ABSTRACT
Cholangiocarcinoma (CCA) is an aggressive tumor that is associated with high rates of recurrence and mortality. This is due, in part, to the fact that CCA cells and their microenvironment secrete immunosuppressive cytokines, transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), that inhibit dendritic cell (DC) functions, which, in turn, results in the decreased anti-tumor activity of T-cells. We hypothesized that the TGF-β receptor and IL-10 blockade on dendritic cells would improve DC function, thereby allowing improved activation of T cells against CCA cells. To test our hypothesis, we generated self-differentiated DCs (SD-DCs) via transduction of human peripheral blood monocytes with lentivirus expressing IL-4 and GM-CSF. SD-DCs were transduced with a second lentivirus containing short-hairpin RNAs (shRNAs) to knock-down TGF-βRII and IL-10RA mRNAs. Immunoblot confirmed the reduced expression levels of TGF-β and IL-10 receptors in both SD-DCs that were transduced with a single and/or combination of lentiviruses containing shRNAs. SD-DCs were thereafter pulsed with tumor antigens extracted from CCA cell lines in an effort to activate DC function. MHC class II (HLA-DR) and co-stimulatory molecules (CD40 and CD86) on SD-DCs were upregulated to levels comparable to those on DCs generated by the conventional method. Suppression of TGF-β and IL-10 receptors on SD-DCs influenced the effector T-cells to produce IFN-γ, which enhanced their ability to kill CCA cells. The preparation of adoptive effector T-cells holds the potential of becoming a novel therapy for cellular immunotherapy in CCA.
Introduction
Cholangiocarcinoma (CCA) is an aggressive tumor of the bile duct that has poor prognosis and treatment options.Citation1 About one-third of CCA cases are eligible for surgery, but the recurrence and mortality rates are high.Citation2 Patients with advanced or metastatic CCA have poor response to standard chemotherapeutic treatment.Citation3 Therefore, the urgency to develop novel treatments for CCA is high.
The tumor microenvironment is being increasingly investigated in a number of different cancers since it plays an important role in suppressing antitumor immunity.Citation4 The production of immunosuppressive cytokines, including transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), which are mechanisms that are known to allow cancer to escape host immune responses has attracted attention as a possible target for intervention.Citation5 These immunosuppressive cytokines impair dendritic cell (DC) functions relative to the induction of effector CD4+ and CD8+ T-cells, which results in a decrease in and perhaps even failure of host anti-cancer immunity.Citation6-9 It has been reported that downregulation of TGF-β and IL-10 receptors on DCs by siRNA cocktail significantly stimulated tumor-specific CD8+ T-cell immune response in the mouse model of cervical cancer.Citation10 Consistent with that, we recently reported that inhibition of TGF-β and IL-10 receptors on DCs by using specific neutralizing antibodies could enhance effector T-cells to kill CCA cells,Citation11 which clearly suggests TGF-β and IL-10 as playing a key role in the suppression of host immune responses. We hypothesized that suppression of TGF-βR and IL-10R on DCs by knocking down TGF-βR and IL-10R mRNAs with lentiviruses containing specific shRNAs would be a novel approach to improving DC function, which would, in turn, facilitate improved activation of effector T-cells for CCA cells. The results of this study revealed that the knocking down of self-differentiated DCs (SD-DCs) derived from monocytes transduced with lentivirus containing IL-4 and GM-CSF cDNA significantly enhanced the ability of effector T-cells to produce IFN-γ, which increased the efficacy of effector T-cells to potentially kill CCA cell lines.
Materials and methods
Lentiviral constructs
Lentiviruses expressing human GM-CSF and IL-4 (LV-GM/IL4) were constructed in pCDH lentiviral expression vector, as previously described.Citation12 MISSION® pLKO.1-puro non-mammalian shRNA control (SHC002V), MISSION® pLKO.1 plasmid containing shRNA against TGF-βRII, and MISSION® pLKO.1 plasmid containing shRNA against IL-10RA constructs were purchased from Sigma-Aldrich Corporation. The sequences of these shRNA are as follows: shRNA against IL-10RA, CCGGGCAGTATTTCACCGTGACCAACTCGAGTTGG TCACGGTGAAATACTGCTTTTTG and shRNA against TGF-βRII: CCGGGACCTCA AGAGCTCCAATAT CCTCGAGGATATTGGAGCTCTTGAGGTCTTTTTTG. The transfer and packaging lentiviral vectors, including pCDH, psPAX2, and pMD2.G, were kindly provided as gifts by Dr. Naravat Poungvarin, Department of Clinical Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Production of lentiviral particles
Briefly, two million human embryonic kidney (HEK) 293T-cells were plated in a 10 cmCitation2 dish and cultured in completed Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco; Thermo Fisher Scientific) with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2 environment overnight. CaCl2 transfection was performed using the transfer lentiviral plasmid and the packaging lentiviral plasmids. After 48 h, culture supernatant containing lentiviruses was collected, clarified through a 0.45 μm filter, aliquoted 1 mL per vial, and stored at −80°C until use. Lentivirus titer was determined by p24 ELISA kit (Clontech Laboratories), and then adjusted to an equal amount for further experiments.
