ABSTRACT
We compared the antibody responses and persistence of the reduced-dose, 9 µg hemagglutinin (HA)/strain intradermal (ID) injection via the Mantoux technique and the 15 μg HA/strain intramuscular (IM) injection of the repeated annual identical trivalent, inactivated, split-virion vaccine 2011–2012 in chronic obstructive pulmonary disease (COPD) patients. Eighty patients were randomized to ID (n = 41) and IM (n = 39) groups. Four weeks post-vaccination, the antibody responses of the two groups were similar; those for influenza A(H1N1)pdm09 and influenza A(H3N2)–but not influenza B–met the criteria of the Committee for Proprietary Medicinal Products (CPMP). The antibody responses for influenza A(H1N1)pdm09 rapidly declined in both groups, especially with the ID injection, whereas those for influenza A(H3N2) maintained above the CPMP criteria throughout 12 months post-vaccination. The geometric mean titres for influenza A(H1N1)pdm09 persisted above the protective threshold (≥ 40) until 6 months post-vaccination in both the ID and IM groups. The seroprotection rates of the ID and IM groups were above 60% until 3 months and 6 months post-vaccination, respectively. In conclusion, the 9 μg HA/strain ID injection of vaccine 2011–2012 elicited antibody responses similar to the standard dose of 15 μg of the HA/strain IM injection at 4 weeks post-vaccination. However, the antibody responses for influenza A(H1N1)pdm09 rapidly declined, especially in the case of the ID injection, whereas they were comparable for influenza A(H3N2). Additional strategies for increasing vaccine durability should be considered, especially for new pandemic strains affecting elderly COPD patients.
Introduction
Annual influenza vaccinations are recommended for chronic obstructive pulmonary disease (COPD) patients to reduce influenza-associated serious illness and mortality.Citation1,Citation2 Most COPD patients are elderly. The standard recommended dose of influenza vaccination for those elderly patients is a 15 μg hemagglutinin (HA)/strain intramuscular (IM)injection or a 15 μg HA/strain intradermal (ID) injection via a licensed microinjection system.Citation3 However, in the case of vaccine shortage, dose-sparing ID vaccination may help to increase vaccine availability. The reduced-dose ID vaccination has been studied in various vaccines, such as rabies, hepatitis B, and influenza, since it can improve immune response. The dermis has abundant antigen-presenting cells, such as dendritic cells (DCs) and plenty of lymphatic networks connecting to regional draining lymph nodes in which the DCs trigger T- and B-cell activation, whereas fewer circulating DCs may capture antigens from the IM vaccinationCitation4,Citation5.
Before the pandemic, studies of reduced-dose ID influenza vaccination administered by the Mantoux technique in the elderly, including COPD patientsCitation6-Citation8 demonstrated that the immunogenicity met the Committee for Proprietary Medicinal Products (CPMP) requirement for annual re-licensure. Soon after the pandemic, we compared the reduced-dose 9 µg of HA/strain ID injection via the Mantoux technique with the standard-dose 15 µg of HA/strain IM injection of seasonal influenza vaccine (2010–2011) containing influenza A(H1N1)pdm09 in COPD patients aged≥60 years Citation9.These patients were naïve to influenza A(H1N1)pdm09 vaccine. The study showed that the immunogenicity of all 3 influenza strains of both the reduced-dose ID and the standard-dose IM injections at 4 weeks post-vaccination met the CPMP criteria. However, the seroprotection rates of the new influenza A(H1N1)pdm09 vaccine in both the ID and IM groups tended to be lower than those of influenza A(H3N2) and B, especially in the ID group. These might be the effects of previous vaccinations, which is attributed to the booster effect of influenza A(H3N2) or influenza B, or to the lower immunogenicity of the influenza A(H1N1)pdm09 strain. Prior studiesCitation10-Citation13 have shown that previous vaccination determined the antibody responses of the influenza vaccine, but the results were mixed, showing either increased or decreased responses.
