ABSTRACT
Since 2006, some Italian Regions introduced the active offer of measles, mumps, rubella, and varicella (MMRV) vaccine for all newborns during the second years of life. In 2011, Italian Drug Authority (AIFA) recommended the discontinuation of the MMRV use for an increased risk of febrile seizures following vaccination; furthermore, some Regions (such as Apulia, that introduced MMRV offer in 2009) chose to continue the use of MMRV and Ministry of Health recommended to guarantee supplemental monitoring of safety of the vaccine. In Italy, the surveillance of Adverse Events following immunization (AEFIs) is currently carried out by AIFA and Regional Health Authorities; this paper aims to summarize the results of MMRV-vaccine surveillance of AEFIs program carried out in Apulia. From the AIFA database, we selected MMRV AEFIs that occurred in Apulia (about 4,000,000 inhabitants) from 2009 to 2017. For serious AEFIs, we applied the WHO causality assessment algorithm, using for cases hospitalized information from individual medical records. In the 8 years of observation, 155 MMRV-AEFIs (reporting rate: 37.9×100,000 doses) occurred of which 26 were classified as serious (6.3×100,000 doses) and 22 led to hospitalization. Performing causality assessment, for 10 the classification was “consistent causal association to immunization” (reporting rate: 2.4×100000 doses), for 2 indeterminate, for 13 “inconsistent causal association to immunization” and for 1 not-classifiable. No case of febrile seizure resulted consistent to vaccination. All consistent serious AEFIs were completely resolved at subsequent follow-up.
Introduction
Immunization is probably the most successful and cost-effective public health intervention; however, due to the advanced control of several vaccine preventable diseases, the topic of vaccine safety has become as important as the efficacy.1
Adverse events following immunization (AEFIs) are medical occurrences following immunization, and they do not necessarily have a causal relationship with the vaccine. Adverse events may be unfavorable or unintended markings, an abnormal laboratory finding, a symptom or a disease: benefits of vaccines are significantly higher than the risks because AEFIs are absolutely rare.Citation1,Citation2
Indeed, vaccines are held to a higher standard of control than other medical products, both through pre-licensure safety evaluations and post-marketing surveillance activities.Citation3 In the post-marketing life of the vaccines, National and International Drug Authorities (such as FDA or for Italy AIFA) monitor the safety by the collection and analysis of spontaneous reports of adverse events carried out by health-care workers and patients or by ad hoc active surveillance studies.Citation4
AEFIs surveillance, reporting, and communication are essential parts of the pharmacovigilance process which is defined as a public health activity aimed at the identification, quantification, evaluation, and prevention of risks associated with the use of marketed drugs and vaccines.Citation5 This activity is crucial in the actual scenario, in which a resurgence of anti-vaccination movements and a diffuse “vaccine hesitancy” has been noted. For “anti-vax” and “vaccine sceptic” the vaccine safety is the topic most discussed when doubts about vaccination strategies are proposed.Citation6
Although international guidelines recommended that all adverse events must be detected and reported to improve product safety and management, the passive surveillance system adopted by national authorities is affected by an high risk of AEFIs underreporting, especially for not serious events.Citation7 For this reason, recently active surveillance and supplemental strategies are being incorporated into vaccine safety programs: these include active screening for targeted conditions of interest (e.g., hospitalization), monitoring of new data sources and real-time methodologies to detect changes in vaccine safety data in these sources.Citation8–Citation10
Several criteria are relevant to define the association between a vaccine and a single adverse event: temporal relationship (vaccination must precede adverse event), alternative etiological explanations, proof association, biological plausibility (the association should be compatible with existing theory and knowledge related to how the vaccine works), prior evidence or population-based evidence, and de-challenge or re-challenge.Citation8,Citation11,Citation12
Since 2005, WHO recommends the systematic evaluation of causal link between vaccinations and serious AEFI; according to the European law in pharmacovigilance, causality assessment must be generally part of the routine work in the pharmacovigilance centers, both in individual cases and in aggregate cases (signal detection). Some studies about the use of causality assessment in the evaluation of safety profile of vaccines have been carried out in Italy but the systematic use of this evaluation is mandatory only since 2017.Citation13,Citation14
In 2018, WHO published the last update of systematic and standardized causality assessment algorithm for serious AEFI to be used by the staff of national immunization programs, regulatory authorities, and pharmacovigilance or surveillance departments.Citation8 According to the WHO algorithm, the association between vaccine and AEFI can be considered not-classifiable when the forms lack of essential data (e.g., the name of the vaccine administered); consistent casual association or inconsistent causal association in presence or absence of a defined causal relationship between adverse event and immunization; indeterminate if there isn’t clear evidence for a causal link, or conflicting trends, or inconsistency with causal association to immunization (this is a potential signal and needs to be considered for further investigation; reviewing factors result in conflicting trends of consistency and inconsistency with causal association to immunization, i.e., it may be vaccine-associated as well as coincidental and it is not possible clearly to favor one or the other).Citation8,Citation15,Citation16
Post-marketing surveillance is crucial to study the safety of vaccines recently marketed, for which the safety pattern could be not clear defined in the registration studies.
