ABSTRACT
The link between stress, other psychological factors and response to cancer, or even the cancer incidence and metastasis, is well established. The inhibition of β-Adrenergic receptors (β-AR) using β-blockers was demonstrated to have an inhibitory effect on cancer recurrence. Direct effects on the stress-induced suppression of anti-tumor immune responses were also shown. In a recent issue of Cancer Immunology Research, Daher and colleagues studied the molecular mechanism behind this protective effect in the context of cancer vaccination. They provided evidence that the β-AR signaling affected the priming of naïve CD8 + T cells in their myeloma model, rather than effector CD8 + T cells which downregulated the expression of β-AR after activation and became insensitive to such signaling. Blocking the β-adrenergic signaling during vaccination led to increased expansion and effector functions of antigen-specific CD8 + T cells and reduced tumor growth. This has implications for the clinical use of β-blockers as adjuvants to enhance cancer vaccination and other types of immunotherapy.
There is still an important debate around the clinical efficacy of therapeutic cancer vaccines. A main issue resides in the various influence of the tumor microenvironment (TME) that will determine the therapeutic outcome. The type of cancer, the immune infiltration or “temperature” of the tumor (hot versus cold), the immune status of the patient and their HLA genotype can all actively influence the clinical response to vaccination. However, an aspect that is rarely mentioned is the psychological status of the patient; more precisely their stress level. Indeed, our sympathetic autonomous nervous system controls our response to external aggression by releasing catecholamine molecules, primarily leading to a “fight-or-flight” response. These molecules bind to β-AR and regulate quick response mechanisms. Thus, it is accepted that this stimulation should be sporadic rather than continuous. Therefore, in case of prolonged or chronic stimulation, deleterious effects have been reported.Citation1,Citation2 This is especially dramatic in the case of tumor growth where activation of β-AR has been shown to affect gene expression inducing tissue invasion and metastasis.Citation3
Immune cells such as lymphocytes also express β-AR, and have been reported to react to sympathetic nervous system stimulation, but only little was known on the implication, in particular during cancer treatment. β-blockers, or β-adrenergic blocking agents, work by blocking the binding of stress hormones (epinephrine and norepinephrine) to the β-AR. Different generation β- blockers interact with various β-ARs; β-1 adrenergic receptor (ADRB1), β-2 adrenergic receptor (ADRB2) and third generation β-blockers also have vasodilative effects.Citation4 ADRB1 is predominantly expressed in cardiac tissue whereas ADRB2 is more widely expressed in other tissues, predominantly in lymphocytes in blood and frequently upregulated in cancer cells (source: Human Protein Atlas). The most widely tested β-blocker, the first generation propranolol, is nonselective and inhibits both ADRB1 and ADRB2. This was the first blocker to show clinical effect in cancer treatment as a single agent, both in retrospective and prospective studies.Citation5,Citation6 The effects of β-blockers can be measured by normalizing NK cell distribution and cytotoxicity,Citation7 endothelial nitric oxide (NO) production and the effect on CD8 + T cells.Citation4 β-blockers are mainly used to treat cardiac diseases such as ischemic heart disease, hypertension, arrhythmia and heart failure, but also have other clinical applications such as glaucoma, migraines and anxiety.Citation8,Citation9 The use of β-blockers can cause major side effects as β-ARs are expressed on many tissues and their blockade affects multiple metabolic and physiologic functions. They can prevent bronchodilation in asthmatic subjects,Citation10 exacerbate peripheral artery disease with cold extremities, absent pulses, and, in some cases, cyanosis and gangrene.Citation11 As β-ARs are also important in glucose metabolism, nonselective β-blockers can facilitate hypoglycemia which can be severe for diabetic patients.Citation12 Furthermore, catecholamines have important effects on potassium balance and blockade of β-ARs can therefore cause hyperkalemia.Citation13,Citation14 Finally, depression, fatigue, and sexual dysfunction are commonly reported side effects of β-blockers, but have been shown to be rare in randomized studies.Citation15 The use of β-blockers, like any drug, carries the risk of side effects, and any complicating factors or disease should be examined before use, especially because they also interact with several other drugs and are now likely to have wider therapeutic applications, not only in cancer.
Bucsek and colleagues described how decreasing housing temperature increased stress levels in preclinical mouse tumor models.Citation16 Using a first generation β-blocker and β-AR knockout mice, the authors demonstrated that the differences in anti-tumor response could be ascribed to β-AR signaling. Reducing the β-AR signaling increased intra-tumoral CD8 + T cells and enhanced the efficacy of checkpoint blockade in these mouse models.
The paper from Daher and colleagues elegantly presented the influence of β-AR signaling in the context of cancer vaccination using a murine HP-E6 and -E7-expressing myeloma model, TC1.Citation17 Mice were vaccinated with an HPV vaccine (STxBE7) where the combination with β-blockers was shown to dramatically reduce tumor growth compared to vaccination alone. The effect was abrogated in mice where CD8 + T cells were depleted. The authors investigated how β-AR signals affected the priming phase versus the effector phase of an HPV-specific CD8 + T cell anti-tumor response and observed increased numbers of CD8+ tumor infiltrating lymphocytes (TIL) after vaccination in the presence of the first generation β-blocker, propranolol.