In the post-pandemic era, the World Health Organization (WHO) recommended identical annual seasonal trivalent influenza virus strains for 2010–2011 and 2011–2012 vaccines. Therefore, we seized the opportunity to evaluate the effects of a previous vaccination on the antibody responses, especially for influenza A(H1N1)pdm09 in the vaccine 2011–2012.We aimed to compare the antibody responses of the reduced-dose 9 µg of HA/strain ID vaccination to the standard-dose 15 µg of HA/strain IM vaccination for vaccine 2011–2102 in the elderly COPD patients. Previous studiesCitation14,Citation15 have reported that the long-term antibody responses of the elderly do not rapidly decline, as had previously been concerned, with antibody responses still exceeding the CPMP criteria for ≥ 4 months after the annual influenza vaccinations. Therefore, the second aim of our study was to evaluate antibody persistence, especially for the relatively new influenza A(H1N1)pdm09, among elderly COPD patients.
Materials and methods
Subjects
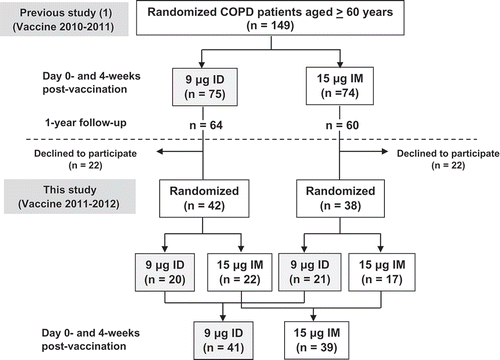
A prospective, randomized, open-label study was conducted to compare the immunogenicity and safety of the reduced-dose 9 µg of HA/strain ID injection via the Mantoux technique and the standard dose 15 µg of HA/strain IM injection of a repeated annual identical trivalent influenza vaccine (vaccine 2011–2012) in COPD patients. The COPD patients, aged ≥ 60 years, were recruited from the COPD clinic of the Faculty of Medicine Siriraj Hospital, Mahidol University in Bangkok, Thailand. All of the enrolled patients had participated in a prior study on the immunogenicity of the ID and IM injections for the seasonal influenza vaccine (2010–2011),Citation9and they provided written, informed consent to participate in this second year study. The Institutional Review Board of the Faculty of Medicine, Siriraj Hospital reviewed and approved the study protocol.
In all, 80 patients from the first-year study were recruited. Computerized block randomization was used to allocate the patients to either the reduced-dose 9 μg of HA/strain ID injection group or the standard dose 15 µg of HA/strain IM injection group, stratified by routes of injection in the previous year (). Forty-one patients in the ID group and 39 in the IM group were enrolled.
Vaccinations
The trivalent, inactivated, split-virion influenza vaccine contained influenza A/California/7/2009(H1N1)-like virus, A/Perth/16/2009 (H3N2)-like virus, and B/Brisbane/60/2008-like virus, according to the annual recommendation of the WHO for use in 2011–2012. All 3 strains of viruses were identical to the previous recommendation in 2010–2011. The final bulk vaccine from Sanofi-Pasteur, France, was distributed by the Government Pharmaceutical Organization–Merieux Biological Products Co., Ltd. It was supplied in 5-mL multi-dose vials (15 µg of HA/strain/0.5 mL) and supported by the Department of Disease Control of the Ministry of Public Health, Bangkok, Thailand (lot number 07B1209).
The details of the vaccine preparation for the injections have been described in previous studies.Citation8,Citation9 The ID injection used the Mantoux technique, and this was done by a trained person who had experience in the ID injection for tuberculin testing. A 0.3-mL dose of vaccine (9 µg HA/strain) was filled in a 1-mL tuberculin syringe attached to a 25-gauge needle, 5/8 inch (16 mm) in length. The tip of the needle, with the bevel facing up, was inserted away from visible vessels and almost parallel to the stretched skin so that the needle tip was visible beneath the skin. A half dose (approximately 0.15 mL) of the vaccine was slowly injected into the dermis of the ventral surface of the right forearm. The needle was slowly withdrawn; however, to prevent leakage, its removal was stopped for a few seconds, and then the needle was completely withdrawn from the skin. The rest of the vaccine (approximately 0.15 mL) was injected into the left forearm using the same technique. A pale, orange-peel appearance bleb immediately appeared, which confirmed the ID injection had been properly administered. The bleb formation, leakage, and bleeding were observed. The IM injection used a 1-mL tuberculin syringe attached to a 25-gauge needle, 5/8 inch in length, for convenience. The vaccine (0.5 mL) was injected perpendicularly into the deltoid muscle of the non-dominant arm.