In 2005, Food and Drug Administration and European licensed the quadrivalent measles, mumps, rubella, and varicella (MMRV) vaccine for use among children aged 12 months–12 years and Advisory Committee on Immunization Practices (ACIP) recommends a 2-dose vaccine schedule in childhood, with the first dose administered at age 12–15 months and the second dose at 4–6 years.Citation17,Citation18 MMRV vaccine was authorized in Europe for the first time in 2006.Citation19
Some post-licensure studies on MMRV reported an increased risk for febrile seizures 5–12 days after vaccination among children aged 12–23 months who had received the first dose of the vaccine compared to children of the same age who had received the first dose of MMR vaccine and varicella vaccine administered as separate injections at the same visit.Citation20,Citation21
This topic has been debated in the literature; other studies suggested that the occurrence of febrile seizures did not increase the risk of death, brain damage, or leaning disorders. However, parents generally consider febrile seizure a severe adverse event very important and this event is related to a negative impact on vaccination coverage.Citation22
In 2009, ACIP adopted new recommendations regarding the use of MMRV vaccine and identifying a personal or family (i.e., sibling or parent) history of seizure as a precaution for use of MMRV vaccine. ACIP considered that post-marketing studies are still needed in order to evaluate the real risk and prognosis of serious AEFIs (above all febrile seizures) after the MMRV vaccine.Citation23–Citation25
In last update fact sheet, the WHO reported that in 2018 MMRV vaccine was adopted by five countries in Europe and two countries in America.Citation26
Since 2006, MMRV vaccine is marketed in Italy and some Regions (such as Apulia, in 2009) recommended its use into Universal Vaccination Mass strategies for the elimination of measles and rubella and for the control of varicella.Citation27
In 2011, Italian Drug Authority (AIFA) and Ministry of Health discouraged the continuation of the use of MMRV vaccine, in particular, for the first dose administered to children aged 12–23 months, for the risk of febrile seizure; Regions that choose to continue the use of MMRV have to guarantee supplemental surveillance of the safety of the vaccine.Citation28,Citation29
Puglia is a Region in the south of Italy (4,000,000 of inhabitants) in which the Universal Mass Vaccination using the MMRV vaccine started in 2009. The vaccine is offered actively and free of charge for children of the second years of life (first dose) and the second dose is administered at 5–6 years.Citation27 The vaccine is also used for catch up strategies. Since 2017, vaccination against measles, mumps, rubella, and varicella is mandatory in Italy.Citation30 The coverage achieved in the 2009–2015 target cohorts ranged from 81.7% (2012 cohort) and 91.1% (2010 cohort) for the first dose while for the second dose, the maximum coverage was achieved in 2010 cohort (85.5%) and the minimum in 2004 cohort (45.6%)Citation31,Citation32
Since 2013, in Puglia supplemental activities to improve the sensitivity of the surveillance of AEFIs have been adopted and systematic causality assessment of AEFIs has been carried out.
This paper summarizes the MMRV AEFIS scenario in Puglia in 2009/2017.
Methods
In Apulia, vaccination strategies for children and adolescents are cared by Vaccination Services, that are established in each town. The surveillance of AEFIs is carried out both by health-care workers of Vaccination Services both by Family Pediatricians and Hospital physicians who have to notify every cases of AEFIs noted in their patients. The notification could also be carried out directly by the parents of children.
From National Pharmacovigilance Network cared by AIFA, we selected MMRV AEFIs reported in Apulia from 01 June 2009 to 31 May 2017 while from the regional immunization database (GIAVA) the number of MMRV vaccines administered per year was obtained. To compare the safety profile of MMRV vs. MMR, we also selected from the same sources of information MMR AEFIs and number of doses of MMR administered, for the same period; in fact, also after the MMRV introduction in the Regional Immunization Schedule, MMR was available in the Vaccination Services and used for subjects immune for varicella for natural infection or according to official recommendations (e.g., subjects with a personal or family history of seizures).
For every subject who have reported an adverse event following MMRV vaccine, a specific form was built, including information on date of birth, gender, date of the vaccine administration, other vaccines administered in the same visit (in Apulian region the first dose of MMRV-immunization is scheduled in the same visit of first anti-hepatitis A vaccination) and information about the AEFI (date of onset and date of computing in National Pharmacovigilance Network, characteristics of the adverse events, case description, duration and treatment, hospitalization or emergency room access, final outcome). For serious AEFIs, the causality assessment was carried out according to WHO guidelines by two independent physicians, an expert in vaccinology; in case of disagreement, a third physician reviewed the evaluation. Information on causality assessment evaluation was added in the form.
Compiled forms were putted in a database created by Excel spreadsheet.
The total reporting rate was calculated as the total number of AEFIs/number of MMRV doses administered while the annual reporting rate was calculated using in the numerator the number of AEFIs occurred in the year and in the denominator the number MMRV doses administered in the same year.
WHO guidelines have been used to classify AEFIs as “serious” or “not serious”.Citation8 An AEFI is considered serious, if: it results in death; it is life-threatening; it requires in-patient hospitalization or prolongation of existing hospitalization; it results in persistent or significant disability/incapacity; it is a congenital anomaly/birth defect, or requires intervention to prevent permanent impairment or damage. Additionally, in 2016, AIFA published a list of particular health conditions that must be considered as serious AEFIs, if happened after vaccination. This list is the Italian edition of EMA IME list.Citation33,Citation34
For serious AEFIs, we retrospectively applied the WHO causality assessment algorithm to classify AEFI as ‘consistent causal association’, ‘inconsistent causal association’, indeterminate’ or ‘not-classifiable’.Citation8,Citation16 For serious AEFIs, 1 month after notification, a follow up was been carried out in order to guarantee a supplemental surveillance of vaccine safety.
