test bivalent HPV-16/18 vaccine has been described in the previous study.Citation9,Citation10,Citation21 A 0.5 mL dose of test vaccine contains 40 μg of HPV-16 and 20 μg of HPV-18 L1 VLPs expressed in E. coli which were adsorbed to 208 μg of aluminum adjuvant. Three Lots of test bivalent HPV vaccine (Lot1, B20120404; Lot2, B20120405; Lot 3, B20120506) were used in the study. The placebo vaccine was commercialized hepatitis E vaccine (Hecolin®) contains 30 μg of E. coli-produced recombinant hepatitis E virus capsid protein and 277 μg of aluminum adjuvant (0.5mL/dose). The test vaccine and placebo vaccine were repackaged and supplied in coded single-dose vials with identical appearance.
Randomization
Participants were stratified by age with 2 age groups (18–26 y and 27–45 y) and then randomly assigned 1:1 to receive either the HPV-16/18 vaccine or the control hepatitis E vaccine. The randomization lists were generalized by SAS software (version 9.1, SAS Institute, Cary, NC) based on block size of 12, including 6 random numbers for 3 lots of the study vaccines (2 random numbers per lot) and 6 numbers for the control vaccines; thus, the participants had been also randomized to receive the three lots of HPV vaccine, with ratio of 1:1:1. All participants and investigators were blinded during the whole study.
Immunogenicity assessments
Serum samples of all the participants were collected at day 0 and month 7 and serum samples of those from the Xinmi site were also collected at month 42. Month 7 and 42 serum samples were subjected to secondary blinding by persons not involved in the clinical study under the supervision of non-blinded statisticians before testing. HPV16/18 IgG antibodies were tested using the same reagent lot by the National Institute for the Control of Pharmaceutical and Biological Products within the same time period by the same testing team. Levels of IgG antibody against HPV-16 and −18 were measured by E. coli-expressed HPV L1 VLP-based ELISA with a cutoff value of 3.0 IU/mL for HPV-16 and 2.1 IU/mL for HPV-18.Citation11 Briefly, 96-well microtiter plates were coated with HPV 16 VLPs or HPV 18 VLPs produced in E. coli. Diluted serum samples were added and after incubation and wash, horse-radish peroxidase-conjugated goat anti-human IgG was added. After incubation and a second wash, tetramethylbenzidine was added. Following incubation, the reactions were stopped with the addition of H2SO4, and the optical density (OD) was read at 450/620 nm. Serum dilutions were adjusted so that the readings were within the linear range of the assay and the titers were calculated using freeze-dried serum as standard which is calibrated by World Health Organization international standards for antibodies against HPV-16 (NIBSC code 05/134) or HPV-18 (NIBSC code 10/140). The antibody level for negative serum sample was arbitrarily set to half of the cutoff value. HPV16/18 neutralizing antibodies were tested using same reagent lot by Xiamen Innovax Biotech CO., LTD within same time period by same testing team. PBNA was employed to measure neutralizing antibodies against HPV-16 and −18 with a cutoff value of 20 YU/mL.Citation20,Citation22 The serum samples to be tested were serially diluted and dilutions incubated at room temperature with HPV-16 or HPV-18 pseudovirions produced by co-transfecting human embryonic kidney (293FT) cells with plasmids encoding for HPV L1, HPV L2, and green fluorescent protein (GFP). Then, the serum-pseudovirus mixtures were added to 293FT cell monolayers and cultured for three days. A positive sample was defined as one that caused a 50% reduction in green fluorescent protein (GFP) expression compared with the negative control, and the neutralizing titers were defined as the highest dilution of the positive samples. The neutralizing titers of the negative samples were set at 1:10, which is half of the starting serum dilution. All the serum samples at day 0 were qualitatively tested for IgG antibodies, and all the samples of month 7 and month 42 were quantitatively tested for IgG antibodies. Only samples from the Xinmi site at month 0 were further quantitatively tested for IgG antibodies, and only samples from Xinmi site at month 0 and month 7 were measured for neutralizing antibodies.