Assessment of side effects
To avoid the possibility of any discomfort being felt by patients when responding to questions about their pain, they were asked to rate their pain by an investigator who had not administered the vaccine injection. The assessment was made immediately after the injection. The patients used a visual-analogue scale ranging from 0 mm (no pain) to 100 mm (worst possible pain), with each patient drawing a vertical line on the scale; the distance from zero was subsequently measured.Citation6Patients were closely observed for 30 minutes after their vaccination to detect any acute serious reactions. Patients and their relatives were reminded how to record side effects. Patients were asked to record side effects in a diary for the first 7 days post-vaccination, and the diary was returned on the next visit 4 weeks post-vaccination.
Immunogenicity
Venous blood was drawn before the vaccinations and 4 weeks, 3 months, 6 months, and 12 months afterward. Sera were separated and stored at −20°C until analysis. The hemagglutination inhibition (HI) titre was used to evaluate the immunogenicity. The details of the HI test procedures were described in previous studies.Citation12,Citation16 Pre- and post-vaccination sera were titrated in duplicate. The antigens for the HI titre testing comprised influenza antigen A/California/7/09 (H1N1)v(NYMCX-179A)(Cell Derived) NIBSC code:09/174;influenza antigen A/Victoria/210/2009 (H3N2)(NYMCX-187) NIBSC code:12/112;and influenza antigen B/Brisbane/60/08 NIBSC code:13/234. They were supplied by the National Institute for Biological Standards and Control (NIBSC, UK).
HI titres of ≥ 1:10 were considered to contain HI antibody. Undetectable HI titres were recorded as 5 for analysis. The immunogenicity was evaluated in terms of geometric mean titre (GMT); seroconversion factor (the ratio of the HI titre post-vaccination to the HI titre pre-vaccination); seroconversion rate (the percentage of post-vaccination HI titre ≥ 1:40 in patients with pre-vaccination HI titres < 1:10 or ≥ 4-fold increase in the post-vaccination HI titres in patients with pre-vaccination titres ≥ 1:10); and seroprotection rates (the percentage of patients with HI titres ≥ 1:40). At least one of the following criteria must be met for each strain 4 weeks after vaccination for adults aged over 60 years: seroconversion factor >2.0, seroconversion rate >30%, or seroprotection rate >60%.Citation17 For the convenience of patients, our study collected blood for the HI titres at 4 weeks post-vaccination instead of 3 weeks post-vaccination, as recommended by the CPMP. The decision to do so was based on a study by Gross et al,Citation18 who demonstrated that HI titres at 2 weeks post-vaccination are identical to those at 4 weeks post-vaccination in patients above 65 years of age. Therefore, HI titres at 3 and 4 weeks post vaccination should be similar.
Statistical analysis
Continuous data and categorical data were described as mean ± standard deviation and percentages, respectively. Analysis of variance of log-transformed results was used to compare GMTs of the ID and IM injections. Student’s t-test and Mann-Whitney U test were used to compare the normally and non-normally distributed continuous variables, respectively, of the ID and IM groups. The chi-squared (χ2) test or Fisher’s exact test was used to compare the categorical variables of the ID and IM groups. One-way analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables, and χ2 test for categorical variables, were used to compare 3 or more unmatched groups. Analysis of covariance (ANCOVA) for continuous variables and binary logistic regression for categorical variables were used to compare antibody responses of the first-year and second-year vaccinations’ adjusted baseline GMTs. Repeated ANOVA for continuous variables and χ2 for trend for categorical variables were used to analyze the decline in antibody responses from 4 weeks post-vaccination across all time points (3 months, 6 months, and 12 months post-vaccination). A p-value of < 0.05 or no overlap of 95% confidence interval (CI) was statistically significant. Data were analyzed using PASW Statistics for Windows, version18 (SPSS Inc., Chicago, IL, USA).