Only for AEFIs that required hospitalization, we requested and obtained medical records; then we repeated the causality assessment using additional data from the medical record.
A summary for each serious AEFIs and results of causality assessment is presented in the results section. AEFIs are described by date of onset.
Results
Since June 2009 to May 2017, 409929 doses of MMRV vaccine were administered in Apulia to people identified as a target of immunization strategies in Regional Immunization Schedules.
In this period, 155 AEFIs after MMRV-immunization (rate: 37.8 × 100000 doses) were notified of which 26/155 (16.7%) were classified as serious (reporting rate: 6.3 × 100000 doses); 22/26 serious AEFIs (84.6% of serious AEFIs) led to hospitalization.
The reporting rate of MMRV-adverse events resulted higher than Italian ratio as indicated in 2017 in AIFA report (11.1 × 100000 doses), while the proportion of serious AEFIs was similar (16.7% vs. 16.0% in AIFA report).Citation14
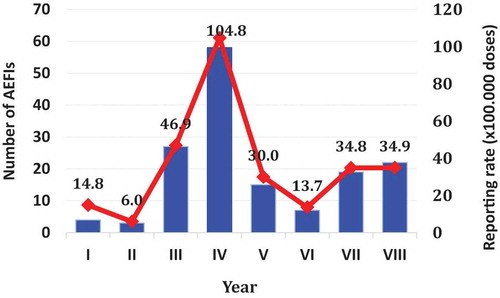
In , the number of AEFIs occurred and annual reporting rate was described: from June 2012 to May 2013 the highest number of adverse events post-MMRV-immunization (58/155) and reporting rate (108,4 × 100000 doses) were registered.
Graph 1. Distribution of MMRV AEFIs, per year of onset and annual reporting rate × 100.000 doses. Apulia Region (Italy), 1/6/2009-31/5/2017
Performing causality assessment based on AEFIs report, for 10 serious AEFIs the classification was “consistent causal association to immunization”, for 2 indeterminate, for 13 “inconsistent causal association to immunization” and for 1 not-classifiable []. Reporting rate of consistent AEFIs resulted in 2.4 × 100000 doses or 2.9 × 100000 doses if we consider both consistent both indeterminate AEFIs.
Table 1. Serious AEFIs after MMRV vaccine. Apulia Regio (Italy), 2009–2017.
24/26 serious AEFIs (92.3%) regarded the first MMRV dose, while 2/26 (7.7%) involves the second dose. 14/26 (58.3%) serious AEFIs occurred in male child, while 12/26 (41.7%) in female. The median age at the time of AEFI onset resulted in 22.0 ± 15.3 months.
In the same period, 674.474 doses of MMR were administered in Apulia and 31 AEFIs were notified (rate 4.6 × 100000 doses) of which 4 were serious (0.59×100000 doses). All serious MMR AEFIs regarded cases of seizure; for 1 a consistent causal association to immunization was established (rate 0.15 × 100000).
Case 1
The first case involved a 23-months male. Few minutes after the vaccination, the child presented a vagal reaction with dizziness and loss of consciousness for which he was admitted to the hospital. After 48 h, he was discharged for the complete remission of symptoms. Performing the Causality Assessment, the relation was “consistent causal association to immunization”.Citation35
Case 2
The second case regarded a 15-months-old female who simultaneously received MMRV and anti-HAV vaccine: 6 days after immunization, she presented fever and hyperpyrexia (39.5°C–40.0°C). For the persistence of symptoms, she was hospitalized: after medical examinations, Kawasaki Syndrome was diagnosed. Through the application of the WHO algorithm for AEFI causality assessment, the cause–effect relationship between vaccination and adverse events should be considered as indeterminate.Citation36
Case 3
The third case involved a 17-months-old male who received the MMRV and anti-HAV vaccines simultaneous administration. Right after vaccination, he presented an immediate allergic reaction with skin manifestations (urticaria, angioedema with face swelling, itching), respiratory manifestations (cough, difficulty breathing) and a reduction in blood pressure (weakness, transient loss of consciousness). He was immediately admitted to the emergency room where allergic reaction resolved completely a few hours later. Applying the algorithm for Causality Assessment, the cause–effect relationship is consistent.Citation37
Case 4
The fourth case regarded a female child aged 12 months who received MMRV and anti-HAV vaccine in the same visit. After a week, she presented fever (T = 38°C) and erythema on the face, trunk, and limbs. For these symptoms, she was admitted to the hospital with a resolution after 2 days. Applying the Causality Assessment algorithm, the cause/effect relationship is consistent.
Case 5
The fifth case involved a 28-months male who received MMRV and anti-HAV vaccines simultaneously administered. A few hours after vaccination, he presented fever and hyperpyrexia that needed hospitalization. Applying the AEFI Causality Assessment algorithm, the association with the MMRV-vaccine is inconsistent because the time from vaccination to adverse reaction onset is too brief for a live-attenuated vaccine.