Generation of control DCs
The protocol for collection of human blood samples for this study was approved by the Siriraj Institutional Review Board (SIRB) of the Faculty of Medicine Siriraj Hospital, Mahidol University (COA no. Si 517/2016). Human peripheral blood mononuclear cells (PBMCs) were prepared by gradient density centrifugation using LymphoprepTM solution (Alere Technologies) according to the manufacturer’s instructions. Cells were cultured in AIM-V (Gibco; Thermo Fisher Scientific) and monocytes were allowed to adhere to the wells of a plastic plate at 37°C for 2 h. Non-adhering cells, which were mainly lymphocytes, were harvested and stored at −70°C as the source of T-cells. Adherence cells were stained with specific antibody conjugated with fluorescence dye and analyzed by flow cytometry. The percentage of CD11c−CD14+ cells, which is monocyte population, was more than 90% (data not shown). Control DCs were generated by conventional method. Briefly, monocytes in a 6-well plate were cultured in AIM-V medium supplemented with GM-CSF (50 ng/ml) (Invitrogen) and IL-4 (25 ng/ml) (Immunotools) at 37°C for 5 days in a humidified 5% CO2 environment to generate immature DCs. On day 5, the immature DCs were pulsed with tumor protein lysate extracted from CCA cell line (KKU-213) and cultured in medium containing TNF-α (50 ng/ml) and IFN-γ (50 ng/ml) (both from Immunotools) for 2 days to generate mature DCs.
Generation of self-differentiated dendritic cells (SD-DCs)
To generate SD-DCs, monocytes in a 6-well plate were transduced with lentiviruses (LV-GM/IL4) using 10 μg/mL protamine sulfate (Sigma-Aldrich) in AIM-V medium without cytokine supplement. After 16 h, culture supernatants were removed and the cells were transduced for a second time with lentiviruses containing different shRNAs.
Suppression of IL-10RA and TGF-βRII on SD-DCs
SD-DCs were transduced for a second time with lentiviruses containing LV-shCtrl, LV-shTGF-βRII, LV-shIL-10RA, or a combination of LV- shTGF-βRII and LV- shIL-10RA for 16 h. The four experimental conditions of SD-DCs included: (i) shCtrl SD-DCs, (ii) shTGF-βRII SD-DCs, (iii) shIL-10RA SD-DCs, and (iv) combined shTGF-βRII/shIL-10RA SD-DCs. The transduced cells were cultured in AIM-V medium for 5 days. Because lentiviral constructs containing shRNAs were used to transduce into monocytes, these lentiviral constructs could integrate into monocyte genome resulting in long-term silencing of the targeted genes. Transduced cells were then pulsed with protein lysate extracted from KKU-213 CCA cell line for 2 days.
Enzyme-linked immunosorbent assay (ELISA)
After lentivirus transduction, the cell culture supernatants from the four conditions of SD-DCs were collected on day 5 to determine IL-4 and GM-CSF production by using Human IL-4 Quantikine ELISA Kit (R&D Systems) and Human GM-CSF Quantikine ELISA Kit (R&D Systems).
Immunoblot analysis
At 7 days post-transduction, protein lysate extracted from shCtrl SD-DCs, shTGF-βRII SD-DCs, shIL-10RA SD-DCs, and combined shIL-10RA/shTGF-βRII SD-DCs was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted using goat anti-TGF-βRII antibody (Clone ab61213; R&D Systems), rabbit anti-IL-10RA antibody (Clone ab93470; Abcam, Cambridge, United Kingdom), and mouse anti-β actin antibody (Clone C4; Santa Cruz Biotechnology). Secondary antibodies conjugated with horseradish peroxidase (HRP) that were specific to primary antibodies were used for enzymatic detection. Light signals were generated by adding SuperSignal® West Pico Chemiluminescent Substrate (Thermo Fisher Scientific), which was detected by exposure to X-ray film. Finally, the protein band intensities were determined by the ImageJ program (open source) to calculate the protein expression levels.
Activation and expansion of effector T-cells by DCs
After transduction for 5 days, SD-DCs-shCtrl, SD-DCs-shTGF-βRII, SD-DCs-shIL-10RA, and combined shTGF-βRII/shIL-10RA SD-DCs were pulsed with protein lysate from KKU-213 cell line for 2 days. Pulsed SD-DCs were co-cultured with autologous T-cells in AIM-V medium supplement with 5% human serum (Sigma-Aldrich) for 3 days. The medium was then replaced with AIM-V containing IL-2 (20 ng/mL), IL-7 (10 ng/mL), and IL-15 (20 ng/mL) (all from Immunotools) for 10–20 days to promote the proliferation of effector T-cells. Fifty percent of the medium was replaced with fresh medium every other day.
