ABSTRACT
Pneumococcal disease is a potentially fatal bacterial infection that is vaccine-preventable. Malaysia has yet to adopt a pneumococcal conjugate vaccine (PCV) into its national immunization program (NIP). In 2016, pneumonia was the 3rd leading cause of death in children under five in Malaysia, accounting for 3.8% of under-five deaths. Introducing a pneumococcal conjugate vaccine (PCV) is an effective strategy to reduce the disease burden. This study used a decision-analytic model to assess the potential impacts of introducing the available PCVs (13-valent and 10-valent) in Malaysia. Epidemiological and costs inputs were sourced from published literature. For each vaccination program, health outcomes and associated healthcare costs were estimated. The scenarios of initiating PCV13 vs. PCV10 and the status quo (no pneumococcal vaccine) were compared. Serotype trends of Finland and the U.K. were used to model the clinical impacts of PCV10 and PCV13 respectively. The base-case analysis used a societal perspective over a 5-year time horizon. Compared with PCV10, PCV13 was projected to avert an additional 190,628 cases of pneumococcal disease and 1126 cases of death. The acquisition of PCV13 was estimated to cost an incremental US$89,904,777, offset by a cost reduction of -US$250,219,914 on pneumococcal disease-related medical care and lost productivity. PCV13 demonstrated a higher cost-saving potential over PCV10. Compared with no vaccination, PCV13 was estimated as cost-saving. Results were robust across a series of sensitivity analyses. The introduction of PCV13 in a NIP was estimated to reduce a significant burden of disease and to be a cost-saving for the Malaysian health system.
Introduction
Streptococcus pneumoniae, a bacterium that colonizes the nasopharynx, causes serious invasive diseases, such as meningitis and bacteremia, and noninvasive mucosal infections, such as acute otitis media (AOM, middle ear infection) and pneumonia (lung infection).Citation1 Both invasive and noninvasive pneumococcal disease presents a significant health and economic burden worldwide. In Malaysia, pneumonia was the 3rd leading cause of death in children under five, accounting for 3.8% of under-five deaths in 2016.Citation2 Introducing a pneumococcal conjugate vaccine (PCV) is an effective strategy to reduce the disease burden. In 2007, the World Health Organization recommended the inclusion of PCVs into the national immunization program of countries with a high disease burden. Following the increased uptake of PCVs globally, a substantial decline in pneumococcal-related morbidity and mortality has been observed between 2000–2015.Citation3-Citation6 Yet, countries with a high disease burden remain without a national PCV program, in part due to financial constraints and funding gaps. Middle-income countries predominately finance vaccines from national budgets and are ineligible for funding from programmes by the Global Alliance on Vaccination and Immunization (GAVI).Citation7,Citation8
Malaysia is an upper-middle income country that has not adopted PCVs into the national immunization program. To inform decisions on introducing a PCV program, several local studies have previously reported the cost-effectiveness of the two licensed PCVs – PCV10 (Synflorix®) and PCV13 (Prevnar 13®) in addition to assessing the introduction of a PCV program versus no program. The findings consistently showed a cost-effectiveness ratio favoring the adoption of a national PCV program.Citation9-Citation12 However, findings from the comparative analyses between PCV10 and PCV13 remain equivocal. The lack of agreement stems from a divergence in model assumptions as well as the modeling approach.Citation13 For instance, the efficacy of PCV10 against acute OM caused by non-typeable Haemophilus influenzae (NTHi) were considered in Aljunid et al. and Wang et al.Citation11 but not Wu et al.Citation10 Indirect effect (herd protection) and cross-protection were inconsistently modeled.Citation9-Citation11 In addition, the conventional use of a Markov model among the existing evaluations might have overly simplified the complexity of the pneumococcal transmission dynamics.Citation13 Hence, the true impact of a PCV vaccination program may be mis-estimated by this approach.