Case 6
The sixth case involved a 14-months-old female vaccinated with MMRV and anti-HAV vaccine at the same time. Eighteen days after the vaccinations, she presented bruising and petechiae. She was hospitalized and, after medical examinations, diagnosis of immune thrombocytopenic purpura has been formulated (PLT = 19000/mmc). Applying the Causality Assessment algorithm, cause/effect relationship between vaccination and adverse events is consistent: there is a biological plausibility even if it is a very rare adverse event (2,6 case of insurgence of thrombocytopenia (ITP)/100000 MMR doses) and alternative causes are not found.Citation38
Case 7
The seventh case involved a 17-months-old male child. Five days after MMRV vaccination, he presented fever (T = 38°C), dizziness and erythema on the face, trunk, and limbs. For these symptoms, he was admitted to the hospital. The resolution of symptoms after a week was noted. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is consistent.
Case 8
The eighth case involved a 13-months-old male vaccinated with MMRV and anti-HAV vaccines at the same time. After a week, he presented fever and hyperpyrexia (T = 39,8°C); the child needed hospitalization. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is consistent.
Case 9
The ninth case involved a 12-months-old female. A week after the vaccination, she presented a sudden loss of consciousness with staring eyes, hypertonic for about 10 min, modest hypersalivation. She was hospitalized and, after medical examination, she was discharged with the diagnosis of hyporesponsive episode in patient with vomiting and metabolic acidosis. Applying the Causality Assessment algorithm, cause/effect relationship between vaccination and adverse events is inconsistent, because an alternative cause (gastrointestinal infectious disease) has been recognized.
Case 10
The 10th case regarded a 25-months-old female vaccinated with MMRV and anti-HAV vaccines at the same time. Twelve days after vaccination, she was affected by a hypotonic-hyporesponsive episode with sudden onset of hypotonia, pallor, and cyanosis, reduced stress reactivity. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is inconsistent, because hypotonic-hyporesponsive episodes are very rare AEFIs that can occur within 48 h after the vaccine administration.Citation39
Case 11
The 11th case involved a 69-months-old female vaccinated with MMRV (second dose) and anti-dTap vaccine at the same time. Iridocyclitis is the adverse event detected and AEFI caused severe and permanent disability. Applying the AEFI Causality Assessment algorithm, the association with the MMRV-vaccine is not-classifiable because of the lack of information about the date of vaccine administration and case definition (medical examination, therapy after adverse event onset).
Case 12
The 12th case regarded a 17-months-old female: 20 days after vaccination, she presented bruising and petechiae on the face, trunk and limbs, and hospitalization were needed.
After medical examination, thrombocytopenia was diagnosed and Parvovirus B19 infection was recognized as determining causal event. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is inconsistent because of the presence of an alternative explanation of AEFI.
Case 13
The 13th case regarded a 15-months-old male. Nine days after vaccination, he reported hyperpyrexia and febrile seizure associated with eyes rolling, limbs twitchings, and loss of consciousness. This episode ended after a few minutes: for these symptoms, he was admitted to the hospital and discharged after 3 days for the complete AEFI resolution.
During hospitalization he presented fever but he did not report another episode of febrile seizures. After medical examination, a final diagnosis of febrile seizure caused by viral pharyngotonsillitis was formulated.
Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is inconsistent for the presence of an alternative disease (viral pharyngotonsillitis).
Case 14
The 14th case involved a 13-months-old male immunized with MMRV and anti-HAV vaccines at the same time. Fifteen days after the vaccination he presented fever, bruising and petechiae on the face, trunk, and limbs, who needs hospitalization. After medical examinations, the diagnosis of immune thrombocytopenic purpura has been formulated (PLT = 25000/mmc); other infectious diseases were not detected.
Applying the Causality Assessment algorithm, cause/effect relationship between vaccination and adverse events is consistent: there is a biological plausibility even if purpura is a very rare adverse event (2,6 case of ITP/100000 MMR doses) and alternative causes are not found.
Case 15
The 15th case regarded a male child aged 14 months vaccinated with MMRV and anti-HAV vaccines at the same time. After a week, he developed erythema on the face, trunk, and limbs and he accessed to the emergency room, where exanthema post-MMRV vaccine was diagnosed. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is classifiable as consistent.
Case 16
The 16th case involved a 23-months-old female vaccinated with MMRV and anti-HAV vaccines at the same time. Three days after immunization, she presented fever and hyperpyrexia and needed hospitalization. After a medical examination and antibiotic therapy, she was discharged with the diagnosis of sepsis and hypertransaminasemia. Applying the Causality Assessment algorithm, the cause/effect relationship between MMRV-vaccination and adverse events is inconsistent, because of the lack of biological and temporal plausibility.
Case 17
The 17th case involved a 16-months-old male: 25 days after immunization he presented bruising and petechiae on the face, trunk, and limbs. He was hospitalized and, after medical examination, diagnosis of immune thrombocytopenic purpura has been formulated (PLT = 20000/mmc) and other infectious diseases were ruled out. Applying the Causality Assessment algorithm, the cause/effect relationship between MMRV-vaccination and adverse events is consistent.
Case 18
The 18th case regarded a 21-months-old male vaccinated with MMRV and anti-HAV vaccines at the same time. Twenty-four days after immunization, he reported a hypotonic-hyporesponsive episode and short-term memory loss: for these symptoms he need hospitalization. Applying the Causality Assessment algorithm, cause/effect relationship between vaccination and adverse events is inconsistent, because hypotonic-hyporesponsive episodes are very rare AEFIs that occur within 48 h after the vaccine administration.
Case 19
The 19th case involved a 15-months-old female vaccinated with MMRV and anti-HAV vaccines. Ten days after immunization, she developed fever and hyperpyrexia and strabismus, which was classified as serious and permanent invalidity. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is not consistent, because of the absence of biological plausibility between strabismus and vaccine administration.