Interestingly, Daher and colleagues actually found that CD8+ TILs were not sensitive to β-AR signaling as previous reports would indicate. Naïve CD8 + T cells, however, seemed to be highly sensitive to stress and β-AR signaling strongly inhibited their activation. The benefit of β-blocker use was therefore seen at the site of priming, namely the tumor-draining lymph node (). The β-blocker did not affect dendritic cell (DC) maturation or antigen presentation supporting its action through a direct effect on CD8 + T cells. It was previously proposed that β-AR signaling also affect monocytes and dendritic cells as an anti-inflammatory drug (reviewed inCitation18) which caused a shift from Th1 to Th2 differentiation of CD4 + T cells.Citation19 However, here the authors tested the effect of the β-blocker on DCs using a setup with transgenic ovalbumin (OVA)-specific OT-I cells which may be a system too robust to show subtle differences in priming.
Figure 1. Blocking stress hormone signaling through β-adrenergic receptors improves CD8 + T-cell priming in cancer vaccination.
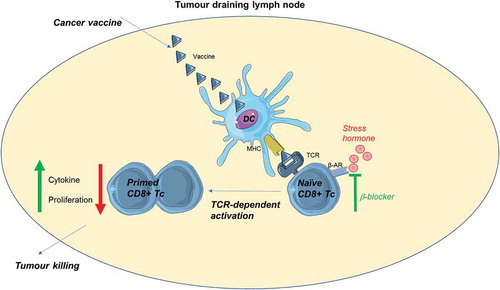
Daher and colleagues further presented data supporting that the difference in sensitivity to stress between naïve CD8 + T cells and TILs was due to downregulation of β-AR after activation. This could also be confirmed in vitro where naïve CD8 + T cells became insensitive to β-AR signaling upon activation, in the same manner as TILs.
The precise mechanisms of immune inhibition by β-AR signaling and its modulation by β-AR antagonists will need further testing as there are several important parameters to consider. First, the expression of β-AR differs between species and can be influenced by other physiological factors such as cytokine levels, and timing.Citation20–Citation23 Human memory CD8 + T cells express higher levels of β-AR than the naïve CD8 + T cells, in contrast to mice.Citation24 This could mean that the impact of inhibition of β-AR signaling in combination with checkpoint blockade could have a higher impact in a patient setting compared to mouse studies. Second, a recent report demonstrated that β-AR stimulation inhibited CD8 + T-cell activation by suppressing metabolic reprogramming during priming.Citation25 TCR-induced activation is accompanied by a switch to glycolysis and oxidative phosphorylation in order to provide sufficient energy to the cells acquiring effector functions. Failing to meet this energetic requirement would blunt the activation and expansion of antigen-specific CD8 + T cells. Daher and colleagues showed reduced calcium response, proliferation and cytokine production of naïve T cells after anti-CD3/CD28 activation when cells were treated with stress hormones. This inhibition was largely abrogated in CD8 + T cells from β-AR knock-out mice, as was the inhibition of glycolysis in such β-AR knockout cells after activation when exposed to β-AR signaling in the report from Qiao and colleagues.Citation25
The balance of CD4 + T helper subsets is also influenced by β-AR signaling.Citation26 Th2 cells downregulate the expression of β-AR, whereas a β-AR agonist, terbutaline, was previously shown to modulate the level of IL-17 and IFN-γ in T helper cells.Citation27 It would be interesting to investigate the effect of β-blockers on CD4 + T-cell priming in vaccination.
In conclusion, this paper paves the way to a better understanding of the influence of our psychological status on molecular mechanisms taking place during T-cell priming and cancer vaccination. An improved understanding of patient stress and how this may affect therapeutic response may support rational design of more efficient treatments. Combining cancer vaccination in patients with the administration of β-blockers as adjuvant could lead to a more powerful anti-tumor immune response.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–44. doi:10.1038/nm1447.
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5(8):466–75. doi:10.1038/ncponc1134.
- Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB, Bottsford-Miller J, et al. Stress effects on fosb- and interleukin-8 (il8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285(46):35462–70. doi:10.1074/jbc.M110.109579.
- Chung JF, Lee SJ, Sood AK. Immunological and pleiotropic effects of individual beta-blockers and their relevance in cancer therapies. Expert Opin Investig Drugs. 2016;25(5):501–05. doi:10.1517/13543784.2016.1164141.
- Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: A population- based study. J Clin Oncol. 2011;29(19):2635–44. doi:10.1200/JCO.2010.33.5422.
- Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628–38. doi:10.18632/oncotarget.v1i7.
- Kanemi O, Zhang X, Sakamoto Y, Ebina M, Nagatomi R. Acute stress reduces intraparenchymal lung natural killer cells via beta-adrenergic stimulation. Clin Exp Immunol. 2005;139(1):25–34. doi:10.1111/cei.2005.139.issue-1.