This study intended to estimate the population health benefits and economic impact associated with the introduction of PCV10 and PCV13 in Malaysia, and to calculate the incremental cost-effectiveness ratio of the PCV10 vs. PCV13. To build upon the literature and address existing gaps, we applied an alternative approach to modeling the potential population impact of PCVs on pneumococcal disease. This approach used surveillance data from countries which have implemented PCVs to derive the post-implementation trends for an assessment of the real-world impact of vaccination. The observed vaccine impact trends were then applied to the Malaysian setting for a cost-effectiveness analysis. With this approach, unverifiable assumptions, such as indirect effects and serotype replacement, underlying conventional cost-effectiveness models were avoided based on the use of real-world data.
The objective of this analysis was to assess the public health and economic impact of introducing a PCV program compared with the status quo (i.e. no vaccination), as well as comparing the PCV10 (Synflorix®) vs. PCV13 (Prevnar 13®).
Methods
Model design
An existing pneumococcal disease forecasting model was adapted.Citation14 The model was built using real-world pre- and post-PCV surveillance data from PCV10 (e.g. Finland and the Netherlands) and PCV13 (e.g. the United Kingdom (UK)) experienced countries with robust surveillance systems. The model forecasts the changes in pneumococcal disease incidence under each PCV selection (i.e. PCV13 vs. PCV10) as well as the scenario of no vaccine, based on the current disease incidence in Malaysia. The simulated changes were then applied to compute the population health and economic impact of each vaccination strategy.
All serotypes contained within PCV13 and PCV10, and the non-PCV13 serotypes were modeled separately for each of the seven age groups (0–2, 3–4, 5–17, 18–34, 35–49, 50–64, and 65+ years). The serotype-specific disease incidence trends following PCV use were primarily derived using surveillance data from Finland (PCV10) and the UK (PCV13) each. Trends from these countries were selected due to their robust surveillance systems and used to match the 2 + 1 vaccination schedule, which may be the schedule adopted by the Malaysia Health Ministry. An alternative 3 + 0 PCV schedule may be beneficial for Malaysia but could not be modeled in this current analysis. Nonetheless, both schedules utilize 3 doses in informing the vaccine acquisition costs. In the absence of local data, we made equivalent assumptions around vaccine implementation for each vaccine with uptake at 90%.
Given the country and serotype-specific forecasts, the number of cases of IPD, pneumococcal pneumonia, and pneumococcal AOM were predicted for a period of 5 years in Malaysia. Rates of pneumococcal pneumonia and AOM were calculated based on all-cause disease incidence. The rates of mucosal disease were assumed to change proportionally according to the same serotypes causing IPD. This assumption was similarly used elsewhere.Citation15,Citation16 The model inherently captures indirect effects (i.e. effects in those over 5 years of age who are unvaccinated) on invasive disease since observed serotype trends include invasive disease behavior in both vaccinated and unvaccinated groups.
Using the model, we calculated the costs and outcomes for each PCV selection. The outcomes included the following: (1) the number of disease cases and deaths avoided, and (2) the number of quality adjusted life years (QALYs) gained or lost. Costing was performed using the societal perspective.
Epidemiological setting and inputs ()
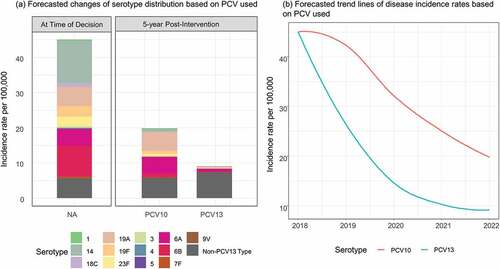
The most recently available data on incidence of IPD and pneumococcal pneumonia for children <5 were obtained primarily from Wahl et al.Citation3 IPD incidence rates for bacteremia and meningitis were adjusted to the remaining age groups based on age structures from Rhodes et al.Citation17 and Thai National Disease Surveillance ReportCitation18 respectively. Serotype-specific IPD incidence rates for children <5 were subsequently generated using the serotype distributions based on Arushothy et al.Citation19 and regional estimates from a systematic review of evidence across South East Asia for those aged >5.Citation20 At the year of potential switch, the incidence of IPD in 0–2 year olds was 45 per 100,000, of which serotype 19A (14%), serotype 14 (27%), and serotypes 6A/6B (30%) caused the majority of residual disease (). For individual’s ≥65 years of age, non-covered serotypes comprised the largest proportion of remaining disease (21%) (). All-cause AOM was obtained from the global burden of disease dataCitation21 and 36%Citation22 was assumed to be due to pneumococcal.