Case 20
The 20th case regarded a 67-months-old female vaccinated with MMRV and anti-dTap vaccines. A few minutes after the immunization, she presented a vagal reaction with transient loss of consciousness for which she was admitted to the emergency room; she was not hospitalized and after few hours she was discharged for the complete remission of symptoms. According to Causality Assessment, the relation is found to be consistent.
Case 21
The 21st case regarded a 14-months-old male: few hours after vaccination, he presented fever and dyspnea. For these symptoms, he was admitted to the hospital and discharged after 3 days with the diagnosis of bronchiolitis caused by the Human respiratory syncytial virus (RSV). Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is not consistent, because of the evidence of an alternative cause (RSV infection).
Case 22
The 22nd case involves a 12-months-old female: since the day after the vaccination, her mother reported the occurrence of increasing and permanent disorder in child neurologic development with deficits in social communication and social interaction, mutism, and language problems (signs and symptoms of autism spectrum disorders). Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is inconsistent, because of the absence of biological plausibility between MMRV vaccination and the AEFI reported. Several studies and reviews excluded the casual link between vaccine and autism supposed in this AEFI report.Citation40
Case 23
The 23rd case involves a male child aged 30 months: few hours after vaccination, he developed hyperpyrexia with an episode of febrile seizure. He was hospitalized and symptoms persisted for 9 days. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is classifiable as inconsistent: even the biological plausibility of AEFI, the time window between vaccination and adverse reactions (hyperpyrexia and febrile seizure) is not compatible (too short).
Case 24
The 24th case involves a 13-months-old male: 25 days after immunization, he presented bruising and petechiae on the face, trunk, and limbs. He was hospitalized and, after medical examination, the diagnosis of immune thrombocytopenic purpura has been formulated (PLT = 25000/mmc). The anamnesis reported a recent human herpes virus 6 infection that is a confounding element. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is indeterminate, because all alternative causes can not be completely ruled out.Citation41
Case 25
The 25th case involves a 13-months-old female: 2 days after vaccination she presented fever, vomiting, and jaundice. She was hospitalized and, after medical examinations, diagnosis of the atypical uremic hemolytic syndrome was formulated. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is inconsistent, because of the absence of biological plausibility. However, the time window between vaccination and AEFI is not compatible with a causal association.
Case 26
The case 26th regards a 43-months old female: few hours after vaccination, she developed hyperpyrexia (T = 40°C) and erythema. Hospitalization was not necessary. Applying the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is classifiable as inconsistent because of the absence of temporal plausibility between MMRV vaccination and adverse events.
Discussion and conclusion
In our study, we tested the use of last updated WHO algorithm of causality assessment to verify the casual association between MMRV and detected AEFIs: our analysis provides a picture of vaccine safety, even if limited by data from spontaneous reporting (e.g., missing or incomplete data).Citation7,Citation16
Using the AIFA database, all serious adverse events following MMRV vaccination notified in the Apulia region since vaccine authorization were analyzed: only for 10/26 (38.4% of serious AEFIs) a casual association was noted and all serious adverse events were already known; therefore no emerging signals were detected.Citation42 The number and the rate of AEFIs increased over time and a peak in the fourth is noted that could be related with a higher number of MMRV doses administered and an improvement of the sensitivity of the surveillance system, that has been noted also for other vaccines.Citation14
Follow-up procedures demonstrated that all serious adverse events judged consistent with immunization (10/10) were completely and spontaneously resolved without sequelae.
Fever and hyperpyrexia are the adverse events most frequently detected in serious AEFI reports (4/10, 40.0%) classified as consistent with immunization. Likewise, thrombocytopenic signs were notified in 3/10 consistent AEFI-reports (30.0%) and erythema – rush were reported in 3/10 (30.0%) while vaso-vagal event were described in 2/10 (20.0%) consistent AEFI reports.
The ITP after MMR and MMRV vaccines has been analyzed in a 2010 review of 12 studies.Citation37 Authors estimated an incidence of MMR-associated ITP from 0.087 to 4 (median 2.6) cases per 100000 vaccine doses. In our study, the general incidence of ITP MMRV is 0.98 x 100000, that begins 0.73 × 100000 if we considered only AEFIs with a consistent causal association to immunization; this difference could be related to the under-reporting. Review shows that severe bleeding manifestations were rare, and MMR-associated thrombocytopenia resolved within 6 months from diagnosis in 93% of the children and also patients of our samples did not report sequelae.
None case of febrile seizure resulted in consistent causal relation with vaccination: e.g., the 13th case regards a possible case of febrile seizure after the MMRV vaccine, but medical records do not support this hypothesis and final diagnosis of febrile seizure caused by viral pharyngotonsillitis was formulated. We have also to consider that seizure is a common event in children aged <3 years also before MMRV vaccine introduction, as shown in an article by Gabutti et al., that reports an hospitalization rate of 618 × 100000.Citation43
As discussed, during the study period 674.474 doses of MMR were administered in Apulia with an AEFIs rate of 4.6 × 100000 doses; MMR was used for subjects with a natural history of varicella (in particular, for immunization of susceptible adolescents and adults, such as Health-Care Workers) or showing contraindication for MMRV use (e.g., personal or family history of seizures). The difference between MMR and MMRV AEFIs rates (4.6 vs. 37.8 × 100.000 doses) could be partially explained by the difference in the attitude of AEFIs notification between Family Pediatricians (who cared infants receiving MMRV) and General Practitioners (who cared adolescents and adults receiving MMR). This difference is yet noted for other vaccines, as in AIFA reports.Citation14
The MMR AEFIs rate for serious events with a consistent causal association seemed lower than MMRV (0.15 x 100000 vs. 2.4 × 100000) but we have to underline that the only febrile seizure with a consistent causal association recognized was detected after MMR immunization. The lack of cases of febrile seizures after MMRV in an 8-year period is probably related to the low number of reports (high under-reporting).