- Poirier L, Tobe SW. Contemporary use of beta-blockers: clinical relevance of subclassification. Can J Cardiol. 2014;30(5 Suppl):S9–S15. doi:10.1016/j.cjca.2013.12.001.
- Brooks AM, Gillies WE. Ocular beta-blockers in glaucoma management. Clinical pharmacological aspects. Drugs Aging. 1992;2(3):208–21. doi:10.2165/00002512-199202030-00005.
- Singh BN, Whitlock RM, Comber RH, Williams FH, Harris EA. Effects of cardioselective beta adrenoceptor blockade on specific airways resistance in normal subjects and in patients with bronchial asthma. Clin Pharmacol Ther. 1976;19:493–501.
- Frohlich ED, Tarazi RC, Dustan HP. Peripheral arterial insufficiency. A complication of beta-adrenergic blocking therapy. JAMA. 1969;208(13):2471–72. doi:10.1001/jama.1969.03160130055016.
- Antonis A, Clark ML, Hodge RL, Molony M, Pilkington TR. Receptor mechanisms in the hyperglycaemic response to adrenaline in man. Lancet. 1967;1(7500):1135–37. doi:10.1016/S0140-6736(67)91710-2.
- Lim M, Linton RA, Wolff CB, Band DM. Propranolol, exercise, and arterial plasma potassium. Lancet. 1981;2(8246):591. doi:10.1016/S0140-6736(81)90987-9.
- Nowicki M, Miszczak-Kuban J. Nonselective beta-adrenergic blockade augments fasting hyperkalemia in hemodialysis patients. Nephron. 2002;91(2):222–27. doi:10.1159/000058396.
- Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002;288(3):351–57. doi:10.1001/jama.288.3.351.
- Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JW, Messmer MN, et al. Beta-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in cd8(+) t cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77(20):5639–51. doi:10.1158/0008-5472.CAN-17-0546.
- Daher C, Vimeux L, Stoeva R, Peranzoni E, Bismuth G, Wieduwild E, Lucas B, Donnadieu E, Bercovici N, Trautmann A, et al. Blockade of β-Adrenergic receptors improves CD8+T-cell priming and cancer vaccine efficacy. Cancer Immunol Res. 2019;7(11):1849–63. doi:10.1158/2326-6066.CIR-18-0833.
- Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: A review. Front Pharmacol. 2015;6:171.
- Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100(6):1513–19. doi:10.1172/JCI119674.
- Sloan EK, Capitanio JP, Cole SW. Stress-induced remodeling of lymphoid innervation. Brain Behav Immun. 2008;22(1):15–21. doi:10.1016/j.bbi.2007.06.011.
- Treinys R, Bogdelis A, Rimkute L, Jurevicius J, Skeberdis VA. Differences in the control of basal l-type ca(2+) current by the cyclic amp signaling cascade in frog, rat, and human cardiac myocytes. J Physiol Sci. 2016;66(4):327–36. doi:10.1007/s12576-015-0430-3.
- Zhao Y, Li X, Yang L, Eckel-Mahan K, Tong Q, Gu X, Kolonin MG, Sun K. Transient overexpression of vascular endothelial growth factor a in adipose tissue promotes energy expenditure via activation of the sympathetic nervous system. Mol Cell Biol. 2018;38(22). doi:10.1128/MCB.00242-18.
- Shusterman V, Usiene I, Harrigal C, Lee JS, Kubota T, Feldman AM, London B. Strain-specific patterns of autonomic nervous system activity and heart failure susceptibility in mice. Am J Physiol Heart Circ Physiol. 2002;282(6):H2076–2083. doi:10.1152/ajpheart.00917.2001.
- Estrada LD, Agac D, Farrar JD. Sympathetic neural signaling via the beta2-adrenergic receptor suppresses t-cell receptor-mediated human and mouse cd8(+) t-cell effector function. Eur J Immunol. 2016;46(8):1948–58. doi:10.1002/eji.201646395.
- Qiao G, Bucsek MJ, Winder NM, Chen M, Giridharan T, Olejniczak SH, Hylander BL, Repasky EA. Beta-adrenergic signaling blocks murine cd8(+) t-cell metabolic reprogramming during activation: A mechanism for immunosuppression by adrenergic stress. Cancer Immunol Immunother. 2019;68(1):11–22. doi:10.1007/s00262-018-2243-8.
- Ramer-Quinn DS, Baker RA, Sanders VM. Activated t helper 1 and t helper 2 cells differentially express the beta-2-adrenergic receptor: A mechanism for selective modulation of t helper 1 cell cytokine production. J Immunol. 1997;159:4857–67.
- Carvajal Gonczi CM, Tabatabaei Shafiei M, East A, Martire E, Maurice-Ventouris MHI, Darlington PJ. Reciprocal modulation of helper th1 and th17 cells by the beta2-adrenergic receptor agonist drug terbutaline. Febs J. 2017;284(18):3018–28. doi:10.1111/febs.14166.