Table 1. Epidemiological and cost inputs used in the cost-effectiveness of infant pneumococcal vaccination program.
Cost inputs ()
Cost inputs were sourced from similar references used in Wu et al.Citation10 Direct medical costs associated with IPD, pneumonia and AOM were sourced from a published cost- effectiveness study of PCV7Citation12 and an economic burden studyCitation23 in Malaysia. Indirect costs among children under five were extrapolated using data from a study on children receiving pneumococcal vaccination in a middle-income country.Citation24 Indirect costs associated with children over five were determined by multiplying the average length of hospital stay (unpublished local study) by the median average daily salary in Malaysia.Citation10 In the absence of public sector pricing between the two PCVs, we used July 2019 private sector pricing for each vaccine and calculated a price disparity of 40% between PCV13 and PCV10 . Since public pricing for an NIP often differs from the private sector due to volume agreements, our base case provides a conservative estimate of budget impact for a PCV program. In comparing PCV programs, a 40% price disparity is also conservative since other public estimates such as PAHO (a public price of USD$12.85 and $14.5 for PCV10 and PCV13, respectively) estimate a 10% price disparity between vaccines. Additional costs (e.g. wastage, storage, delivery) were not accounted for in the current analysis. All costs were reported in 2018 United States Dollar (USD).
Utilities
Baseline utilities for Malaysia were sourced from published literature.Citation25 Utility decrements were applied for each occurrence of disease relative to an age-specific baseline utility weight for individuals who did not experience a case of the disease. Annual decrement of 0.0070 and 0.0232 were assumed for bacteremia and meningitis,Citation26 respectively. Decrements of 0.005, 0.006, and 0.004 were assumed for OM, inpatient pneumonia, and outpatient pneumonia.Citation27 Sequelae such as neurologic impairment and hearing loss following a case meningitis were assumed to occur with a probability of 7% and 13%,Citation28,Citation29 respectively, and carried a lifetime QALY decrement of 0.40 and 0.20.Citation30,Citation31
Analysis
In the base case analysis, cases of disease, deaths, and associated costs and QALYs were estimated over a 5-year time horizon. A scenario of PCV13 use was compared with PCV10. An incremental cost-effectiveness ratio (ICER) was then calculated. Costs and outcomes were discounted at a rate of 3.0% per annum.Citation32
Sensitivity analyses
We conducted the following sensitivity analyses: (1) vary the study time horizon (i.e. five year vs. ten years), (2) vary the assumed indirect effects due to pneumonia for both vaccines, (3) alternate to the payer perspective, and (4) further consider the potential impact of NTHi and Moraxella catarrhalis (M. catarrhalis) on AOM. Additionally, we explored the impact of PCV13 alone as well as both vaccines have on the NTHi and M. catarrhalis AOM (single- species and co- colonized episodes). This additional exploration was motivated by the possibility that PCV13 may have broader benefits in averting early onset non-pneumococcal AOM hence avoiding the downstream complex cases. This consideration was suggested by an earlier investigation.Citation33 This analysis was accomplished by applying an annual rate of change in disease for PCV13 against the residual AOM caused by each pathogen. The rates applied were 0.755 for NTHi and 0.759 for M. catarrhalis. Due to limited evidence on the impact of PCV10 against non-pneumococcal OM, an annual rate of change in disease for PCV10 of 0.785 for NTHiCitation34 and 0.0 for M. catarrhalis was applied.Citation35 NTHi and M. catarrhalis were estimated to cause 31.7% and 1.6% of AOM disease.Citation35
Additional scenarios were evaluated using serotype trends from the Netherlands, which implemented PCV10 in a 2 + 1 schedule in 2011. Given the natural variation in vaccine implementation and uptake, these scenarios present potential serotype trajectories under different vaccine pressures. Lastly, we also assessed the robustness of our results by applying the incidence rates of IPD, pneumonia and AOM used in Wu et al.Citation10 (Supplementary Tables 1 and 2). We also performed a probabilistic sensitivity analysis (second-order Monte Carlo simulations) over 5,000 random draws to test model certainty. The proportion of IPD that is meningitis, vaccination rate, the percentage of all-cause OM that is pneumococcal, the percentage of all-cause pneumonia that is pneumococcal, utilities, and disease-specific case fatality rates were drawn from beta distributions. Limits on forecasted incidence, pre-vaccine incidence, direct costs, vaccine acquisition costs, vaccine administration costs, sequelae costs, lost productivity, and all-cause mortality rates were drawn from gamma distributions.