Results
Demographic data
The mean ages of the COPD patients in the ID and IM groups were 72 ± 5.9 years and 75.3 ± 9.7 years, respectively, and the p-value was 0.077 (). Most patients in both groups were male. The baseline characteristics in the ID group (n = 41) and the IM group (n = 39) were not significantly different, except for the body mass index (BMI). The mean BMIs of the ID group (23.2 ± 3.8 kg/m2) and the IM group (20.4 ± 4.2 kg/m2) were significantly different, with p-value = 0.002. The percentage of patients who were underweight (BMI < 18.5 kg/m2)Citation19 in the IM group was significantly higher than that for the ID group, with 38.5% (15 patients)and 4.9% (2 patients), respectively, and p-value < 0.001. Since being underweight might affect immunogenicity,Citation20 the antibody responses between BMI < 18.5 kg/m2 and BMI ≥ 18.5 kg/m2 in the IM group were compared (). There was no difference in the antibody responses of both BMI groups; therefore, we included the data of the underweight patients in the analysis.
Table 1. Baseline characteristics of COPD patients in the intradermal (ID) and intramuscular (IM) groups.
Table 2. The hemagglutination inhibition (HI) titres† of pre-vaccination and 4 weeks post-vaccination of COPD patients in the intramuscular (IM) group compared between BMI < 18.5 kg/m2(n = 15) and BMI ≥ 18.5 kg/m2(n = 24).
Immunogenicity
At 4 weeks post-vaccination
The antibody responses of the ID and the IM groups were similar at 4 weeks post-vaccination (), except that the seroconversion rate for influenza A(H1N1)pdm09 in the ID group (82.9%, 95% CI 68.7% – 91.5%) was higher than that for the IM group (61.5%, 95% CI 45.9% – 75.1%), and p-value = 0.032. The seroprotection rates of influenza A(H1N1)pdm09 in the ID group were 95.1% (95% CI 83.9% – 98.7%) and those in the IM group were 92.3% (79.7% – 97.3%), and p-value = 0.671. The immunogenicity for influenza A(H1N1)pdm09 and influenza A(H3N2) in both the ID and IM groups met all three criteria of the CPMP requirement for annual re-licensure of age > 60 years,Citation17 whereas the hemagglutination inhibition (HI) titres of influenza B were quite low in both the ID and IM groups. Therefore, most further analyzes in the current study focused on the antibody responses of influenza A(H1N1)pdm09 and influenza A(H3N2).
Table 3. Hemagglutination inhibition (HI) antibody titres pre- and 4 weeks post-vaccination of repeated identical influenza virus strains (vaccine 2011–2012) in the intradermal (ID, n = 41) and intramuscular (IM, n = 39) groups.
All patients of this study had been vaccinated in the previous year (2010–2011 vaccine), which was the first-year vaccination with influenza A(H1N1)pdm09. In the ID group, the pre-vaccination GMTs for influenza A(H1N1)pdm09 of the second-year vaccination (19.3, 95% CI 14.0–26.6) were not significantly different from those of the first-year vaccination (13.8, 95% CI 10.2–18.7),with p-value = 0.159, whereas in the IM group, those of the second-year vaccination (24.8, 95% CI 17.1–35.9) were significantly higher than those of the first-year vaccination (13.0, 95% CI 10.0–17.0), with p-value = 0.005 (). After adjusting the pre-vaccination GMTs, the antibody responses of influenza A(H1N1)pdm09 in the ID group were significantly increased for the second-year vaccination. The seroprotection rate for influenza A(H1N1)pdm09 of the ID injection significantly increased from 64% (52.7% −74.0%) for the first-year vaccinationCitation9 to 95.1% (83.9% – 98.7%) for the second-year vaccination, with p-value < 0.001. The antibody responses for influenza A(H1N1)pdm09 in the IM injection tended to increase from 78.4% (67.6%-86.3%) to 92.3% (79.7–97.3%), but they were not significantly different (p-value = 0.115).
Table 4. Hemagglutination inhibition (HI) antibody titres† pre- and 4 weeks post-vaccination of annual repeated identical influenza virus strains (2011–2012 vaccine) and previous study (vaccine 2010–2011 vaccine)Citation9 in the intradermal (ID) and intramuscular (IM) groups.
