ABSTRACT
The recent licensure of two different serogroup B recombinant protein meningococcal vaccines in Brazil emphasizes the importance of a better knowledge of the real burden of serogroup B meningococcal (MenB) disease to establish evidence-based vaccination policies. We performed an observational, descriptive study, from 2001 to 2015, analyzing the incidence and case fatality rates (CFR) of MenB disease in Brazil, according to age group and region. In the absence of any vaccine use targeting MenB disease, a significant decline of 90% in the overall incidence rates of MenB disease was observed (from 0.55 cases/100,000 habitants in 2001 to 0.05 in 2015), with declines found in all age groups during the study period. The highest incidence rates were consistently observed in infants and children 1–4 year of age, whereas adults ≥ 60 years experienced the highest CFR (33.9%). The proportion of cases with serogroup identified increased from 37.1% in 2001 to 51.5% in 2015. Despite an improvement in recent years, the quality of diagnosis is highly heterogeneous in the diverse regions, presenting important deficiencies that still prevent the possibility of a robust and reliable analysis of the burden of the meningococcal disease in Brazil. Based on the findings of this study and taking in account the unlikely indirect effect associated with the use of the new recombinant serogroup B protein vaccines, infants < 1 year is the age group to be prioritized when considering the implementation of routine immunization programmes with MenB vaccines.
Introduction
Despite being associated with low and decreasing incidence trends in the last decades, few diseases still have the capacity to cause panic among the population as invasive meningococcal disease (IMD), mainly because of its potential epidemic nature, the rapid onset of illness, its high case fatality rates (10% – 20%), morbidity and risk of complications (up to 20% of survivors of MD may develop long-term sequelae, including neurological deficit, ocular and hearing impairment or limb amputation).Citation1–Citation3
The causative agent of MD, Neisseria meningitidis, is a gram-negative, aerobic, non-mobile diplococcus belonging to the Neisseriaceae family. The antigenic composition of the polysaccharide capsule enables the classification of N. meningitidis into 12 different Serogroups: A, B, C, H, I, K, L, W, X, Y, Z and E. Currently, Serogroups A, B, C, Y, W and X are responsible for nearly all cases of disease, infecting only humans.Citation3–Citation5
In Brazil, IMD has been considered a major public health problem and remains a leading cause of meningitis and septicemia. Most of the cases reported are sporadic, with seasonal variations and periodic outbreaks occurring in several cities.Citation6 During the last 50 years, several meningococcal vaccination programmes were implemented in Brazil, providing important lessons. In the early 1970s, an epidemic of serogroup A disease, with incidence rates as high as 179 cases/100,000 population in determined regions provided the first major experience in the world with a large-scale vaccination of a polysaccharide A and C vaccine, resulting in the successful control of the epidemic one year after its implementation.Citation7 During the 1980s and 1990s, epidemics of serogroup B meningococcal (MenB) disease in several locations around the country motivated a reactive vaccination campaign in the early 1990s with the Cuban serogroup B outer membrane vesicle vaccine.Citation7However, although achieving high coverage among the age groups targeted for the vaccination programme in the State of Sao Paulo, the observed impact on MenB disease rates was limited, if any. More recently, in the early 2000s, a significant rise in the number and proportion of cases due to serogroup C, associated with the ST-103 complex, was registered, with several outbreaks affecting different regions across the country, motivating the health authorities to incorporate the meningococcal C conjugate (MCC) vaccine into the National Immunization Program in late 2010, initially targeting only infants and from 2017 also including adolescents, providing the first experience of a MCC vaccination programme against MenC disease associated with the hypervirulent clone belonging to the ST-103 complex, unrelated to ST-11 complex strains.Citation8
Despite the availability of safe and effective conjugate vaccines against serogroups A, C, W and Y for several years, only recently two serogroup B recombinant protein meningococcal vaccines were licensed for the prevention of serogroup B MD across different age groups.Citation9 Although recommended by the scientific committees (Brazilian Society of Pediatrics and Brazilian Society of Immunization) to infants, children and adolescents, protein-based serogroup B meningococcal vaccines are not yet included in publicly funded programmes in Brazil or in any other Latin-American country, emphasizing the importance of a better knowledge of the real burden of MenB disease to establish evidence-based vaccination policies.