Flow cytometric analysis
At 7 days post-transduction, control DCs and SD-DCs were harvested and stained with monoclonal fluorescent-conjugated antibodies, including anti-CD14 FITC (Clone 18D11; Immunotools), anti-CD11c APC (Clone BU15; Immunotools), anti-CD86 PE (Clone IT2.2; eBiosciences), anti-CD40 FITC (Clone HI40a; Immunotools), anti-HLA-DR FITC (Clone MEM12; eBiosciences), and anti-CD83 PE (Clone HB15e; eBiosciences). Activated effector T-cells were stained on day 5 with anti-CD3 FITC (Clone UCHT-1), anti-CD4 APC (Clone OKT-4), anti-CD8 APC (Clone UCHT-4), anti-CD16 APC (Clone LNK16) (all from Immunotools), anti-CD28 PE (Clone CD28.2; eBiosciences), and anti-CD56 PE (Clone HCD56; BioLegend). Stained cells were then fixed with 1% formaldehyde in 1x phosphate buffered saline (PBS) containing 2% FBS. The immunophenotypes of DCs and effector T-cells were analyzed by FACSCalibur using CellQuest™ software (BD Biosciences).
Determination of intracellular IFN-γ
After co-culturing with different SD-DCs for 7 days, the activated effector T-cells were harvested and then stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) and ionomycin (Sigma-Aldrich) for 5 h at 37°C. The protein transport blocker monensin (eBioscience) was then added to the activated effector T-cells for 5 h at 37°C. Cells were harvested, stained with anti-CD3 FITC, washed with 1x PBS containing 2% FBS, fixed with 4% paraformaldehyde in 1x PBS containing 2% FBS, and then permeabilized with 0.5% saponin (Sigma-Aldrich) in 1x PBS containing 2% FBS. Cells were stained for intracellular IFN-γ with anti-IFN-γ PE antibody (Clone B27; Immunotools). Finally, the cells were fixed and analyzed by flow cytometry. Isotype-matched antibodies were used to staining the cells used as controls. The percentages of IFN-γ-producing T-cells (CD3+ IFN-γ) were analyzed using FACSCalibur and CellQuest software (BD Biosciences).
Cytolytic activity assay
Briefly, 3 × 10Citation4 cells from CCA cell lines (KKU-213 or KKU-100) and immortalized cholangiocyte cell line (MMNK-1) were plated into wells of a 24-well plate. Activated effector T-cells were added to co-culture with the target T-cells at various effector-to-target (E:T) ratios (2.5:1, 5:1, and 10:1) for 24 h. Cells were harvested, stained with AnnexinV-APC-PI (Immunotools), and analyzed by flow cytometry using CellQuest™ software (BD Biosciences). The target T-cell population that showed higher FSC and SSC than effector cells was gated to analyze cell apoptosis (Annexin V+ alone and Annexin V+/PI+).
Statistical analysis
Statistical analysis was performed using GraphPad Prism Software (GraphPad Software, Inc., v.5.0, San Diego, CA, USA). Mean ± standard error of the mean (SEM) of at least three independent experiments were calculated from all experiments. The experimental data sets from the control and investigated groups were compared and tested using Student’s t-test. A p-value<0.05 was considered to be statistically significant.
Results
Lentiviral vector (LV) construction and self-differentiated DC (SD-DC) generation
We initially constructed four different lentiviral vector (LV) constructs, including LV-GM/IL4, LV-shCtrl, LV-shTGF-βRII, and LV-shIL-10RA ()). The lentiviruses in the culture supernatants were harvested at 48 h to determine viral particles by p24 ELISA kit. Equal amounts of different kinds of lentiviruses were adjusted for DC generation.