Results
Epidemiologic results are presented for children < 2 years old and those over 65 years old as these age groups are most susceptible to pneumococcal disease and form the largest proportion of the total burden, while cost implications are based on population-level impacts.
Epidemiologic model results
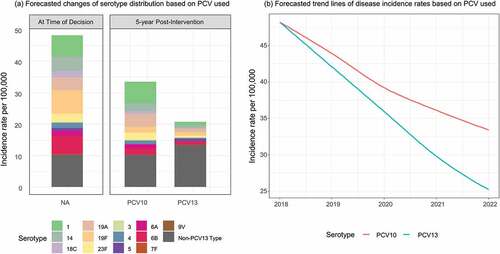
Under a PCV13 program, 5 years after the introduction, total IPD in children 0–2 years was estimated to decline in children by 80% from 45 to 9.8 per 100,000. All PCV13 serotypes were estimated to decrease between 60-95%, while non-PCV13 serotypes were estimated to increase by 30% based on observed disease trends under vaccine pressure in the UK (). Specifically, serotype 19A was estimated to decrease by 94% from 5.3 to 0.3 per 100,000. A similar trend was observed in adults > 65 years old, where total IPD decreased by 48% due to indirect effects of the pediatric program ().
Under a PCV10 program, 5 years after the introduction, total IPD among the 0–2 years old was estimated to decrease by 56% (from 45 to 19.8 per 100,000). PCV10- type disease was estimated to decrease between 70-100% for covered serotypes with no impact on serotypes 3, 6A, 19A or non-PCV13 type disease, based on observed disease trends in Finland. A similar trend was observed among the > 65 years old, where total IPD decreased by 31% with most of the remaining diseases being caused by the serotype 19A (4.0 per 100,000) and non-PCV13 serotypes (10 per 100,000).
Base case cost- effectiveness results
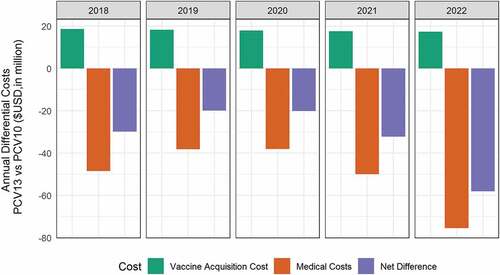
After five years, for all ages, compared with PCV10, PCV13 was estimated to reduce an additional 4,086 cases of IPD, of which 276 were due to serotype 19A alone, 160,833 cases of AOM, 25,708 cases of pneumonia, and 1,126 deaths. () An estimation of 2280 quality-adjusted life years (QALYs) were gained. PCV13 were estimated to have an incremental acquisition costs at $89.9 million, which were offset by the -$250 million of direct and indirect cost savings. Over a 5 years period, PCV13 was estimated to save an additional $160 million compared to the use of PCV10. The annual differential costs of PCV13 vs. PCV10 is illustrated in .
Table 2. Incremental cases, deaths, and costs under a PCV13 versus PCV10 vaccination program, over a 5- year time horizon.