We have also to consider that the recommendations from AIFACitation24 could influence the level of attention of health-care workers to the MMRV vaccine, and this could partially explain the different rate.
In a recent study published by Cocchio et al., fever was the most common systematic reaction after the MMRV vaccine and the incidence of febrile seizure was 0.2%. This study was based on active surveillance (than, the reporting rate was expected as higher) but causality assessment was not carried out, than we cannot evaluate the link between vaccine and AEFIs.Citation44
In a 2014 study on 3112 children, 10 febrile seizures were reported following MMRV administered alone or with other vaccines (rate: 3.21 × 1000 doses). Three of the seizures were reported by the investigator as related to vaccination (rate 1/1000 doses) and seven were reported as probably or definitely not related to vaccination: also, in this case, the authors did not use the WHO algorithm and it makes difficult to compare the results.Citation25
Our results suggested that, considering the low rate of AEFI, the passive surveillance system would be useful to detect safety emerging signals which are expected following changes in the immunization program, but specific active post-marketing surveillance programs would be implemented to gain a complete figure of vaccine safety.Citation15,Citation45
Data confirmed that MMRV vaccine is safe and the choice of Apulia Region of continuing its use was right: consulting the 2017 AIFA report, the incidence of MMRV AEFIs was no higher than among children of the same age who received the first dose of MMR vaccine and varicella vaccine administered as separate injections at the same visit.Citation14
The use of the Causality Assessment algorithm must be implemented in the view of accountability of the National Health System, because of the importance of continuous updating of vaccines safety profiles, performed also by the analysis of post-marketing surveillance data. This good practice could also support Public Health Authority decisions, in order to avoid vaccines retraction from the market decided only for temporal coincidence, without a complete assessment.Citation46
According to this observation, AIFA in 2017 “Vaccine report”, for the first time, published the result of causality assessment evaluation in order to avoid an inappropriate image of vaccine safety.Citation14
It is crucial to improve the quality of the notifications, because some AEFI reports lacked of essential information, such as date of vaccine administration or the clinical characteristics of the disease signalized after the vaccination (case 11 and case 13), while, in the case of hospital admission, clinical documentation could be always added to AEFI report because it represents a fundamental element of causal link evaluation protocol.Citation47
The main strength of our pilot study is the systematic use of the causality assessment and the analysis of medical records for serious AEFIs. MMRV-vaccine is an exceptional case study, because of the relatively recent introduction in a restricted target population (1–12 years aged people). We have also to consider that recommendations of AIFA and the Ministry of Health increased the attention of physicians and parents about the safety profile of this vaccine.Citation27,Citation28
The main goal of spontaneous reporting systems is to identify signals, serious/unknown AEs, or any unexpected increase of known reactions, but this model of surveillance is highly affected by an important risk of under-reporting. The data presented in our study show a high variation of the reporting rate in different years (10-fold increase in year IV in comparison to I, II, and VI), suggesting a very high level of under-reporting in the Apulia region.
In general, the number of serious AEFIs examined is low and this is an important limitation; so it would be important to set up multicentric studies to improve the level of evidence about the post-marketing safety profile of MMRV vaccine. Finally, in almost all serious cases, the MMRV vaccine is administered together with the HAV vaccine, leading to a more difficult evaluation of causality for many events.
Future studies have to focus the theme of the reliability of causality assessment and the importance of adequate and updated evaluation protocols; in this scenario, an alliance between physician, parents, an expert in vaccine-vigilance and institutions (such as National Drug Authority) is crucial in the view of increasing the accountability of vaccination system.
Highlights
MMRV AEFIs surveillance is a Public Health issue for the increased risk of seizure
None case of febrile seizure resulted consistent to vaccination
Performing WHO causality assessment, <50% of MMRV serious AEFIs could be related to vaccine
References
- Di Pasquale A, Bonanni P, Garçon N, Stanberry L, El-Hodhod M, Tavares Da Silva F. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34(52):6672–80. doi:10.1016/j.vaccine.2016.10.039.
- Principi N, Esposito S. Adverse events following immunization: real causality and myths. Expert Opin Drug Saf. 2016 Jun;15(6):825–35. doi:10.1517/14740338.2016.1167869.
- Chen RT, Davis RL. Rhodes PH Special methodological issues in pharmacoepidemiology studies of vaccine safety. Pharmacoepidemiology. 4th ed. John Wiley & Sons, Ltd. 2007. 455–85. doi:10.1002/9780470059876.ch30.
- Shimabukuro T, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–405. doi:10.1016/j.vaccine.2015.07.035.
- Haider N, Mazhar F. Factors associated with underreporting of adverse drug reactions by nurses: a narrative literature review. Saudi J Health Sci. 2017;6(2):71. doi:10.4103/sjhs.sjhs_37_17.