During 12 months post-vaccination
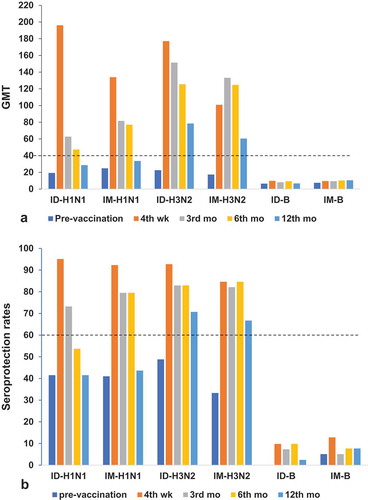
The HI titres at pre-vaccination and 4 weeks, 3 months, 6 months, and 12 months post-vaccination were evaluated for the sustainable antibody responses. The GMTs for influenza A(H1N1)pdm09 declined with time from 4 weeks post-vaccination in both the ID and IM groups (, ). However, those in the ID group significantly decreased earlier than those in the IM group (at 3 months post-vaccination vs at 12 months post-vaccination, respectively). The GMTs for influenza A(H1N1)pdm09 in both the ID and IM groups remained above the protective threshold (≥40) until 6 months post-vaccination, whereas those for influenza A(H3N2) in both the ID and IM groups maintained above 40 throughout the12-month post-vaccination period (). The seroprotection rates for influenza A(H1N1)pdm09remained above 60%, as per the CPMP criteriaCitation17 until 3 months and 6 months post-vaccination in the ID and IM groups, respectively (, ). In contrast, the seroprotection rates for influenza A(H3N2) maintained above 60% throughout the 12 months of follow-up in both the ID and IM groups.
Table 5. Geometric mean titres (GMTs) and seroprotection rates for influenza A(H1N1)pdm09 and influenza A(H3N2) at baseline and 4 weeks, 3 months, 6 months, and 12 months post-vaccination in the ID and IM groups.
Figure 2. (a)Geometric mean titres (GMTs) with SD and (b) seroprotection rates (HI titre ≥ 1:40) at pre-vaccination,4 weeks,3 months, 6 months, and12tmonths post-vaccination in the ID and IM groups of COPD patients aged >60 years. Numbers inthe ID group: at pre-vaccination = 41,4weeks = 41,3 months = 37, 6months = 37,12months = 35. Numbers in the IM group: at baseline = 39, 4weeks = 39, 3 months = 34, 6months = 36, and 12months = 32.(a) Reference line is the seroprotective HI titre ≥ 40; (b) reference line is the CPMP criterion of seroprotection rate >60% for patients aged >60 yearsCitation17.
Side effects
There were no serious side effects of either the ID or IM injections within 7 days post-vaccination. The level of pain at the injection site, which was immediately assessed by a blinded observer after the injection, was similar for the two-site ID injections and the IM injection with visual analogue scale scores of 17.2 ±10.6 and 9.4 ± 12.6, respectively (p-value = 0.779). The diaries used to record the side effects during the first 7 days post-vaccination were returned for evaluation by 36 patients (87.8%) from the ID group and 35 patients (89.7%) from the IM group. All patients with the ID injection developed erythema and swelling at the injection sites (). The local reactions in the ID group (erythema, itching, swelling, and ecchymosis) were significantly higher than those in the IM group, with p-value < 0.01. The systemic reactions were not significantly different between the ID and IM groups. Fever tended to be higher with the ID than the IM injection, but there was no significant difference (13.9% vs 2.9%, p-value = 0.097). Myalgia was reported by 30.6% of the participants receiving the ID injection and 25.7% of the participants receiving the IM injection.
Table 6. Local and systemic side effects of the intradermal (ID, n = 36) and intramuscular (IM, n = 35) injection.