Figure 1. Lentiviral vectors and workflow for self-differentiated DC (SD-DC) generation. (a) Schematic representation of lentiviral vectors in this study. (b) Schematic diagram showing the generation of self-differentiated DC (SD-DC) with suppression of TGF-β and IL-10 receptor expression by LV transduction. (c) After transduction for 7 days, cells were harvested and then subjected to immunoblot analysis using antibodies specific to TGF-βRII and IL-10RA
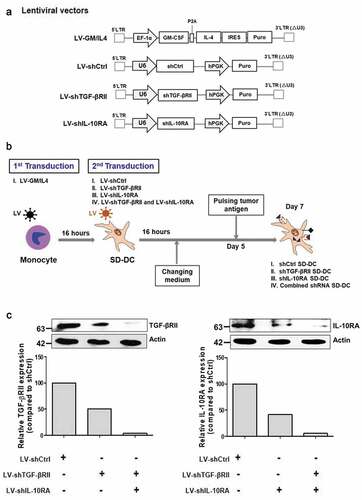
After establishing the lentivirus constructs and virus particles, we tested their ability to transduce. Monocytes isolated from human peripheral blood mononuclear cells (PBMCs) were first transduced with LV-GM/IL4 using protamine sulfate to generate self-differentiated DCs (SD-DCs). Those SD-DCs were subsequently transduced with LV-shCtrl, LV-shTGF-βRII, LV-shIL-10RA, or combination of both LV-shTGF-βRII and LV-shIL-10RA to generate four types of SD-DCs, including: (i) shCtrl-SD-DCs (negative control), (ii) shTGF-βRII-SD-DCs, (iii) shIL-10RA-SD-DCs, and (iv) combined shRNAs-SD-DCs ()).
The efficiency of shRNAs to suppress the expression of TGF-β and IL-10 receptors in SD-DCs was determined by immunoblot analysis compared with shCtrl-SD-DCs, which was used as a negative control. The expressions of TGF-βRII and IL-10 receptors were notably decreased approximately 50% in the shTGF-βRII-SD-DCs and shIL-10RA-SD-DCs, respectively. In the combined shRNAs-SD-DCs condition, the expressions of TGF-βRII and IL-10 receptors were decreased approximately 90% when compared to control. The results indicated that shRNA specific to each receptor could generally reduce the expression of the target protein ()). Accordingly, these lentivirus constructs were used in the ensuing experiments.
Characteristics of SD-DCs
Cell culture supernatants from SD-DCs were harvested after 5 days of transduction in order to determine the GM-CSF and IL-4 levels by enzyme-linked immunosorbent assay (ELISA). The IL-4 in the cell culture supernatants of shCtrl-SD-DCs, shIL-10RA-SD-DCs, shTGF-βRII-SD-DCs, and combined shRNAs-SD-DCs were detected, with levels of IL-4 production of 0.67 ± 0.067, 0.80 ± 0.023, 0.57 ± 0.15, and 0.61 ± 0.18 ng/mL, respectively ()). Similarly, the GM-CSF levels of shCtrl-SD-DCs, shTGF-βRII-SD-DCs, shIL-10RA-SD-DCs, and combined shRNAs-SD-DCs were 0.59 ± 0.07, 0.67 ± 0.06, 0.83 ± 0.06, and 0.51 ± 0.25 ng/mL, respectively ()).
Figure 2. Monocyte-derived self-differentiated DCs (SD-DCs). (a) SD-DCs were transduced with LV-shRNA. After transduction for 5 days, the culture supernatants from the indicated conditions of these SD-DCs were harvested, and the cytokine production of both IL-4 and GM-CSF was determined by ELISA. (b) After transduction for 7 days, the SD-DC morphologies were observed under microscope and compared with DCs generated with conventional method. (c) The percentages of monocytes (CD14+ cells), SD-DCs (CD11c+ cells) with positively expressed HLA-DR, CD40, CD83, and CD86 were examined by flow cytometry. Results represent the mean ± SEM (bars) of three independent experiments
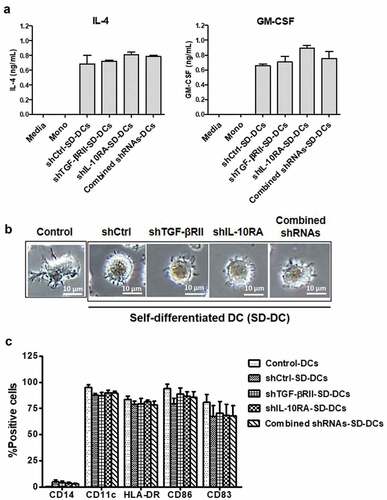
The production of these cytokines did not differ significantly among the generated SD-DCs, which indicated that the transduction efficiencies of lentiviruses carrying cytokine genes were comparable. In contrast, untransduced monocytes could not detect IL-4 and GM-CSF in culture medium. Taken together, these data showed that SD-DCs were successfully generated, and that shRNA sequences did not affect cytokine production.
In cultures extended to 7 days, the morphologies of SD-DCs were observed under microscope and compared with control DC generated by the conventional method using recombinant IL-4 and GM-CSF supplementation. The results showed that all SD-DCs had morphology characterized by roundness and being surrounded by dendrites, while the DCs generated by the addition of recombinant cytokines showed a morphology characterized by roughness and cytoplasmic projections ()). The DCs were phenotypically determined by staining with specific fluorescence-conjugated antibodies and subsequent analysis by flow cytometry. As shown in ), the percentages CD14+ cells, which is monocyte population, were considerably decreased in all SD-DC conditions (2–5%) and in control DC generation. Similarly, the percentages of DC population (i.e., CD11c+CD14− cells) in three donors were significantly increased to approximately 85–90% in all conditions of DC generation ()). These results suggest that monocytes were activated by GM-CSF/IL-4 lentivirus transduction, and that they were then differentiated into SD-DCs.