HPV DNA assessment
Methods of HPV DNA assessment had been described previously.Citation11 In brief, cervical swab was collected for each women pre-vaccination and at month 7 by trained gynecologist. Those with abnormal cytology diagnosis might be referred to colposcopy and biopsy if needed. The satisfying swabs, and biopsy samples with diagnosis of grade I or higher cervical intraepithelial neoplasia (CIN), vulvar intraepithelial neoplasia (VIN), and/or vaginal intraepithelial neoplasia (VaIN), were tested for HPV DNA by HPV DNA enzyme immunoassay method (Labo Biomedical Products, the Netherlands), positive samples would be further typed by reverse hybridization line probe assay (Labo Biomedical Products, Netherlands, based on licensed Innogenetics LiPA technology) to detect 14 oncogenic and 11 nononcogenic HPV types. In addition, all HPV positive samples were also tested by HPV-16 and −18 specific polymerase chain reactions (PCRs) (HPV TS16/18, Labo Biomedical Products, Netherlands). A positive result for HPV-16 or HPV-18 was defined as the presence of the relative type of HPV DNA by LiPA or HPV TS16/18.
Safety assessment
The participants were requested to stay for at least 30 min after each vaccination, any adverse reactions observed were documented by the investigators. All the women were trained to record all of the adverse reactions/events (AEs) that occurred after each vaccination in a diary card, including the solicited local and general symptoms for up to 7 days and unsolicited adverse events in 30 days after each dose. All the women were face-to-face followed-up or through phone call for at least twice in 7 days and for another one time during day 8 to day 30 after each vaccination. Serious adverse events (SAEs) were recorded for the entire duration of the whole trial.
Statistical analysis
Sample size was determined according to the efficacy endpoint assessment as previously described.Citation11 For lot-to-lot consistency analysis, assuming 20% non-evaluable subjects (loss to follow-up, baseline seropositive and DNA positive for HPV-16 or HPV-18), 588 subjects per group were needed to conclude consistency between the three lots with an overall power of over 90%. The total sample size for the phase III clinical trial were over 6000, which is sufficient to observe the differences between the lots. The primary analysis was based on the per-protocol set (PPS) for immunogenicity in the vaccine group which included the participants who were seronegative for the relative antibodies at baseline, were negative for relevant types of HPV DNA from day 0 through month 7, provided evaluable serum samples at day 0 and month 7, received all three doses of vaccine according to the protocol, and had no major protocol deviations. To assess the lot-to-lot consistency of geometric mean concentration (GMC), the 90% confidence intervals (CIs) of the GMC ratios of IgG and neutralizing antibodies at month 7 were calculated for each pair of vaccine lots (Lot 2 vs. Lot 1, Lot 3 vs. Lot 1, Lot 3 vs. Lot 2) with analysis of covariance (ANCOVA) model. GMC ratios for IgG antibodies at month 7 were adjusted by ANCOVA model with logarithmic antibody titer as the dependent variable, and Lot, study site and Lot*study site interaction as independent variable. Consistency was reached if the two-side 90% CIs of GMC ratio within the [0.5, 2] interval.
All participants were included in safety analysis if they received at least one dose of vaccine. All statistical analyses were performed by SAS 9.2 software (SAS Institute, Cary, NC).
Result
Study population
From November 22, 2012, to April 1, 2013, a total of 7372 participants were enrolled and randomly assigned to receive the HPV-16/18 vaccine (3689 women) or hepatitis E vaccine (placebo, 3683 women). Of the 3689 participants in the HPV vaccine group, 1230, 1230 and 1229 women were randomly assigned to the Lot 1, Lot 2 and Lot 3 groups, respectively (). There were one woman in each of the three lot groups who received a wrong vaccine, vaccine of other lot or the placebo, by mistakes during the whole vaccination course, thus, these three women were excluded and leaving 3686 subjects in the safety analysis set. The baseline characteristics of subjects, including age, seroprevalence, and GMC of HPV-16/18 specific IgG and neutralizing antibodies, prevalence of HPV-16/18 DNA was balanced among the three groups, which were summarized in .
Table 1. Baseline characteristics of participants in the three vaccine lot groups.
Immunogenicity
In the immunogenicity PPS cohort who received three doses of the HPV-16/18 vaccine according to protocol and were seronegative for the relative antibody at entry, almost all of the participants seroconverted at 1 month after the last dose (month 7), with seroconversion rates for HPV-16 IgG of all the three lots was between 99.9%-100.0% and for HPV-18 were between 99.8%-100.0%. At month 42, all the participants from Xinmi site in the PPS cohort remained seropositive for HPV-16 IgG, and more than 98.8% of them were seropositive for HPV-18 IgG. For neutralizing antibodies, all the participants except for one in Lot 2 group seroconverted for both HPV types at month 7 in the PPS cohort of Xinmi site ().
Table 2. Immuno-response at month 7 among the three lot groups (PPS for immunogenicity).