The objective of this study was to provide a detailed epidemiologic profile of the burden of meningococcal disease in Brazil, analyzing the incidence and case-fatality rates of MenB disease in Brazil from 2001 to 2015, according to age group and region.
Patients and methods
Type of study, population and period
We conducted an observational, descriptive ecological study, including all confirmed cases of MD reported to the Notifiable Diseases Information System (SINAN) database in Brazil, from 2001 to 2015.
Data source
In Brazil, notification of cases of MD (suspected or confirmed) is mandatory.Citation10,Citation11 Information was based on the national SINAN database, sponsored by the Informatics Department of the National Health System (DATASUS), Ministry of Health, Brazil. The analysis also included laboratory results from the Adolfo Lutz Institute database to increase the sensitivity of identified cases. The soundex method was applied to the patient name variable in the SINAN database, codes identified as similar were compared and duplicates were removed. Then, information related to the serogroup existing in the database of the Adolfo Lutz Institute was inserted, increasing the proportion of cases with serogroup identification and creating a more complete database. The population databases were the population estimates of the Brazilian Institute of Geography and Statistics (IBGE).
According to the Ministry of Health Surveillance Guidelines, cases reported to SINAN are classified as meningococcal meningitis, meningococcemia and/or meningococcal meningitis plus meningococcemia. A confirmed case is defined by the presence of at least one of the following: isolation of Neisseria meningitidis from a normally sterile site, detection of bacterial DNA by polymerase chain reaction (PCR), antigen detection, clinical-epidemiological criteria (case of close contact with a laboratory confirmed case), gram-staining, or clinical criteria (patient with suggestive symptoms and petechial or purpuric rash).Citation11
Study variables
Age group, sex, causative serogroup, clinical presentation (meningitis, meningococcemia or both), year of disease occurrence, federated region of residence and outcome (discharge or death).
Data analysis
Incidence rates were calculated using as a numerator the number of cases of MD and as a denominator the population of the reference year. The case fatality rate was calculated using as a numerator the deaths from MD by specific serogroup and as a denominator the cases of MD by specific serogroup. These indicators were analyzed according to the year of notification, region of residence and age of the patient. The analysis was performed using Microsoft Office Excel 2013 and EpiInfo version 7 software. Chi-square and Fisher’s exact tests were used for comparisons and a p-value < 0.05 was considered statistically significant. The analysis of the annual trend was made by the annual percentage change (APC) through Joinpoint modeling, using the calendar year as a regression variable. The Joinpoint Regression Program version 3.3 software was used for this calculation.
Ethical aspects of the research project
The project was approved by the Research Ethics Committee of the Sisterhood of the Santa Casa de Misericórdia de São Paulo under No. 670.434.
Results
During the study period, from 2001 to 2015, a total of 40,411 cases of MD and 8,972 deaths were reported in Brazil. Males accounted for 55.7% of all cases and 57.5% of deaths. Among the total 40,411 cases reported of MD, 17,918 (44.5%) were laboratory-confirmed and with information on serogroup, of which 6,264 (34.9%) were serogroup B, 10,422 (58.2%) serogroup C, 864 (4.8%) serogroup W, 245 (1.2%) serogroup Y and 123 (0.6%) serogroup A. The proportion of cases with serogroup identified increased during the study period, from 37.1% in 2001 to 51.5% in 2015. We found the highest proportion of cases reported with information on serogroup results in the Southeast region (49.3%), followed by the Central-West (47.9%), South (44.3%), Northeast (36.7%) and North (30.2%).
Seasonal variation followed the same pattern during the whole period of the study with peaks during the winter months.
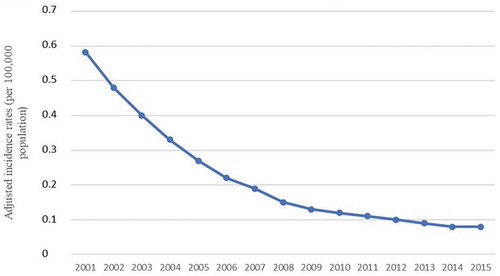
The annual incidence rates of MenB disease decreased significantly, from 0.55/100,000 in 2001 to 0.05/100,000 population in 2015. After adjusting the incidence rates for trends analysis calculations using Joinpoint, we found a significant decreasing trend in incidence rates of MenB disease during the study period, with a mean annual reduction of 17% from 2001 to 2009, and 7.8%, from 2009 to 2015 ().