The percentages of CD11c+HLA–DR+ cells indicated that the maturation of DC was not different among the investigated groups (approximately 80% from all groups) and the control group (83% of total cells). There were some variations in the percentages of CD11c+CD40+ cells, CD11c+CD83+ cells, and CD11c+CD86+ cells among the three donors, as shown in ). Taken together, these data clearly demonstrated that it is possible to express DC cell-related surface markers in SD-DCs generated by lentiviral transduction, and to enable them to produce IL-4 and GM-CSF, both of which are important for monocyte-derived DC generation. In addition, transduction with LV carrying IL-4 and GM-CSF genes could efficiently induce monocytes to differentiate into dendritic cells in vitro. To sum up, DCs could be successfully generated without supplementation of the recombinant cytokines.
Activation of effector T-cells by SD-DCs suppression of TGF-β and IL-10 receptors
We next tested the activation of autologous effector T-cells by SD-DCs with the suppression of TGF-β and IL-10 receptors, which were pulsed with protein lysate from KKU-213 cell line. The percentages of effector CD4 + T-cells in three donors after co-culturing with pulsed-shCtrl-SD-DCs, pulsed-shTGF-βRII-SD-DCs, pulsed-shIL-10RA-SD-DCs, and pulsed-combined shRNAs-SD-DCs were not changed (approximately 50% of CD3+ cells in all conditions) compared to that of naïve or non-activated CD4+ T-cells (49.4 ± 1.99%) ()). In contrast, the effector CD8+ T-cells activated with pulsed-shCtrl-SD-DCs, pulsed-shTGF-βRII-SD-DCs, pulsed-shIL-10RA-SD-DCs, and pulsed-combined-shRNAs-SD-DCs increased (approximately 30–35% of CD3+ cells in all SD-DC conditions) to higher levels than those observed in non-activated CD8+ T-cells (21.10 ± 0.77%). After co-culturing with SD-DCs, the number of natural killer (NK) cells was decreased compared to the respective non-activated control.
Figure 3. Activation of effector cells by pulsed SD-DCs transduced with shRNAs. Autologous effector cells were co-cultured with pulsed shCtrl-SD-DCs, shTGF-βRII-SD-DCs, shIL-10RA-SD-DCs, and combined shRNAs-SD-DCs for 5 days. (a) Activated effector cells, including CD4 + T-cells, CD8 + T-cells, and NK cells, were stained for cell surface markers and analyzed by flow cytometry. (b) After activation for 7 days, the percentages of IFN-γ-producing T-cells (CD3+ IFN-γ+ cells) were determined by intracellular cytokine staining and then analyzed by flow cytometry. Results represent the mean ± SEM (bars) of three independent experiments. (*p < .05, **p < .01, as analyzed by Student’s t-test)
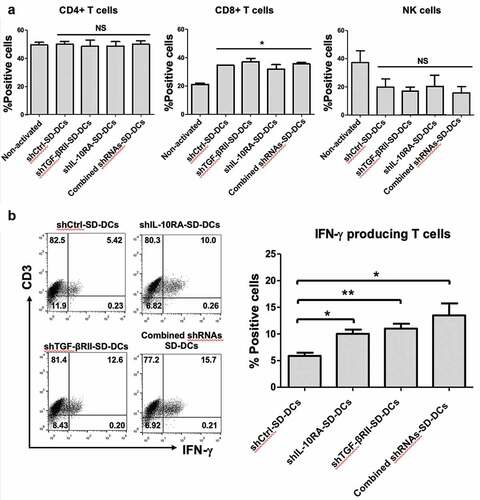
We next evaluated IFN- γ production in SD-DC-activated T cells ()). Effector T-cells activated with pulsed-shTGF-βRII-SD-DCs, pulsed-shIL-10RA-SD-DCs, and pulsed-combined-shRNAs-SD-DCs produced more IFN-γ (10.03 ± 0.80%, 13.49 ± 2.2%, and 11.02 ± 0.9%, respectively) than the effector T-cells activated with pulsed-shCtrl SD-DCs (5.89 ± 0.59%). These results demonstrated that pulsed SD-DCs potently activated the proliferation of CD8+ T-cells. In addition, suppression of TGF-β and IL-10 receptors in pulsed SD-DCs improved T-cell function to produce IFN-γ.