- Hoffman BL, Felter EM, Chu KH, Shensa A, Hermann C, Wolynn T, Williams D, Primack BA. It’s not all about autism: the emerging landscape of anti-vaccination sentiment on Facebook. Vaccine. 2019 Apr 10;37(16):2216–23. doi:10.1016/j.vaccine.2019.03.003.
- Varallo F, Guimarães S, Abjaude S, Mastroianni P. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev Escola Enfermagem USP. 2014;48(4):739–47. doi:10.1590/S0080-623420140000400023.
- WHO. Causality Assessment of an adverse event following immunization (AEFI). User manual for the revised WHO classification. [accessed 2018 Jan]. http://apps.who.int/iris/bitstream/handle/10665/259959/9789241513654-eng.pdf?sequence=1.
- Crawford N, Clothier H, Hodgson K, Selvaraj G, Easton M, Buttery J. Active surveillance for adverse events following immunization. Expert Rev Vaccines. 2013;13(2):265–76. doi:10.1586/14760584.2014.866895.
- Thoon K, Soh S, Liew W, Gunachandran A, Tan N, Chong C, Yung CF. Active surveillance of adverse events following childhood immunization in Singapore. Vaccine. 2014;32(39):5000–05. doi:10.1016/j.vaccine.2014.07.020.
- Vaudry W, Lee B, Roth A. Active hospital based surveillance for meningococcal vaccine adverse events after a community mass immunization program. Paediatr Child Health. 2002;7(suppl_A):33A–34A. doi:10.1093/pch/7.suppl_A.33Aa.
- WHO. Adverse event following immunization. Aide-Mémoire On Causality Assessment. [accessed on 2019 March 15]. http://www.who.int/vaccine_safety/initiative/investigation/New_aide_mem_causal_assmt.pdf.
- Alicino C, Merlano C, Zappettini S, Schiaffino S, Della Luna G, Accardo C, Gasparini R, Durando P, Icardi G. Routine surveillance of adverse events following immunization as an important tool to monitor vaccine safety. Hum Vaccin Immunother. 2014;11(1):91–94. doi:10.4161/hv.34360.
- National Drug Authority (AIFA). Rapporto sulla sorveglianza post-marketing dei vaccini in Italia Anno 2017. [accessed on 2019 March 17]. http://www.aifa.gov.it/sites/default/files/Rapp_Vaccini_2017_0.pdf.
- Stefanizzi P, Calabrese G, Infantino V, Del Matto G, Tafuri S, Quarto M. Systematic Use of causality assessment in AEFI surveillance: a 2013-2016 pilot study in Puglia. EBMJ. 2017;12:154–58.
- Puliyel J, Naik P. Revised World Health Organization (WHO)’s causality assessment of adverse events following immunization—a critique. F1000 Research. 2018;7:243. doi:10.12688/f1000research.
- CDC. Licensure of a combined live attenuated measles, mumps, rubella and varicella vaccineMorbidity and Mortality Weekly Report (MMWR). 2005;54. 1212–14.
- CDC. Measles, mumps, and rubella–vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1998;47(RR–8):1–57.
- European Medicine Agency. Proquad. [accessed on 2019 March 17]. https://www.ema.europa.eu/en/medicines/human/EPAR/proquad.
- Jacobsen S, Ackerson B, Sy L, Tran T, Jones T, Yao J, Xie F, Cheetham TC, Saddier P. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009;27(34):4656–61. doi:10.1016/j.vaccine.2009.05.056.
- Klein N, Fireman B, Yih W, Lewis E, Kulldorff M, Ray P, Baxter R, Hambidge S, Nordin J, Naleway A, et al. Measles-Mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126(1):e1–e8. doi:10.1542/peds.2010-0665.
- Ma S, Xiong Y, Jiang L, Chen Q. Risk of febrile seizure after measles–mumps–rubella–varicella vaccine: A systematic review and meta-analysis. Vaccine. 2015;33(31):3636–49. doi:10.1016/j.vaccine.2015.06.009.
- Marin M, Broder KR, Temte JL, Snider DE, Seward JF Centers for Disease Control and Prevention (CDC). Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010 May 7;59(RR–3). 1–12.
- Macartney K, Gidding H, Trinh L, Wang H, Dey A, Hull B, Orr K, McRae J, Richmond P, Gold M, et al. Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatr. 2017;171(10):992. doi:10.1001/jamapediatrics.2017.1965.
- Klopfer S, Stek J, Petrecz M, Reisinger K, Black S, Goveia M, Nicholson O, Gardner JL, Grosso AD, Brown ML, et al. Analysis of safety data in children after receiving two doses of ProQuad® (MMRV). Vaccine. 2014;32(52):7154–60. doi:10.1016/j.vaccine.2014.08.067.
- WHO. Vaccine-preventable diseases: monitoring system. 2018 global summary. [accessed on 2019 March 20]. http://apps.who.int/immunization_monitoring/globalsummary/schedules.
- Puglia R. Assessorato alle politiche della salute settore assistenza territoriale prevenzione. Bollettino Ufficiale n. 124 del 12-8-2009 – verbale Commissione Regionale Vaccini n. 2/2009 dell’8 giugno. 2009. [accessed on 2019 March 22]. http://www.regione.puglia.it/documents/10192/5083710/N124_12_08_09.pdf/4da63ac5-cd29-4794-9f6c-5417081a120a.