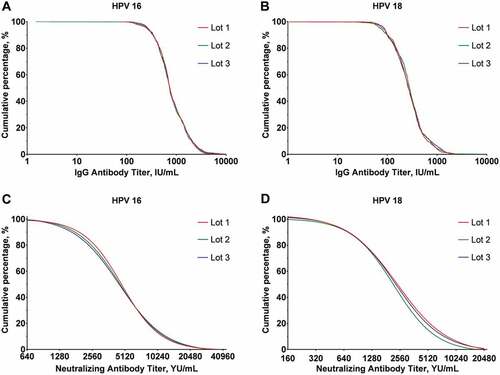
At month 7, the GMCs and 95%CI for HPV-16 IgG was 779.7 IU/mL (741.9, 819.3), 789.2 IU/mL (749.0, 831.4), and 802.9 IU/mL (763.1, 844.8); for HPV-18 IgG was 265.7 IU/mL (253.5, 278.5), 270.3 IU/mL (256.7, 284.6) and 267.7 IU/mL (255.1, 280.9) in the PPS cohort of Lot 1, Lot 2 and Lot 3 group separately. The GMCs of neutralizing antibodies to HPV-16 were 3313.8 YU/mL (2977.0, 3688.6), 3165.9 YU/mL (2798.7, 3581.1) and 3171.2 YU/mL (2835.7, 3546.5), and to HPV-18 were 1720.2 YU/mL (1516.6, 1951.2), 1476.5 YU/mL (1297.9, 1679.7) and 1658.5 YU/mL (1460.8, 1883.0) for the three lots (). For each paired comparison of the three Lot groups (Lot 2 vs. Lot 1, Lot 3 vs. Lot 1 and Lot 3 vs. Lot 2), the two-sides of 90% CIs of GMC ratios for both IgG and neutralizing antibodies for both HPV-16 and HPV-18 at month 7 were within the equivalence interval [0.5, 2] (). Reverse cumulative curves for IgG and neutralizing antibody titers of the three lots groups at month 7 were almost overlapped (). At month 42, both HPV-16 and HPV-18 IgG concentrations of the three lot groups decreased to around 10% of the peak levels at month 7, while they remained consistent with GMC ratios remaining within the equivalence interval (). In those baselines seropositive participants who received three doses vaccine according to the protocols and had no major protocol deviations, HPV-16 and HPV-18 IgG antibody levels were also comparable at month 7 and month 42 ().
Table 3. Equivalence analysis of GMC at month 7 (PPS for immunogenicity).
Table 4. Immuno-response at month 7 and 42 among three lots groups in participants who were seropositive at baseline*.
Safety
As shown in , the majority of solicited and unsolicited events were mild or moderate. The proportion of women reported solicited AEs of grade 3 or more were 0.7%, 1.1% and 0.8% in Lot 1 to Lot 3 groups (P = 0.41), which were mainly induration, redness, swelling, and fever. The incidence of any solicited adverse events of Lot 2 group (65.6%) were similar to Lot 3 (63.0%), while slightly higher than Lot 1 (60.5%). However, the incidence of solicited adverse events by symptoms were all similar among three lots groups. Injection site pain was the most frequent local adverse event, reported in 33.3% to 35.2% of participants in the three lots groups. Fever was the most common systemic adverse events (33.4% to 36.2%). During the entire study, SAEs were reported in 6.3%, 5.4% and 5.0% participants in Lot 1 to Lot 3 groups (P = .39). Three deaths reported during the entire study, with one in Lot 1 group and two in Lot 3 group. One participant with a history of type 2 diabetes (for >10 years) in Lot 1 died from diabetic ketoacidosis at 14 months post the third injection. In Lot 3, one participant made suicide by taking poison at 13 days post the first injection and one died from traffic accidents at 24 months after last injections. None of the SAEs was considered related to vaccination by the investigator.
Table 5. Incidence of adverse events (safety analysis).
Discussion
In this multi-center, randomized, double-blind, placebo-controlled phase III clinical trial, we demonstrated the safety and immunogenicity consistency of the three consecutive lots of E. coli-produced HPV-16/18 bivalent vaccines, which indicated the stability in the production process of this novel vaccine.