Enhancement of anti-tumor activity by SD-DC suppression of TGF-β and IL-10 receptors
Lastly, we evaluated the ability of protein lysate (from KKU-213 cells) pulsed SD-DCs to activate T-cells. The cytolytic activities of activated T-cells against three cell lines, including KKU-213 (specific CCA target-cells), immortalized cholangiocytes (MMNK-1), and KKU-100 (CCA-related cell line), were assayed by AnnexinV-PI staining.
As shown in ), the anti-tumor effects of activated effector T-cells against KKU-213 were significantly enhanced in the conditions where effector T-cells were activated by pulsed-shTGF-βRII-SD-DCs (p = .0019), pulsed-shIL-10RA-SD-DCs (p = .0325), and pulsed-combined-shRNAs-SD-DCs (p = .0044). The anti-tumor activity of effector T-cells activated by pulsed-shTGF-βRII-SD-DCs (47.44 ± 1.79%), the anti-tumor activity of T-cells activated by pulsed-shIL-10RA-SD-DCs (42.44 ± 4.43%), and pulsed-combined-shRNAs-SD-DCs (49.46 ± 3.25%) were significantly higher than the activities of pulsed-shCtrl-SD-DCs (26.47 ± 2.27%) (effector cell to target cell [E:T] ratios – both 10:1). In addition, the cytolytic activity of T-cells activated by pulsed-combined-shRNAs-SD-DCs (43.24 ± 1.253%) was significantly higher than that of T-cells activated by pulsed-shIL-10RA-SD-DCs (31.21 ± 3.945%) at the 5:1 ratio. The cytolytic activity between T cells activated by pulsed-shTGF-βRII-SD-DCs and T cells activated by pulsed-combined-shRNAs-SD-DCs did not have significant difference in all ratios.
Figure 4. Cytolytic activities of activated effector cells after co-culturing with pulsed SD-DCs transduced with shRNAs to suppress TGF-β and IL-10 receptors against CCA cell lines. (a) Cytotoxicity activities of activated effector T-cells co-cultured with various conditions of pulsed SD-DCs on KKU-213 CCA cell line (at effector to target (E:T) ratios of 2.5:1, 5:1, and 10:1) examined by flow cytometry using AnnexinV-PI staining. (b) Cytolytic activities of activated effector T-cells co-cultured with pulsed SD-DCs against KKU-100 cells (E:T ratio 10:1). (c) Cytolytic activities of activated effector T-cells co-cultured with pulsed SD-DCs against MMNK-1 cells (E:T ratio 10:1). Results represent the mean ± SEM (bars) of three independent experiments. (*p < .05, **p < .01, as analyzed by Student’s t-test)
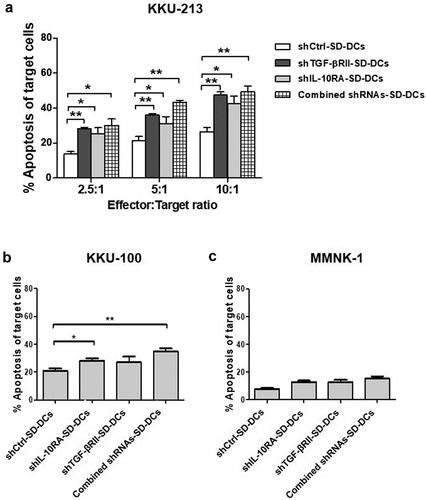
We then tested these cytolytic activities in a different CCA cell line (KKU-100). At an E:T ratio of 10:1, our results revealed the percentages of KKU-100 apoptosis to be significantly greater in the conditions where effector T-cells were activated by pulsed-shTGF-βRII-SD-DCs (28.27 ± 1.78%) and pulsed-combined-shRNAs-SD-DCs (34.85 ± 2.20%) than in the condition where effector T-cells were activated by pulsed-shCtrl-SD-DCs (20.96 ± 1.65%) ()). These results suggest that DC-activated effector T-cells selectively kill CCA cells. Cytolytic activity assay of these activated effector T-cells against cholangiocytes (MMNK-1) showed lower percentages of apoptotic cells (approximately <15% at an E:T ratio of 10:1 ()).
Discussion
A Self-differentiated Myeloid-derived Antigen-presenting-cells Reactive against Tumors DC (‘SmartDC’) was recently developed for the large-scale DC production.Citation12 SmartDCs can be initiated by transduction with lentivirus construct carrying cDNAs expressing IL-4, GM-CSF, and tumor-associated antigen (TAA). Our group recently developed self-differentiated DCs encoding IL-4, GM-CSF, and TAA – cAMP-dependent protein kinase type I-alpha regulatory subunit (PRKAR1A) or SD-DC-PR for anti-tumor activity testing against CCA. Employing SD-DC, autologous effector T-cells (CD3+CD8+) displayed greater anti-tumor activity against CCA cells than those activated by the conventional method.Citation13 In the present study, we have broadened these findings by successfully producing SD-DCs that counteract the immunosuppressive effects of TGF-β and IL-10. These findings have great potential importance relative to the development of therapies to treat several different types of cancer.