Discussion
The antibody responses at 4 weeks post-vaccination of trivalent, inactivated split-virion influenza vaccine (2011–2012) of the total dose 9 µg of HA/strain, 2-site ID injections by the Mantoux technique were similar to those of the standard dose 15µg of HA/strain IM injection (). Other post-pandemic studies of reduced-dose (3, 6, or 9 μg HA/strain) ID influenza vaccination with different ID injection systems (Becton Dickinson’s SoluviaTM, micronJet600TM, or Immucise®)Citation21,Citation22 showed comparable withCitation21 and superior antibody responses withCitation22 the reduced-dose ID vaccination in the elderly and/or patients with chronic illness, including COPD patients compared to the standard-dose influenza IM or subcutaneous vaccination. Although some studies used only 3 μg (20% of the dose) to achieve the same response, others used 60%, as was done in this study, suggesting that the delivery devices or reliability of the injection may have an effectCitation21-Citation24.
All COPD patients in our study had been vaccinated in the previous year with the 2010–2011 vaccine containing influenza A(H1N1)pdm09 for the first time. The antibody responses to influenza A(H1N1)pdm09 in the patients who were naïve to influenza A(H1N1)pdm09 exposure were low even though they met the CPMP requirement.Citation9However, after repeating with the annual identical influenza vaccination (vaccine 2011–2012), the antibody responses for influenza A(H1N1)pdm09 increased in both the ID and IM groups, but only significantly in the ID group (). The increasing antibody responses of the repeated identical influenza A(H1N1)pdm09 suggested the booster effect of the previous vaccination. Other post-pandemic studies with the standard dose of influenza vaccine containing influenza A(H1N1)pdm09 have also demonstrated that a repeated annual vaccination of influenza A(H1N1)pdm09 boosted immune responsesCitation25,Citation26.
We also evaluated the persistence of the antibody responses over 12 months of follow-up. We found that the GMTs for influenza A(H1N1)pdm09 in both the ID and IM groups significantly declined over the year, persisting above the protective threshold (≥ 40) until 6 months post-vaccination(). The seroprotection rates remained at over 60% until 3 months and 6 months post-vaccination in the ID and IM groups, respectively. In contrast, the GMTs kept above the seroprotective level (≥40), and the seroprotection rates were > 60% throughout 12 months post-vaccination for influenza A(H3N2) in both the ID and IM groups (, ). Our findings were similar to the study by Leung et al. on health care workers aged 20–75 years.Citation27It showed that the HI titres for influenza A(H1N1)pmd09 declined significantly, whereas those for influenza A(H3N2) did not significantly decline 6 months post-vaccination.
The COPD patients who participated in our study were elderly. The reduced-dose 9 µg of HA/strain ID injection elicited antibody responses that met the requirement at 4 weeks post-vaccination, but the antibody responses could not maintain the protective level after 3–6 months post-vaccination for influenza A(H1N1)pdm09. However, the CPMP criterionCitation17 is defined for 3 weeks post vaccination. It is not a formal criterion for immunogenicity levels at 3, 6, or 12 months post vaccination, even though it is used as a benchmark for those time points. With the elderly, other strategies, such as a high-dose vaccine, an adjuvanted vaccine, a booster vaccination, or alternate delivery routesCitation22,Citation28-Citation35 may be needed to improve and maintain the antibody responses, especially in the case of low-immunogenicity pandemic strains. Hung et al.Citation32 showed that pretreatment with topical imiquimod ointment, a synthetic Toll-like receptor 7 agonist, before ID vaccination significantly expedited, augmented, and prolonged the immunogenicity of influenza vaccinations for the elderly. This might be an affordable strategy for middle-income countries to counter the relatively reduced durability of standard unadjuvanted and non-HD vaccines, in both seasonal and pandemic eras. However, it would have been of great value to test durability using adjuvanted or high-dose vaccines as well.
Limitation
The ID injection by Mantoux technique is routinely used for tuberculin test. It requires trained personnel and requires time to be perfectly injected into the dermis. The Mantoux method in our study was performed by a very well-trained individual with a small group of patients, which means that the results may not be generalizable to mass vaccinations. Among the 41 patients with the two-sided ID injections in our study, we found only one patient was injected too deep without bleb formation at one site of the injection. However, the HI titres in that case were increased ≥ 4-fold at 4 weeks post-vaccination for influenza A(H1N1)pdm09 and influenza A(H3N2). The Mantoux method may be suitable for use in the event of a vaccine shortage in countries that are very familiar with the technique. The ID Mantoux method is technique-dependent and thus may improve with the use of more reliable delivery devices, such as SoluviaTM by BD, MicronJet600TM by NanoPass, ID adaptor by West Pharmaceutical Services, and Immucise by Terumo. The other limitation of this study is that the small sample size of each subgroup may attribute the potentially high variability and potentially the lack of statistical significance of some of the results. Thus, it would be useful to conduct a study on elderly and/or chronic patients using a larger sample size.