Figure 1. Serogroup B meningococcal disease adjusted incidence rates in Brazil between 2001 and 2015.
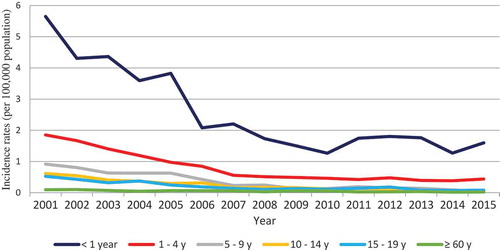
The highest incidence rates of MenB disease were observed in infants under 1 year of age, ranging from an annual peak of 5.6/100,000 in 2001 to a low of 1.1/100,0000 population in 2015. Approximately 60% of the cases of MenB disease in infants occurred between 0 and 6 months of age. No second peaks were observed in adolescents or the elderly.
Reductions in incidence rates were observed in all age groups, ranging from 80% to 90%, between 2001 and 2015 ().
Figure 2. Incidence rates of serogroup B meningococcal disease according to age group in Brazil between 2001 and 2015.
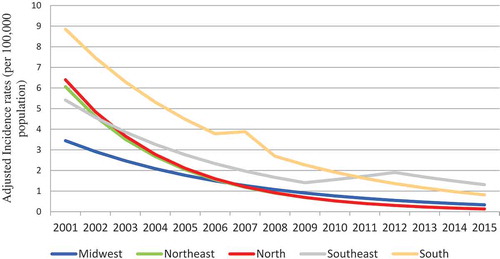
The States of the Southern region (Santa Catarina, Rio Grande do Sul and Parana) consistently presented the highest incidence rates of MenB disease from 2001 to 2011. MenB disease adjusted incidence trends followed a similar pattern across the country, with significant decline trends evident in all geographic regions during the whole study period, except in Southeast region, where after a decline from 2001 to 2009, adjusted incidence rates remained stable, at low levels, from 2009 to 2015 ().
Figure 3. Serogroup B meningococcal disease adjusted incidence rates according to geographic region in Brazil between 2001 and 2015.
According to clinical presentation, among the 6,264 confirmed cases of MenB disease, 3,076 cases (49.1%) presented with meningitis, followed by 2,493 cases (39.8%) with meningitis plus meningococcemia, and 695 cases (11.1%) with meningoccemia. The frequency of the different clinical presentations during the study period was similar.
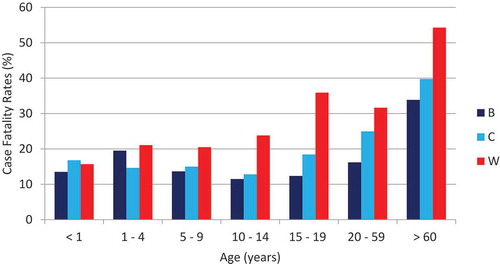
The mean CFR associated with MenB disease between 2001 and 2015 was 15.7% (varying from 13.5% in infants to 33.9% in adults ≥ 60 years), slightly lower compared to the mean CFR observed for MenC disease (18.1%. p = .23) and significantly lower compared to MenW disease (26.8% p < .01). For both MenC disease (varying from 16.7% in infants to 39.7% in adults ≥ 60 years) and MenW disease (varying from 15.7% in infants to 54.3% in adults ≥ 60 years), we also observed increasing CFR in older age groups, particularly the elderly ().