Importantly, the biological properties and morphologies of these SD-DCs were only marginally different from the DCs generated by the conventional method. These SD-DCs retained their immunophenotype, including expression of CD83 (maturation markers), HLA-DR (MHC class II), CD40 and CD86 (co-stimulatory molecules) ()), which are all required for the activation of T-cells.Citation14 In addition to the DC morphology and phenotype, the inhibition of TGF-βRII and IL-10RA using specific shRNA did not affect cytokine production. Although transduction efficiencies vary from person to person, we propose that these data constitute proof-of-concept that transduction of lentiviral construct encoding for IL-4 and GM-CSF into human monocytes can drive DC differentiation, and that in vitro SD-DCs could be successfully generated without supplementation of the recombinant cytokines.
The results showing that SD-DCs could promote the proliferation of effector CD8+ T-cells broaden data from a phase I/II trial in which vaccination of autologous tumor lysate-pulsed dendritic cells in recurrent glioma patients showed no changes in the percentages of CD4+ cells between before and after therapy; however, four of five patients showed slight increase in the percentages of CD8+ cells.Citation15 In the co-culture condition, SD-DCs constitutively secreted IL-4 and, GM-CSF and one, the other, or both may play an important role in activating T-cells. Our data also extend the data from rodent studies related to the role of IL-4 and GM-CSF in the activation and proliferation of T-cells, where the endogenous expression of IL-4 had direct effect on the stimulation of antigen-specific CD8+ T-cell proliferation in a MHC class-I restricted antigen through the IL-4 signaling pathway.Citation16,Citation17 In a study using GM-CSF−/- mice,Citation18 cytotoxic CD8+ T-cells did not respond to specific antigen, and CD4 + T-cells impaired cytokine production and its proliferation via delayed immunoglobulin production. Taken together, these results suggest IL-4 and GM-CSF as having roles in adaptive immune responses against cancer cells.
The data also show that blocking of the two receptors, TGF-βRII and IL-10RA, on DCs was accomplished by siRNAsCitation10 and neutralizing antibodies,Citation11 both of which increased the number of IFN-γ-producing CD3 + T-cells. In this study, the suppression of TGF-βRII and IL-10RA by shRNA in SD-DCs individually or in combination significantly increased the percentages of IFN-γ-producing CD3 + T-cells compared to the experiment was conducted by shCtrl-SD-DCs ()). The fact that these activated effector T-cells were more cytotoxic to KKU-213 cells than to KKU-100 cells (,)) might be explained by the fact that the source of the tumor antigen used for pulsing SD-DCs was KKU-213 cells. It was previously reported that the activation of effector T-cells by KKU-213 cell lysate pulsed DCs had more killing ability against KKU-213 cells than against KKU-100 cells.Citation19 The explanation was postulated to be that only some tumor antigens were shared between these two CCA cell lines.Citation19 It was then to be expected that activated effector T-cells displayed less cytolytic effect on cholangiocytes (MMNK-1 cell line) at the highest ratio ()). These results suggest the specificity and safety of adoptive T-cells that can selectively kill only tumor cancer cells. These results also suggest a more effective strategy for preparation and modification of DCs without recombinant cytokine supplementation for in vitro activation of effector T-cells in CCA and other cancers. To apply this procedure into clinical trials or clinical setting, SD-DCs with the suppression of IL-10RA or TGF-βRII by shRNAs can be pulsed with the tumor cell lysate extracted from cancer cell line or the patient’s tumor tissue. Autologous effecter T cells are activated by these SD-DCs. The activated T cells against cancer cells are then transfused into the patient for adoptive T-cell therapy. In addition, allogeneic SD-DCs prepared from healthy donors pulsed with tumor cell lysate could also activate cancer-specific T-cells for adoptive T-cell therapy. The cytolytic activity of T cells activated by allogeneic SD-DCs with IL-10RA or TGF-βRII suppression by shRNAs should be assessed to compare with that activated by autologous SD-DCs. For future clinical treatment, the autologous effector T cells activated by monocyte-derived DCs or SD-DCs can be cryopreserved and later thawed and expanded in the culture medium with appropriate cytokines for adoptive T-cell therapy. The cytotoxic activity of effector T cells activated by pulsed-combined-shRNAs-SD-DCs was dependent on the efficiency of lentivirus transduction. The cytolytic activity between T cells activated by pulsed-shTGF-βRII-SD-DCs and T cells activated by pulsed-combined-shRNAs-SD-DCs did not have significant difference in all ratios. This may be explained by the variation in transduction efficiency using combined-shRNAs resulting in lower anti-tumor activity of T-cells activated by pulsed-combined-shRNAs-SD-DCs than somewhat expected.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Professor Peter Hokland, Department of Clinical Medicine, Arhus University Hospital, Denmark, and Mr. Kevin Jones for critical reading and editing of this manuscript.