Conclusion
At 4 weeks post-vaccination, the reduced-dose 9 μg of HA/strain ID injection via the Mantoux technique of the repeated annual identical trivalent, inactivated, split-virion vaccine 2011–2012 elicited antibody responses similar to the standard dose of 15 μg of HA/strain IM injection. However, the antibody responses for influenza A(H1N1)pdm09 rapidly declined, especially with the ID injection, whereas they were comparable for influenza A(H3N2). Additional strategies such as high doses, adjuvanted vaccines, or other delivery devices to increase vaccine durability should be considered, especially for new pandemic strains affecting elderly COPD patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank the Department of Disease Control of the Ministry of Public Health of Thailand for supplying the vaccine. The authors also thank Professor KhunNanta Maranetra for proofreading the article and Miss Khemajira Karaketklang for review of the statistical analysis.
Additional information
Funding
References
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO.
- Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;125(6):2011–2020. doi:10.1378/chest.125.6.2011.
- Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ. 2011;89(3):221–226. doi:10.2471/BLT.10.079426.
- McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? J Infect Dis. 2008;198(5):632–634. doi:10.1086/592362.
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7(8):1201–1214. doi:10.1586/14760584.7.8.1201.
- Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351(22):2286–2294. doi:10.1056/NEJMoa043555.
- Chi RC, Rock MT, Neuzil KM. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin Infect Dis. 2010;50(10):1331–1338. doi:10.1086/652144.
- Chuaychoo B, Wongsurakiat P, Nana A, Kositanont U, Maranetra KN. The immunogenicity of intradermal influenza vaccination in COPD patients. Vaccine. 2010;28(24):4045–4051. doi:10.1016/j.vaccine.2010.04.006.
- Chuaychoo B, Kositanont U, Rittayamai N, Niyomthong P, Songserm T, Maranetra KN, Rattanasaengloet K, Nana A. The immunogenicity of the intradermal injection of seasonal trivalent influenza vaccine containing influenza A(H1N1)pdm09 in COPD patients soon after a pandemic. Hum Vaccin Immunother. 2016;12(7):1728–1737. doi:10.1080/21645515.2016.1149276.
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ’s Hospital. Lancet. 1979;1(8106):33–35. doi:10.1016/S0140-6736(79)90468-9.
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15(10):1114–1122. doi:10.1016/S0264-410X(97)00003-0.
- Kositanont U, Assantachai P, Wasi C, Puthavathana P, Praditsuwan R. Kinetics of the antibody response to seasonal influenza vaccination among the elderly. Viral Immunol. 2012;25(6):471–476. doi:10.1089/vim.2012.0024.
- Nabeshima S, Kashiwagi K, Murata M, Kanamoto Y, Furusyo N, Hayashi J. Antibody response to influenza vaccine in adults vaccinated with identical vaccine strains in consecutive years. J Med Virol. 2007;79(3):320–325. doi:10.1002/(ISSN)1096-9071.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197(4):490–502. doi:10.1086/587088.
- Song JY, Cheong HJ, Hwang IS, Choi WS, Jo YM, Park DW, Cho GJ, Hwang TG, Kim WJ. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine. 2010;28(23):3929–3935. doi:10.1016/j.vaccine.2010.03.067.
- Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25(4):659–663. doi:10.1016/j.vaccine.2006.08.026.
- Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonization of requirements for influenza vaccines (CPMP/BWP/214/96). Euro Agency Eval Med Products; 1997 Mar 12. [accessed 2010 Mar 8]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf.
- Gross PA, Russo C, Teplitzky M, Dran S, Cataruozolo P, Munk G. Time to peak serum antibody response to influenza vaccine in the elderly. Clin Diagn Lab Immunol. 1996;3:361–362.
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi:10.1016/S0140-6736(03)15268-3.