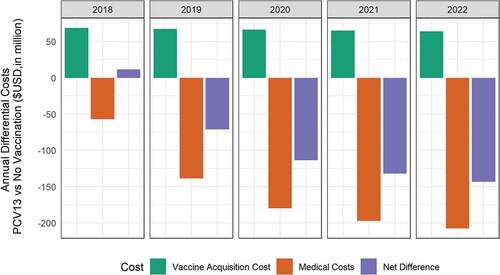
Compared with the status quo of no vaccination, introducing PCV13 was estimated to reduce (over 5 years) an additional 10,018 cases of IPD, 524,966 cases of AOM, 81,443 cases of pneumonia and 3,046 deaths (). This population impact can be translated into an increment of 6,008 QALYs gained. PCV13 acquisition costs were estimated at $331.9 million, which were offset by the $780.2 million of direct and indirect cost savings. This can be translated to a significant net savings of $448 million in a 5-year PCV13 program for Malaysia. The annual differential costs of PCV13 vs. no vaccination is illustrated in .
Table 3. Incremental cases, deaths, and costs under a PCV13 versus no vaccination program, over a 5- year time horizon.
Sensitivity analyses
Sensitivity analyses predicted PCV13 to remain dominant over PVC10 for all the analyses conducted (). The probabilistic sensitivity analysis (Supplementary Figure 1) showed that a PCV13-based program remained cost-saving in 100% of the simulations.
Table 4. Sensitivity analyses and the incremental costs and quality-adjusted life years (QALY) under a PCV13 versus PCV10 vaccination program.
Discussion
Our study compared the potential public health and economic impact of the available PCV options for Malaysia. The results show that the implementation of a national PCV program can potentially avert a significant number of pneumococcal-related diseases and deaths. Compared with PCV10, PCV13 was estimated to reduce an additional 4,086 cases of IPD, 160,833 cases of AOM, 25,708 cases of pneumonia, and 1,126 deaths over a 5-year implementation in Malaysia. In Malaysia, a PCV program with the 13-valent vaccine was estimated to have greater population health and economic value than using the 10-valent vaccine from both societal and payer perspectives.
The results of our study supported the findings of earlier economic analyses,Citation10 suggesting higher public health and economic impacts with the adoption of PCV13 into the NIP over PCV10 in Malaysia. Contrary to our results, previous studies by Aljunid et al.Citation9 and Wang et al.Citation11 suggested more cost-savings with a PCV10 program as compared with PCV13. The differences in the predicted outcomes are driven by the differing assumptions used in the economic evaluations. For instance, Wang et al.Citation11 assumed cross- protection of PCV10 against non- vaccine serotypes such as serotypes 6A and 19A. Although there were some earlier case-control studies which demonstrated the possibility of cross- protection against 19A with PCV10, recent surveillance evidence demonstrates an increasing trend in cases due to 19A in both vaccinated and unvaccinated individuals in countries with a PCV10 program.Citation36 Additionally, the authors acknowledged that the evidence on the cross-protection effect of PCV10 against serotype 6A was less conclusive.Citation11 Wang et al.Citation11 also assumed the additional benefit of PCV10 in preventing AOM due to NTHi based on evidence for its 11-valent precursor. However, real-world evidence over the past few years presented mixed results on the effectiveness of PCV10 against NTHi AOM. To date, no study of PCV10 has demonstrated similar effectiveness as seen for 11-valent vaccine in reducing NTHi- caused AOM.Citation14 In our analysis, we explored the impact of PCV13 alone as well as both PCV10 and PCV13 on NTHi-related AOM. PCV13 remained dominant over PCV10 in both scenarios.
To our knowledge, this study was the first economic evaluation of PCVs in Malaysia using a disease-forecasting model. Previous studies examining the cost-effectiveness of PCVs in Malaysia relied largely on the conventional approach of static disease modeling.Citation9-Citation11 Static models assume constant risk of infection and their accuracy in modeling an infectious disease such as pneumococcal infection have been questioned.Citation37 Real world observational data indicate that the incidence of IPD has declined among both vaccinated and non-vaccinated populations due to indirect effects.Citation38 Conversely, the implementation of PCVs can also lead to undesired, negative population effects such as serotype replacement.Citation39 This further complicates the estimation of the full value of a PCV program. A dynamic model is preferred as it captures the complexity of disease transmission over time, and the interaction between the vaccinated and unvaccinated individuals within a population, though dynamic transmission models are complicated to implement. The forecasting model used in our analysis leveraged the surveillance data that captured the impact of a pediatric PCV program on disease incidence across all age groups. As a result, the model inherently captures serotype replacement and indirect effects based on the disease incidence trends reported in the surveillance data. This approach circumvents the pitfalls of conventional modeling approaches where concerns on incorporating the herd immunity and serotype replacement effects had been raised.Citation37 The direction and magnitude of the indirect effects cannot be ascertained without the support of high-quality surveillance data and a solid understanding of the disease dynamic.