- Ministero della Salute – Dipartimento della Sanità Pubblica e Innovazione. DGPRE 0021509-P-10/10/2012. Indicazioni in merito alla somministrazione della vaccinazione contro la varicella in età pediatrica. 2012.
- AIFA – Working Group Pediatrico. Raccomandazioni del working group pediatrico dell’AIFA in relazione all’ utilizzo dei vaccini MPRV. [accessed on 2019 March 21]. http://www.aifa.gov.it/sites/default/files/raccomandazione_vaccino_mprv_14_novembre_2011.pdf.
- Ministero della Salute. Legge n. 119 del 31 luglio 2017. Pubblicato in Gazzetta Ufficiale n. 182 del 05-08-2017. [accessed on 2019 March 23]. http://www.gazzettaufficiale.it/eli/id/2017/08/5/17G00132/sg.
- Epicentro-Istituto Superiore della Sanità. Vaccini e vaccinazioni - Copertura vaccinale in Italia. [accessed on 2019 March 20]. http://www.epicentro.iss.it/vaccini/dati_Ita#morbillo.
- Gruppo di lavoro Malinf della Regione Puglia. Bollettino delle coperture vaccinali al 31 dicembre 2017- dalla nascita all’adolescenza. [accessed on 2019 March 21]. https://www.sanita.puglia.it/documents/36126/269121/Bollettino+delle+Coperture+Vaccinali+al+31+dicembre+2017+-+dalla+nascita+all%27adolescenza/3b204405-e020-4db3-8ae4-78cb088ed15f?version=1.3&t=1524747097880.
- AIFA – Gruppo di Lavoro sull’analisi dei segnali dei vaccini. Guida alla valutazione delle reazioni avverse osservabili dopo vaccinazione. 2016. [accessed on 2019 March 24]. http://www.aifa.gov.it/sites/default/files/Guida_valutazione_reazioni_avverse_osservabili_dopo_vaccinazione_2.pdf.
- European Medical Agency. Important medical event terms list. [accessed on 2019 March 23]. https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview.
- Myers T, McNeil M, Ng C, Li R, Lewis P, Cano M. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010–2015. Vaccine. 2017;35(14):1758–63. doi:10.1016/j.vaccine.2017.02.030.
- Esposito S, Bianchini S, Dellepiane R, Principi N. Vaccines and Kawasaki disease. Expert Rev Vaccines. 2015;1–8.
- Chung E. Vaccine allergies. Clin Exp Vaccine Res. 2014;3(1):50. doi:10.7774/cevr.2014.3.1.50.
- Mantadakis E, Farmaki E, Buchanan G. Thrombocytopenic purpura after measles-mumps-rubella vaccination: a systematic review of the literature and guidance for management. J Pediatr. 2010;156(4):623–28. doi:10.1016/j.jpeds.2009.10.015.
- Vigo A, Costagliola G, Ferrero E, Noce S. Hypotonic-hyporesponsive episodes after administration of hexavalent DTP-based combination vaccine: a description of 12 cases. Hum Vaccin Immunother. 2017;13(6):1375–78. doi:10.1080/21645515.2017.1287642.
- Taylor L, Swerdfeger A, Eslick G. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32(29):3623–29. doi:10.1016/j.vaccine.2014.04.085.
- Hashimoto H, Maruyama H, Fujimoto K, Sakakura T, Seishu S, Okuda N. Hematologic findings associated with thrombocytopenia during the acute phase of exanthem subitum confirmed by primary human herpesvirus-6 infection. J Pediatr Hematol Oncol. 2002;24(3):211–14. doi:10.1097/00043426-200203000-00010.
- Tafuri S, Gallone M, Calabrese G, Germinario C. Adverse events following immunization: is this time for the use of WHO causality assessment? Expert Rev Vaccines. 2015;14(5):625–27. doi:10.1586/14760584.2015.1029460.
- Gabutti G, Kuhdari P, Ferioli S, Trucchi C. Hospital admissions for seizure in Italy: a decennial retrospective analysis with a special focus on the burden in the pediatric age. Neurol Sci. 2015 Sep;36(9):1667–73. doi:10.1007/s10072-015-2230-1.
- Cocchio S, Zanoni G, Opri R, Russo F, Baldo V. A postmarket safety comparison of 2 vaccination strategies for measles, mumps, rubella and varicella in Italy. Hum Vaccin Immunother. 2015;12(3):651–54. doi:10.1080/21645515.2015.1101198.
- Tafuri S, Fortunato F, Gallone M, Stefanizzi P, Calabrese G, Boccalini S, Martinelli D, Prato R. Systematic causality assessment of adverse events following HPV vaccines: analysis of current data from Apulia region (Italy). Vaccine. 2018;36(8):1072–77. doi:10.1016/j.vaccine.2018.01.018.
- Sukumaran L, McNeil M, Moro P, Lewis P, Winiecki S, Shimabukuro T. Adverse events following measles, mumps, and rubella vaccine in adults reported to the Vaccine Adverse Event Reporting System (VAERS), 2003-2013. Clin Infect Dis. 2015. doi:10.1093/cid/civ061.
- Lei J, Balakrishnan M, Gidudu J, Zuber P. Use of a new global indicator for vaccine safety surveillance and trends in adverse events following immunization reporting 2000–2015. Vaccine. 2018;36(12):1577–82. doi:10.1016/j.vaccine.2018.02.012.