Although neutralizing antibodies measured by PBNA was thought as the gold standard for analyzing protective antibodies,Citation18,Citation19 and should be the most suitable index for evaluating lot-to-lot consistency of immunogenicity, due to its complexity and low throughput, it was set as the secondary endpoint for analysis and measured only samples of a subgroup (Xinmi site) of the whole cohorts. HPV type-specific IgG antibodies were designed as the primary endpoint, as it is a highly reproducible method and correlated well with the neutralizing antibodies when testing post-vaccination serum samples.Citation20 Data showed that all the three consecutive lots of HPV vaccine were highly immunogenic and produced high level of both HPV-16/18 specific IgG and neutralizing antibodies among women aged 18 to 45 years. The GMC ratios of HPV-16 and HPV-18 IgG antibodies at month 7 and month 42 ranged from 0.82 to 1.02, which all met the predefined equivalence criterion. The data also indicated the three lots of HPV vaccines induced consistent protective neutralizing antibodies. The seroconversion rates of all the three consecutive lots for IgG and neutralizing antibodies were over 99.5% at month 7 and seropositive rate for IgG antibodies remained over 98.8% at month 42, which is similar to the marketed HPV vaccine in Chinese womenCitation23,Citation24 and indicated similar immuno-persistency for the three lots.
Due to the different assays used in HPV vaccine clinical trials to measure different subsets of the constellation of antibodies induced by VLP vaccineCitation25 and lack of controlled comparisons, it is difficult to make direct comparison of immunogenicity profiles of test vaccine and market vaccines. However, similar to the market vaccines, peak IgG levels at month 7 were over 100-fold (for HPV-16) and 50-fold (for HPV-18) higher than those induced by natural infection, then declined approximately 10-fold 1 year later and reached a plateau level over the next 2 years.Citation11,Citation25 At month 7 after vaccination, the peak neutralizing antibody levels against HPV-16 and HPV-18 were also over 70-fold and 50-fold higher than after natural infection. In addition, the efficacies against high-grade genital lesions and persistent infection were 100.0% (95%CI: 55.6–100.0%) and 97.8% (95%CI: 87.1–99.9%), respectively, in the per-protocol cohort, which is also similar to the marketed HPV vaccines.Citation26,Citation27 These data indicating the E. coli-produced bivalent HPV-16/18 vaccine is a viable substitute for the commercial vaccine in terms of efficacy profile.
This bivalent HPV vaccines were well tolerated with most AEs mild or moderate and the safety profile was comparable between three lots. Injection site pain and fever were the most frequently reported local and systemic reactions, and the majority of them were mild or moderate. The incidence of ≥ grade 3 AEs among three lots were generally low and comparable from 0.7% to 1.2% for solicited AEs and from 0.4% to 0.7% for unsolicited AEs, in line with findings from clinical trials of marketed HPV vaccines which were conducted in China.Citation23,Citation24,Citation28 No SAE or death was consider vaccine-related during the entire study.
The strength of this study was the randomized controlled design with a large sample size, and both HPV type 16/18-specific IgG by ELISA and neutralizing antibodies by PBNA were assessed to evaluate the lot-to-lot immunogenicity consistency of this E. coli-produced HPV-16/18 bivalent vaccine, which would make the conclusion more reliable. However, the neutralizing antibody levels at month 7 and the IgG antibody levels at month 42 are only available for a subset in this study. Although the sample size for neutralizing antibody at month 7 and IgG antibody at month 42 was small (a smaller sample size will lead to a wider confidence interval), the 90% CIs of GMC ratios were still within the equivalence interval [0.5, 2].
In conclusion, this study demonstrates lot-to-lot consistency of the three consecutive lots of E. coli-produced HPV-16/18 bivalent vaccine, which could induce similar high level of HPV-16/18 type-specific IgG and protective neutralizing antibodies in healthy adult women with similar immune persistence.
Disclosure of potential conflicts of interest
B.-Z.L., Z.-J.L., G.S., H.-R.P. report being current employees of Xiamen Innovax. No other potential conflict of interest relevant to this article was reported.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. doi:10.1016/s2214-109x(16)30143-7. PMID: 27470177.
- Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26. doi:10.1016/j.bpobgyn.2017.08.006. PMID: 29037457.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–70. doi:10.1002/ijc.30716. PMID: 28369882.
- Drolet M, Benard E, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JML, Cummings T, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–80. doi:10.1016/s1473-3099(14)71073-4. PMID: 25744474.
- Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect. 2016;144:449–68. doi:10.1017/s0950268815002198. PMID: 26429676.
- Mesher D, Panwar K, Thomas SL, Edmundson C, Choi YH, Beddows S, Soldan K. The impact of the national HPV vaccination program in England using the bivalent HPV vaccine: surveillance of type-specific HPV in young females, 2010–2016. J Infect Dis. 2018;218:911–21. doi:10.1093/infdis/jiy249. PMID: 29917082.