Additional information
Funding
References
- Mathema VB, Na-Bangchang K. Current insights on cholangiocarcinoma research: a brief review. Asian Pac J Cancer Prev. 2015;16(4):1307–13. doi:10.7314/apjcp.2015.16.4.1307. PMID: 25743790.
- Zabron A, Edwards RJ, Khan SA. The challenge of cholangiocarcinoma: dissecting the molecular mechanisms of an insidious cancer. Dis Model Mech. 2013;6(2):281–92. doi:10.1242/dmm.010561. PMID: 23520144.
- Gatto M, Alvaro D. Cholangiocarcinoma: risk factors and clinical presentation. Eur Rev Med Pharmacol Sci. 2010;14(4):363–67. PMID: 20496549.
- Yu Y, Cui J. Present and future of cancer immunotherapy: A tumor microenvironmental perspective. Oncol Lett. 2018;16(4):4105–13. doi:10.3892/ol.2018.9219. PMID: 30214551.
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi:10.1146/annurev-immunol-031210-101324. PMID: 21219185.
- Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, Grosse-Wilde H, Broelsch CE, Gerken G, Cicinnati VR. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10(21):7260–69. doi:10.1158/1078-0432.CCR-04-0872. PMID: 15534100.
- Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-β1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134(2):270–78. doi:10.1046/j.1365-2249.2003.02295.x. PMID: 14616787.
- Lim DS, Kim JH, Lee DS, Yoon CH, Bae YS. DC immunotherapy is highly effective for the inhibition of tumor metastasis or recurrence, although it is not efficient for the eradication of established solid tumors. Cancer Immunol Immunother. 2007;56(11):1817–29. doi:10.1007/s00262-007-0325-0. PMID: 17443323.
- Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, Baba H, Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101(8):1913–19. doi:10.1111/j.1349-7006.2010.01614.x. PMID: 20545696.
- Ahn YH, Hong SO, Kim JH, Noh KH, Song KH, Lee YH, Jeon JH, Kim DW, Seo JH, Kim TW. The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-beta receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity. Clin Exp Immunol. 2015;181(1):164–78. doi: 10.1111/cei.12620. PMID: 25753156.
- Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-beta receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14(6):1423–31. doi:10.1080/21645515.2018.1431598. PMID: 29420117.
- Sundarasetty BS, Chan L, Darling D, Giunti G, Farzaneh F, Schenck F, Naundorf S, Kuehlcke K, Ruggiero E, Schmidt M, et al. Lentivirus-induced ‘Smart’ dendritic cells: pharmacodynamics and GMP-compliant production for immunotherapy against TRP2-positive melanoma. Gene Ther. 2015;22(9):707–20. doi:10.1038/gt.2015.43. PMID: 25965393.
- Panya A, Thepmalee C, Sawasdee N, Sujjitjoon J, Phanthaphol N, Junking M, Wongkham S, Yenchitsomanus PT. Cytotoxic activity of effector T cells against cholangiocarcinoma is enhanced by self-differentiated monocyte-derived dendritic cells. Cancer Immunol Immunother. 2018. doi:10.1007/s00262-018-2212-2. PMID: 30056600.
- Ni K, O’Neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75(3):223–30. doi:10.1038/icb.1997.35. PMID: 9243286.
- Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89(7):1172–79. doi:10.1038/sj.bjc.6601268. PMID: 14520441.
- Morris SC, Heidorn SM, Herbert DBR, Perkins C, Hildeman DA, Khodoun MV, Finkelman FD. Endogenously produced IL-4 nonredundantly stimulates CD8(+) T cell proliferation. J Immunol. 2009;182(3):1429–38. doi:10.4049/jimmunol.182.3.1429. PMID: 19155490.
- Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. IL-4: an important cytokine in determining the fate of T cells. Biophys Rev. 2014;6(1):111–18. doi:10.1007/s12551-013-0133-z. PMID: 28509961.
- Wada H, Noguchi Y, Marino MW, Dunn AR, Old LJ. T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice. Proc Natl Acad Sci U S A. 1997;94(23):12557–61. doi:10.1073/pnas.94.23.12557. PMID: 9356488.
- Junking M, Grainok J, Thepmalee C, Wongkham S, Yenchitsomanus PT. Enhanced cytotoxic activity of effector T-cells against cholangiocarcinoma by dendritic cells pulsed with pooled mRNA. Tumour Biol. 2017;39(10):1010428317733367. doi:10.1177/1010428317733367. PMID: 29034817.