- Sagawa M, Kojimahara N, Otsuka N, Kimura M, Yamaguchi N. Immune response to influenza vaccine in the elderly: association with nutritional and physical status. Geriatr Gerontol Int. 2011;11(1):63–68. doi:10.1111/j.1447-0594.2010.00641.x.
- Arakane R, Nakatani H, Fujisaki E, Takahama A, Ishida K, Yoshiike M, Nakayama T, Takeshita F. Immunogenicity and safety of the new intradermal influenza vaccine in adults and elderly: A randomized phase 1/2 clinical trial. Vaccine. 2015;33(46):6340–6350. doi:10.1016/j.vaccine.2015.09.010.
- Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong T-Y, Yuen K-Y, et al. Dose sparing intradermal trivalent influenza (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine. 2012;30(45):6427–6435. doi:10.1016/j.vaccine.2012.08.014.
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198(5):650–658. doi:10.1086/592362.
- Levin Y, Kochba E, Hung I, Kenney R. Intradermal vaccination using the novel microneedle device MicronJet600: past, present, and future. Hum Vaccin Immunother. 2015;11(4):991–997. doi:10.1080/21645515.2015.1010871.
- Plant EP, Fredell LJ, Hatcher BA, Li X, Chiang MJ, Kosikova M, Xie H, Zoueva O, Cost AA, Ye Z, et al. Different repeat annual influenza vaccinations improve the antibody response to drifted influenza strains. Sci Rep. 2017;7(1):5258. doi:10.1038/s41598-017-05579-4.
- Trieu MC, Jul-Larsen A, Saevik M, Madsen A, Nostbakken JK, Zhou F, Skrede S, Cox RJ. Antibody responses to influenza A/H1N1pdm09 virus after pandemic and seasonal influenza vaccination in healthcare workers: a 5-year follow-up study. Clin Infect Dis. 2019;68(3):382–392. doi:10.1093/cid/ciy487.
- Leung VKY, Carolan LA, Worth LJ, Harper SA, Peck H, Tilmanis D, Laurie KL, Slavin MA, Sullivan SG. Influenza vaccination responses: evaluating impact of repeat vaccination among health care workers. Vaccine. 2017;35(19):2558–2568. doi:10.1016/j.vaccine.2017.03.063.
- Boraschi D, Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol Lett. 2014;162(1):346–353. doi:10.1016/j.imlet.2014.06.006.
- Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM. Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: A literature review. Hum Vaccin Immunother. 2015;11(3):553–563. doi:10.1080/21645515.2015.1011562.
- Della Cioppa G, Nicolay U, Lindert K, Leroux-Roels G, Clement F, Castellino F, Galli C, Groth N, Levin Y, Del Giudice G, et al. A dose-ranging study in older adults to compare the safety and immunogenicity profiles of MF59(R)-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum Vaccin Immunother. 2014;10(6):1701–1710. doi:10.4161/hv.28618.
- Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol. 2014;29:38–42. doi:10.1016/j.coi.2014.03.008.
- Hung IF, Zhang AJ, To KK, Chan JF, Li C, Zhu HS, Li P, Li C, Chan T-C, Cheng VCC, et al. Immunogenicity of intradermal trivalent influenza vaccine with topical imiquimod: a double blind randomized controlled trial. Clin Infect Dis. 2014;59(9):1246–1255. doi:10.1093/cid/ciu582.
- Seo YB, Choi WS, Lee J, Song JY, Cheong HJ, Kim WJ, Plotkin SA. Comparison of the immunogenicity and safety of the conventional subunit, MF59-adjuvanted, and intradermal influenza vaccines in the elderly. Clin Vaccine Immunol. 2014;21(7):989–996. doi:10.1128/CVI.00615-13.
- Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27(52):7304–7312. doi:10.1016/j.vaccine.2009.10.033.
- Yoo BW, Kim CO, Izu A, Arora AK, Heijnen E. Phase 4, post-marketing safety surveillance of the MF59-adjuvanted influenza vaccines FLUAD(R) and VANTAFLU(R) in South Korean subjects aged >/=65 years. Infect Chemother. 2018;50(4):301–310. doi:10.3947/ic.2018.50.4.301.