Discussion
The evidence gathered from our study highlights that the overall burden of MenB in Brazil is low, with incidence rates of disease declining in all age groups and geographic regions of the country between 2001 and 2015. The declining incidence rates of MenB disease demonstrated in our study, despite the absence of any vaccine use targeting MenB disease, is not a phenomenon exclusive to Brazil. The same situation has been recently reported in several regions of the world.Citation12–Citation16 The reasons behind this phenomenon are not yet clearly known but might be related to the natural cyclical pattern of the meningococcal serogroup distribution, pathogen virulence, environmental and host factors, variations in population immunity and declining smoking rates, particularly among older adolescents and young adults, the age groups usually responsible for the highest rates of meningococcal carriage.Citation12
The overall CFRs associated with MenB disease in Brazil were consistent and stable during the study period, somewhat higher when compared to the rates reported in other countries, including neighboring Latin American countries.Citation15–Citation18 Interestingly, the mean CFRs observed for MenC and MenW disease were also higher when compared to other countries.Citation12–Citation17 This phenomenon may reflect the lower quality of the reporting system, where less severe cases have a higher chance of being missed in the surveillance system. Furthermore, it may reflect the lack of adequate health-care infrastructure in several regions of the country, limiting the timely access of the MD cases to hospital admission, resulting in delayed medical care and increasing the risk of complications and death.
The study has limitations that should be taken into account. First, the use of secondary surveillance data sources, such as SINAN, may underestimate the true burden of disease due to underreporting and inconsistency in the data. However, previous studies have demonstrated that SINAN data was able to detect variations in the historical trends of the incidence and serogroup distribution of invasive disease.Citation19,Citation20 The inclusion of the laboratory results from the Adolfo Lutz Institute database (the National reference laboratory for invasive diseases in Brazil) in the analysis also proved to be an important tool to increase the sensitivity of identified cases.Citation21 Second, despite an improvement in the quality of diagnosis observed during the study period, particularly in determined states from the South, Central-West and Southeast regions, where molecular-based typing diagnosis was incorporated in the surveillance system, the quality of diagnosis is still highly heterogeneous in the diverse regions, with a significant proportion of the cases reported without identification of the causing-serogroup (overall, only 44.5% of the cases reported during the study period were laboratory-confirmed and with information on serogroup), leading to a substantial underestimation of the true serogroup-specific incidence rates of disease. Thus, any differences observed between the different geographical areas can be partly explained by the quality of epidemiological surveillance.
The great success achieved with the incorporation of meningococcal conjugate vaccines in immunization programmes was based not only on the direct protection among the age groups vaccinated but also, and very importantly, on the capacity of these vaccines, once incorporated into high coverage among adolescents and young adults (the age groups responsible for the highest incidence rates of meningococcal carriage), to prevent acquisition of carriage, interrupting transmission of the bacteria in the community and preventing disease even in unvaccinated populations.Citation22,Citation23 However, the data available so far shows that the serogroup B recombinant protein vaccines appear to have limited, if any, impact on the prevention of acquisition of carriage, anticipating the importance of relying on direct protection as the only reliable strategy to protect the population against serogroup B endemic disease.Citation24,Citation25 In this regard, the recent experience from the UK is reassuring.Citation26 Public Health England estimates that three years after being implemented, the MenB vaccination programme, targeting infants with a 2, 4 and 12-month schedule, prevented 277 cases of MenB disease in the vaccine-eligible cohort. Significant reductions of MenB disease cases were demonstrated in infants for 3 consecutive years following the reduced 2-dose priming schedule, with no major safety concerns observed following the vaccination of more than 3 million infants.Citation27,Citation28
The information collected from this study is crucial for a better understanding of the MenB epidemiology in Brazil and to anticipate the potential impact of different immunization strategies with the two novel protein-based serogroup B meningococcal vaccines on disease burden. Unlike what was observed in North America, where the majority of outbreaks are due to serogroup B, occurring in adolescents and young adults from Universities, outbreaks of serogroup B MD were rarely reported in Brazil, apart from sporadic cases in these age groups.Citation29 The highest incidence rates of MenB disease in Brazil were consistently reported in children younger than 5 years of age, particularly among young infants.