- Drolet M, Benard E, Perez N, Brisson M; Group HPVVIS. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi:10.1016/S0140-6736(19)30298-3. PMID: 31255301.
- Clendinen C, Zhang Y, Warburton RN, Light DW. Manufacturing costs of HPV vaccines for developing countries. Vaccine. 2016;34:5984–89. doi:10.1016/j.vaccine.2016.09.042. PMID: 27771183.
- Hu YM, Huang SJ, Chu K, Wu T, Wang ZZ, Yang CL, Cai J-P, Jiang H-M, Wang Y-J, Guo M, et al. Safety of an Escherichia coli-expressed bivalent human papillomavirus (types 16 and 18) L1 virus-like particle vaccine: an open-label phase I clinical trial. Hum Vaccin Immunother. 2014;10:469–75. doi:10.4161/hv.26846. PMID: 24161937.
- Wu T, Hu YM, Li J, Chu K, Huang SJ, Zhao H, Wang ZZ, Yang CL, Jiang HM, Wang YJ, et al. Immunogenicity and safety of an E. coli-produced bivalent human papillomavirus (type 16 and 18) vaccine: A randomized controlled phase 2 clinical trial. Vaccine. 2015;33:3940–46. doi:10.1016/j.vaccine.2015.06.052. PMID: 26100924.
- Qiao YL, Wu T, Li RC, Hu YM, Wei LH, Li CG, Chen W, Huang SJ, Zhao FH, Li MQ, et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2019. doi:10.1093/jnci/djz074. PMID: 31086947.
- Hu Y, Guo M, Li C, Chu K, He W, Zhang J, Gu J, Li J, Zhao H, Wu X, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. 2019. doi:10.1007/s11427-019-9547-7. PMID: 31231780.
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec. 2017; 92:241–68.
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–45. doi:10.1542/peds.2006-0461. PMID: 17079588.
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40:564–71. doi:10.1016/j.jadohealth.2007.02.015. PMID: 17531764.
- Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, Huang L-M, Kim DS, Pitisuttithum P, Chen J, et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28–e39. doi:10.1542/peds.2014-3745. PMID: 26101366.
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793–802. doi:10.1001/jama.2013.1625. PMID: 23632723.
- Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10:681–92. doi:10.1038/nrmicro2872. PMID: 22961341.
- Ferguson M, Wilkinson DE, Zhou T. WHO meeting on the standardization of HPV assays and the role of the WHO HPV laboratory network in supporting vaccine introduction held on 24–25 January 2008, Geneva, Switzerland. Vaccine. 2009;27:337–47. doi:10.1016/j.vaccine.2008.10.062. PMID: 19007840.
- Zhao H, Lin ZJ, Huang SJ, Li J, Liu XH, Guo M, Zhang J, Xia N-S, Pan H-R, Wu T, et al. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum Vaccin Immunother. 2014;10:740–46. doi:10.4161/hv.27619. PMID: 24384608.
- Gu Y, Wei M, Wang D, Li Z, Xie M, Pan H, Wu T, Zhang J, Li S, Xia N, et al. Characterization of an Escherichia coli-derived human papillomavirus type 16 and 18 bivalent vaccine. Vaccine. 2017;35:4637–45. doi:10.1016/j.vaccine.2017.06.084. PMID: 28736197.
- Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi:10.1016/j.virol.2003.12.027. PMID: 15051381.
- Zhu FC, Chen W, Hu YM, Hong Y, Li J, Zhang X, Zhang Y-J, Pan Q-J, Zhao F-H, Yu J-X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18–25 years: results from a randomized controlled trial. Int J Cancer. 2014;135:2612–22. doi:10.1002/ijc.28897. PMID: 24740596.
- Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, Pan Q-J, Zhang W-H, Zhao F-H, Zhang C-F, et al. Efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine in Chinese women aged 18–25 years: event-triggered analysis of a randomized controlled trial. Cancer Med. 2017;6:12–25. doi:10.1002/cam4.869. PMID: 27998015.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–F138. doi:10.1016/j.vaccine.2012.04.108. PMID: 23199956.
- Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–68. doi:10.1016/s0140-6736(07)60852-6.
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi:10.1016/S0140-6736(09)61248-4. PMID: 19586656.
- Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, Wang S, Liao X, Shou Q, Zheng M, et al. Safety of a quadrivalent human papillomavirus vaccine in a Phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90months of follow-up. Vaccine. 2019;37:889–97. doi:10.1016/j.vaccine.2018.12.030. PMID: 30638797.