Our study also included the most recent local serotypes distribution data from the Institute of Medical Research in Malaysia, providing a more robust analysis on pneumococcal disease input. In the setting of high serotype 19A burden in Malaysia, the use of PCV13 may lead to greater reductions than PCV10, as this serotype is contained in PCV13 and cross protection from serotypes in PCV10 may not offer the same magnitude of benefit as those observed from using PCV13.Citation40 Our study was conducted based on a wider societal perspective which could illustrate more comprehensively the values of a public health initiative such as a vaccination program.
Our study was not without limitations. Firstly, in the absence of public sector pricing between the two PCVs, we assumed a price disparity of 40% between PCV13 and PCV10. This price disparity will require further validation in the future for more accurate estimation. However, we believe our assumptions provide a conservative perspective as any volume-based discounts would further improve the cost-savings of the program. Furthermore, our results on PCV implementation may over-estimate the benefit as we did not account for vaccine implementation or wastage costs for either vaccine. Secondly, our analysis was conducted using a 2 + 1 schedule. We were not able to explore the alternative 3 + 0 PCV schedule due to the lack of data. This analysis may be of value to inform the choice of vaccination schedule. For countries that have yet to introduce PCV, the WHO recommends a 3- dose schedule of PCV administration either as 2 + 1 or as 3 + 0.Citation41 Thirdly, our study was unable to examine the protective effect of pneumococcal vaccination arising from the private sector. The private sector is known to have contributed to about 30% of the pediatric vaccination in Malaysia. Lastly, in the absence of Malaysian data to inform the model parameters, we used data from literature with epidemiologically similar population where possible. Post-PCV implementation trends from Finland and the UK were used in this analysis due to their robust surveillance systems. While the data sources from these two countries are reliable, cross-country differences may exist in healthcare system and vaccine delivery strategy between these high-income countries and a middle-income country such as Malaysia. Hence, cautions should be made when interpreting the potential public health and economic impacts of PCVs in this analysis.
If a population- based PCV program was considered in Malaysia, the choice of vaccination schedule (i.e. between 2p+1 and 3p+0) should be further examined and Operational and programmatic factors should considered. These considerations may include the timeliness of vaccination, the coverage expected to be achieved at the third dose, and pneumococcal disease age distribution patterns, if known. The 2p+1 schedule is likely to offer higher benefits over the 3p+0 schedule, when programmatically feasible. A higher level of antibody is known to be associated with 2p+1 schedule at the end of the second year of life. This can be of great importance in the maintenance of an effective herd immunity.Citation40,Citation41 Efforts should also be made in conducting post-PCV introduction surveillance. A sustained and high-quality population-based surveillance should be in place to monitor the epidemiological impact of PCV. Ideally, surveillance should be started at least 1–2 years before the introduction of a PCV program and be continued at least for 5 years after introduction but preferably indefinitely.Citation41 Such surveillance program will be useful in monitoring the changes of pneumococcal diseases and serotypes pattern as the trend may evolve over time following the PCV program. In conclusion, the introduction of PCV13 into the NIP has a potentially high public health and economic impact to the Malaysian population and society. Compared to PCV10 and to status quo (no vaccination), PCV13 is likely to have a higher value in terms of reduction of disease burden as well as net cost saving for the health system.
Disclosure of potential conflicts of interest
SP and JN are employees of Pfizer. SP is a shareholder of Pfizer. CYF and CW are employees of IQVIA. IQVIA received professional fees from Pfizer for the supportive services related to this study. The rest of the authors reported no conflict of interest.