MenB isolates from Brazil were recently assessed using a Meningococcal Antigen Typing System developed to predict the level of protection against a particular strain, resulting in approximately 81% potential coverage with the 4CMenB vaccine.Citation30 Carriage studies performed in Brazil after the implementation of the MCC vaccination programme consistently found genogroup B strains as the most frequent groupable isolate emphasizing the need for continuous MD surveillance to detect changes in the incidence of MenB disease in the future.Citation31,Citation32
Despite the declining incidence rates of Men B disease, currently at historical low levels, our epidemiological data showed that the highest burden of MenB disease is consistently observed in children younger than 5 years, particularly young infants. Incorporation of MenB recombinant protein vaccines into the national immunization programmes targeting infants, the age group with the highest incidence rates of IMD, in the region will depend on continuous surveillance to provide quality information to guide future vaccination strategies. Taking in account the current epidemiologic situation, we anticipate that the protein-based serogroup B Meningococcal vaccines should be considered for persons at increased risk for IMD and to control outbreaks of serogroup B IMD occurring in institutions or at community level.
Disclosure of potential conflicts of interest
M.A.P.S. and J.C.M. have received grants to support research projects and consultancy fee from GSK, Pfizer and Sanofi Pasteur; the other authors have nothing to disclose.
Additional information
Funding
References
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20. doi:10.1007/978-1-61779-346-2_1.
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–1210. doi:10.1016/S0140-6736(07)61016-2.
- Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, Corton C, Care R, Poolman JT, Zollinger WD, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–73. doi:10.3201/eid1904.111799.
- American Academy of Pediatrics. Meningococcal infections. In: Kimberlin DW, Brady M, Jackson MA, Long SS, editors. Red book: report of the 2018 committee on infectious diseases. 31th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2018. p. 550–61.
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. New Engl J of Med. 2001;344(18):1378–88. doi:10.1056/NEJM200105033441807.
- Presa JV, de Almeida RS, Spinardi JR, Cane A. Epidemiological burden of meningococcal disease in Brazil: a systematic literature review and data base analysis. Int J InfectDis. 2019;80:137–46. doi:10.1016/j.ijid.2019.05.006.
- de Moraes JC, Barata RB. Meningococcal disease in São Paulo, Brazil, in the 20th century: epidemiological characteristics. Cad Saúde Pública. 2005;21(5):1458–71. doi:10.1590/S0102-311X2005000500019.
- Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MAP. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(2):B26–36. doi:10.1016/j.vaccine.2011.12.032.
- Villena R, Safadi MAP, Valenzuela MT, Torres JP, Finn A, O´Ryan M. Global epidemiology of serogroup B meningococcal disease and opportunities for prevention with novel recombinant protein vaccines. Hum Vaccin Immunother. 2018;14(5):1042–57. doi:10.1080/21645515.2018.1458175.
- Brazil. Ministry of Health. List of infectious diseases considered of mandatory notification. [accessed 2019 Aug 13]. http://bvsms.saude.gov.br/bvs/saudelegis/gm/2016/prt0204_17_02_2016.html
- Brazil. Ministry of Health. Health surveillance guide; Brasília (DF), 2017 [accessed 2019 Jun 31]. http://portalarquivos.saude.gov.br/images/pdf/2017/outubro/06/Volume-Unico-2017.pdf
- Sridhar S, Greenwood B, Head C, Plotkin SA, Sáfadi MA, Saha S, Taha M-K, Tomori O, Gessner B. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect Dis. 2015;15(11):1334–46. doi:10.1016/S1473-3099(15)00217-0.
- European Centre for Disease Prevention and Control ECDC. Invasive meningococcal disease – annual epidemiological report for 2016. ECDC; 2016. [accessed 2019 May 20]. https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2016-invasive-meningococcal-disease_1.pdf.
- Centre of Disease Control. CDC. enhanced meningococcal disease surveillance report, 2017. [accessed 2019 May 20]. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf
- Safadi MA, O’Ryan M, Valenzuela MT, Brandileone MC, Gorla MC, de Lemos AP, Moreno G, Vazquez JA, López EL, Taha M-K, et al. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine. 2015;33(48):6529–36. doi:10.1016/j.vaccine.2015.10.055.
