ABSTRACT
Background
The study was aimed at comparative evaluation of seasonal influenza vaccine RIBSP versus commercial vaccine VAXIGRIP® for immunogenicity and safety in the course of clinical trial phase II on healthy subjects up to 60 years.
Methods
The trial involved 150 subjects in randomized 2:1 groups that received either RIBSP vaccine or comparator vaccine VAXIGRIP®. One dose (0.5 ml) of either vaccine contained 15 µg of hemagglutinin of each influenza virus strain recommended by WHO for the Northern hemisphere in 2016–2017 flu season. The observation period lasted 21 days. The trial was registered at ClinicalTrials.gov identifier NCT 03016143.
Results
Assessment of immunogenic activity of the vaccine under study showed that in 21 days the portion of participants with 4-fold seroconversions was 80.0% to А/H1N1; 65.0% to А/H3N2 and 64.0% to B virus. Antibody titer increase factor in the group of subjects that received RIBSP vaccine was 13.4 for А/H1N1; 5.2 for А/H3N2 and 5.2 for B virus. The subjects that received RIBSP vaccine demonstrated 88% seroprotection rate against А/H1N1; 75% against А/H3N2 and 61% against B virus. In the course of evaluating the vaccine safety, no serious adverse events were recorded. All changes of laboratory data were slight and single in most cases. All recorded local reactions have been light in character and these have been predicted reactions observed at vaccination against influenza.
Conclusion
Comparison vaccines RIBSP and VAXIGRIP®, showed similar immunogenic activity. The RIBSP vaccine is safe and immunogenic for the elderly and conforms to international criteria in CPMP/BWP/214/96.
Introduction
The most vulnerable to the influenza virus are those with weakened immunity, elderly people and children. People of advanced age (over 60 years) make up the age group which accounts for the greatest number of complications and deaths associated with influenza.Citation1,Citation2
Pneumonia, cardiovascular diseases (myocarditis, pericarditis) and nervous system diseases (meningoencephalitis) are more common in the elderly. The influenza can lead to an exacerbation of chronic diseases and the development of bacterial superinfection. Atrophic processes developing in the mucosa of the upper respiratory tract of elderly lead to easy penetration of influenza virus into the bronchi causing bronchitis and pneumonia.Citation3,Citation4
The incidence of complications associated with influenza leading to hospitalization in adults over the age of 60 is 58.9 per 10 000, compared to 18 per 10 000 in healthy adults. Mortality caused by influenza in elderly is also very high: 75.1 cases per 200 000 of population compared to 7.0–17.6 cases per 200 000 in healthy adults aged 20–60 years. Vaccination of the people over 60 years can reduce mortality due to influenza in almost 90% of cases and the hospitalization rate up to 70%.Citation5,Citation6 All persons over 60 years are classified as a high-risk group for complications and deaths caused by influenza and WHO recommends their mandatory vaccination against seasonal influenza.Citation7
Since the intensity of immunity to influenza after each immunization persists not longer than a year (especially in elderly people), it is reasonable not to include adjuvants (aluminum hydroxide) and preservatives (thiomersal) into vaccines, as these substances can be harmful due to their cumulative action.Citation8,Citation9
Seasonal influenza vaccine preparation not containing adjuvants and preservatives can be recommended for use in high-risk groups.Citation10,Citation11 Besides, these preparations are manufactured in the reduced number of steps and corresponding analytical tests. Having taken all this into consideration the researchers of the RIBSP developed and manufactured the seasonal influenza vaccine.Citation12,Citation13
Until recently, four foreign seasonal influenza vaccines were registered in Kazakhstan, the purchase of which for risk groups cost the state budget a considerable amount. The most available has been VAXIGRIP® vaccine (Sanofi Pasteur, France). This vaccine is a split vaccine which contained thiomersal as a preservative.
In order to ensure the prevention of seasonal influenza in the Republic of Kazakhstan (RK), the Research Institute for Biological Safety Problems of the RK has developed an allantoic split inactivated seasonal influenza vaccine (RIBSP vaccine) containing influenza viruses of subtypes A (H1N1pdm09 and H3N2) and type B.
Preclinical studies and phase I clinical trials in humans under 60 years showed good safety and immunogenic profile of RIBSP vaccine.Citation14,Citation15 RIBSP vaccine was also safe and immunogenic in phase II clinical trials in healthy subjects under 60 compared to the VAXIGRIP® vaccine (widely used for vaccination of the risk groups in the Republic of Kazakhstan).Citation16 The humoral immune response to RIBSP vaccine meets all three requirements of the European Committee for Medical Products for Human Use of the European Medicines Agency (CHMP) CPMP/BWP/214/96 and showed similar profile with VAXIGRIP®.Citation17
Evaluation of the immunogenic activity of RIBSP vaccine showed that the highest seroconversion rate for А/H1N1pdm09 component reached 87.0% 21 days after vaccination, i.e. by 7% higher than the rate of VAXIGRIP® for the same vaccine component. The seroconversion rates for А/H3N2 and B also exceeded the same rates of VAXIGRIP®.Citation16 The obtained results demonstrated that RIBSP vaccine provided seroconversion rates for all influenza vaccine components significantly exceeding CPMP/EWP/214/96 criteria for adults.