Contributors
SP, AAS, JN conceived and designed the analysis; NA, JN, SP collected the data; AAS, NA, JN, CYF, CW, SP, KKT analysed and/or interpreted the results; CYF, CW, SP wrote the first draft; AAS, NA, JN, CYF, CW, SP, KKT critically reviewed the content and approved the final version.
Data sharing
Data used for the analyses are available on request.
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplemental Material
Download MS Word (162.6 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21645515.2019.1701911.
Additional information
Funding
References
- Cohen R, Biscardi S, Levy C. The multifaceted impact of pneumococcal conjugate vaccine implementation in children in France between 2001 to 2014. Hum Vaccin Immunother. 2016;12(2):277–84. doi:10.1080/21645515.2015.1116654.
- Statistics on Causes of Deaths. Putrajaya, Malaysia: Department of Statistics; 2017 [accessed 2019 Oct 09]. https://www.dosm.gov.my/v1/index.php?r=column/pdfPrev&id=Y3psYUI2VjU0ZzRhZU1kcVFMMThGUT09.
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Luksic I, Nair H, McAllister DA, Campbell H, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–e57. doi:10.1016/S2214-109X(18)30247-X.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, McCarthy ND, Petrou S. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–e59. doi:10.1016/S2214-109X(16)30306-0.
- GAVI. Advance Market Commitment for Pneumococcal Vaccines - Annual Report April 2014 - March 2015.
- VIEW-hub Global Vaccine Introduction Report, March 2018. International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health; 2018 Mar [accessed 2019 May 24]. www.jhsph.edu/ivac/view-hub.
- Aljunid S, Maimaiti N, Ahmed Z, Muhammad Nur A, Md Isa Z, Azmi S, Sulong S. Economic impact of Pneumococcal Protein-D Conjugate Vaccine (PHiD-CV) on the Malaysian National Immunization Programme. Value Health Reg Issues. 2014;3:146–55. doi:10.1016/j.vhri.2014.04.008.
- Wu DB, Roberts C, Lee VW, Hong LW, Tan KK, Mak V, Lee KK. Cost-effectiveness analysis of infant universal routine pneumococcal vaccination in Malaysia and Hong Kong. Hum Vaccin Immunother. 2016;12(2):403–16. doi:10.1080/21645515.2015.1067351.
- Wang XJ, Saha A, Zhang XH. Cost-effectiveness analysis of a universal mass vaccination program with a PHiD-CV 2+1 schedule in Malaysia. Cost Eff Resour Alloc. 2017;15:17. doi:10.1186/s12962-017-0079-2.
- Aljunid S, Abuduxike G, Ahmed Z, Sulong S, Nur AM, Goh A. Impact of routine PCV7 (Prevenar) vaccination of infants on the clinical and economic burden of pneumococcal disease in Malaysia. BMC Infect Dis. 2011;11:248. doi:10.1186/1471-2334-11-248.
- Wasserman M, Sings HL, Jones D, Pugh S, Moffatt M, Farkouh R. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018;17:71–78. doi:10.1080/14760584.2018.1409116.
- Wilson M, Wasserman M, Jadavi T, Postma M, Breton MC, Peloquin F, Earnshaw S, McDade C, Sings H, Farkouh R. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018. doi:10.1007/s40121-018-0206-1.
- Thorrington D, van Rossum L, Knol M, de Melker H, Rumke H, Hak E, van Hoek AJ. Impact and cost-effectiveness of different vaccination strategies to reduce the burden of pneumococcal disease among elderly in the Netherlands. PLoS One. 2018;13(2):e0192640. doi:10.1371/journal.pone.0192640.
- van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–13. doi:10.1016/j.vaccine.2012.10.017.
- Rhodes J, Dejsirilert S, Maloney SA, Jorakate P, Kaewpan A, Salika P, Akarachotpong T, Prapasiri P, Naorat S, Areerat P, et al. Pneumococcal bacteremia requiring hospitalization in rural Thailand: an update on incidence, clinical characteristics, serotype distribution, and antimicrobial susceptibility, 2005-2010. PLoS One. 2013;8(6):e66038. doi:10.1371/journal.pone.0066038.
- 2017 National Disease Surveillance (Report 506) Nonthaburi. Thailand: Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health; 2018.