- US centers for disease control and prevention. Active Bacterial Core surveillance (ABCs). [accessed 2019 Jun 20] https://www.cdc.gov/abcs/overview/background.html
- Australian Government, Department of Health. Meningococcal–Australian meningococcal surveillance programme annual reports. [accessed 2019 Jun 20]. https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-menganrep.htm
- Sáfadi MAP, de Los Monteros LEE, López EL, Sàez-Llorens X, Lemos AP, Moreno-Espinosa S, Ayala SG, Torres JP, de Moraes JC, Vázquez JA, et al. The current situation of meningococcal disease in Latin America and recommendations for a new case definition from the global meningococcal initiative. Expert Rev Vaccines. 2013;12:903–15. doi:10.1586/14760584.2013.814879.
- Azevedo LCP, Toscano CM, Bierrenbach AL. Bacterial meningitis in Brazil: baseline epidemiologic assessment of the decade prior to the introduction of pneumococcal and meningococcal vaccines. PLoS One. 2013;8(6):e64524. doi:10.1371/journal.pone.0064524.
- Moraes C, de Moraes JC, Silva GDM, Duarte EC. Evaluation of the impact of serogroup C meningococcal disease vaccination program in Brazil and its regions: a population-based study, 2001–2013. Mem Inst Oswaldo Cruz. 2017;112(4):237–46. doi:10.1590/0074-02760160173.
- Andrade AL, Minamisava R, Tomichet L, Lemos AP, Gorla MC, Brandileone MC, Domingues CMS, de Moraes C, Policena G, Bierrenbach AL, et al. impact of meningococcal C conjugate vaccination four years after introduction of routine childhood immunization in Brazil. Vaccine. 2017;35(16):2025–33. doi:10.1080/21645515.2017.1415682.
- Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike IO, Oeser C, Kent A, et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect. 2015;71(1):43–52. doi:10.1016/j.jinf.2015.02.006.
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2016;16(4):313–28. doi:10.1080/14760584.2017.1258308.
- McNamara L, Dolan Thomas J, MacNeil J, Chang HY, Day M, Fisher E, Martin S, Poissant T, Schmink S, Steward-Clark E, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-FHbp in response to a university serogroup B meningococcal disease outbreak-Oregon, 2015–2016. J Infect Dis. 2017;216:1130–40. doi:10.1093/infdis/jix446.
- Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, Mac-Neil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al. Meningococcal carriage evaluation in response to a Serogroup B meningococcal disease outbreak and mass vaccination campaign at a College-Rhode Island, 2015–2016. Clin Infect Dis. 2017;64(8):1115–22. doi:10.1093/cid/cix091.
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN, et al. Effectiveness and impact of a reduced infant schedule of4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388:2775–82. doi:10.1016/S0140-6736(16)31921-3.
- Public Health England. Invasive meningococcal disease in England: annual laboratory confirmed reports for epidemiological year 2018 to 2019. Available from: www.gov.uk/government/publications/meningococcal-disease-laboratory-confirmedcases-in-england-in-2018-to-2019 [accessed Sept 30 2019]
- Ladhani S Meningococcal protein vaccines - where next? Abstract number 263125 presented at ESPID in Ljubljana, Slovenia. May 9, 2019.
- Centers for Disease Control and Prevention (CDC). Interim guidance for control of serogroup B meningococcal disease outbreaks in organizational settings. [accessed 2019 May 30]. https://www.cdc.gov/meningococcal/downloads/interim-guidance.pdf
- Medini D, Stella M, Wassil J. MATS: global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine. 2015;33:2629–36. doi:10.1016/j.vaccine.2015.04.015.
- De Moraes JC, Kemp B, de Lemos AP, Gorla MC, Marques EG, Ferreira MD, Sacchi C, Marques Pinto Carvalhanas TR, Ribeiro AF, Ferreira CM, et al. Prevalence, risk factors and molecular characteristics of meningococcal carriage among Brazilian adolescents. Pediatr Infect Dis J. 2015;34:1197–202. doi:10.1371/journal.pone.0166475.
- Nunes AMPB, Ribeiro GS, Ferreira ÍE, Moura ARSS, Felzemburgh RDM, de Lemos APS, Reis MG, de Moraes JC, Campos LC. Meningococcal carriage among adolescents after mass meningococcal C conjugate vaccination campaigns in Salvador, Brazil. PLoS One. 2016;11(11):e0166475. doi:10.1371/journal.pone.0166475.