- Arushothy R, Ahmad N, Amran F, Hashim R, Samsudin N, Azih CRC. Pneumococcal serotype distribution and antibiotic susceptibility in Malaysia: a four-year study (2014-2017) on invasive paediatric isolates. Int J Infect Dis. 2019;80:129–33. doi:10.1016/j.ijid.2018.12.009.
- Jauneikaite E, Jefferies JM, Hibberd ML, Clarke SC. Prevalence of Streptococcus pneumoniae serotypes causing invasive and non-invasive disease in South East Asia: a review. Vaccine. 2012;30(24):3503–14. doi:10.1016/j.vaccine.2012.03.066.
- Institute for Health Metrics and Evaluation. Global Burden of Disease (GBD). [accessed 2019 May 29]. http://www.healthdata.org/gbd.
- Hausdorff WP, Yothers G, Dagan R, Kilpi T, Pelton SI, Cohen R, Jacobs MR, Kaplan SL, Levy C, Lopez EL, et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr Infect Dis J. 2002;21(11):1008–16. doi:10.1097/01.inf.0000035588.98856.05.
- Ahmed Z, Aljunid S, Maimaiti N, Nur A, Isa Z, Sulung S. Economic burden of pneumococcal disease in Malaysia. Abstract presented at: the 2012 APCP Conference; 2012; Kuching, Malaysia.
- Nakamura MM, Tasslimi A, Lieu TA, Levine O, Knoll MD, Russell LB, Sinha A. Cost effectiveness of child pneumococcal conjugate vaccination in middle-income countries. Int Health. 2011;3(4):270–81. doi:10.1016/j.inhe.2011.08.004.
- Mittmann N, Trakas K, Risebrough N, Liu BA. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics. 1999;15(4):369–76. doi:10.2165/00019053-199915040-00004.
- Bennett JE, Sumner W 2nd, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154:43–48.
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14. doi:10.1016/j.vaccine.2004.05.003.
- McIntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, Perez CM. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278(11):925–31. doi:10.1001/jama.278.11.925.
- Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323(24):1651–57. doi:10.1056/NEJM199012133232402.
- Cheng AK, Niparko JK. Cost-utility of the cochlear implant in adults: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1999;125(11):1214–18. doi:10.1001/archotol.125.11.1214.
- Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121–27. doi:10.1155/2007/713576.
- Ministry of Health Malaysia. Pharmacoeconomic Guideline For Malaysia 2012. [accessed 2019 May 20]. https://www.pharmacy.gov.my/v2/ms/dokumen/pharmacoeconomic-guideline-malaysia.html.
- Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. 2016;16(4):480–92. doi:10.1016/S1473-3099(15)00549-6.
- Tregnaghi MW, Saez-Llorens X, Lopez P, Abate H, Smith E, Posleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Correction: efficacy of pneumococcal nontypable haemophilus influenzae Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: a double-blind randomized controlled trial. PLoS Med. 2015;12(6):e1001850. doi:10.1371/journal.pmed.1001850.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63(5):611–18. doi:10.1093/cid/ciw347.
- Wasserman M, Palacios MG, Grajales AG, Baez Revueltas FB, Wilson M, McDade C, Farkouh R. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccin Immunother. 2019;15(3):560–69. doi:10.1080/21645515.2018.1516491.
- Nymark LS, Sharma T, Miller A, Enemark U, Griffiths UK. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine. 2017;35(49Pt B):6828–41. doi:10.1016/j.vaccine.2017.10.024.
- Kim YK, LaFon D, Nahm MH. Indirect effects of pneumococcal conjugate vaccines in national immunization programs for children on adult pneumococcal disease. Infect Chemother. 2016;48(4):257–66. doi:10.3947/ic.2016.48.4.257.
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11(10):760–68. doi:10.1016/S1473-3099(11)70090-1.
- Meeting of the strategic advisory group of experts on immunization, October 2017 – conclusions and recommendations. [accessed 2019 May 29]. https://www.who.int/immunization/sage/meetings/2017/october/presentations_background_docs/en/.
- Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper; 2019 Feb [accessed 2019 May 29]. https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1.