In the group of subjects immunized with RIBSP vaccine, the GMT fold increase was 23.3 for А/H1N1 influenza virus, 4.4 for А/H3N2 and 4.5 for influenza B virus. Seroprotection rates were at least 80% for all influenza strains in both groups, the highest one being recorded for А/H1N1 influenza virus. VAXIGRIP® vaccine showed insignificantly higher seroprotection rates compared to the RIBSP vaccine.Citation16 There were shown no significant differences in the immunogenic activity of RIBSP and VAXIGRIP® vaccines.
The aim of this non-inferiority trial was to compare the RIBSP vaccine and the available and widely used in Kazakhstan VAXIGRIP® vaccine for immunization of adults over 60 years as a milestone in the development and introduction for wide use of the domestic flu vaccine in Kazakhstan.
Results
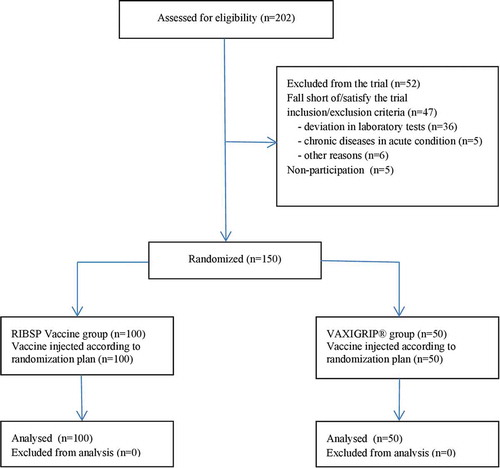
Registration of subjects started on October 24 and completed on November 3, 2016; 202 subjects were registered (). The participants were vaccinated on October 26, the study was completed on November 26, 2016.
All 150 subjects were involved in safety and immunogenicity analysis of the vaccines; none of them stopped his/her participation or was excluded from the trial during the entire period. There were no significant differences in age, gender, weight and height of subjects. The demographic information is displayed in .
Table 1. Summary of demographic data.
Safety
Adverse events of immediate type (AEIT) (local reactions) were recorded in the RIBSP group within 2 hours post vaccination (see ).
Table 2. Adverse events within 21 days after vaccination.
The local adverse events observed during the first 7-day post vaccination were similar to the adverse events of immediate type. Systemic reactions were recorded by one subject in the RIBSP group and one subject in VAXIGRIP® group. Induration at the site of vaccine administration was the most frequent local symptom in both groups. All local reactions to vaccination were infrequent, mild and self-limiting and consisted of expected events. The recorded systemic side reactions such as headache and fever were also mild. All recorded AEs were transient; there was no need in therapy. These events were recorded in similar frequencies in two study groups.
During the study period from D8 to D20 day post vaccination the participants of both groups recorded AEs in their self-observation Diary cards at similar frequency (17% of those who received the tested preparation and 18% of the comparator group). The most often recorded symptoms were typical for acute respiratory infections (ARI), as the autumn season in Almaty is usually characterized by the higher ARI morbidity rate. In most cases the AEs were mild. One subject vaccinated with RIBSP preparation recorded temperature rise up to 38.5°С against the background of ARI on the 14–16th day post vaccination; the fever was considered as moderate AE not associated with vaccination.
Post vaccination changes in laboratory findings (results of clinical and biochemical blood analyses) were observed in both study groups on the 3rd, 7th and 21st-day post vaccination; the frequency rate was comparable for two groups. Changes in lab findings were demonstrated by 14% of subjects in RIBSP group, and 12% in VAXIGRIP® group. All changes were minor and in most of the cases only in one parameter. There were no pathological changes in the results of urine analyses. No serious AEs were observed in the course of the study.
Immunogenicity
Comparison of allantoic split inactivated seasonal influenza vaccine produced at RIBSP versus VAXIGRIP® vaccine in subjects aged over 60 years demonstrated similarity of their immunogenic activity against all vaccine components ().
Table 3. Immune response for the RIBSP vaccine and comparator preparation licensed vaccine VAXIGRIP®.
Evaluation of RIBSP vaccine immunogenic activity showed that 4-fold seroconversions were observed in 80.0% to А/H1N1pdm09 influenza virus; 65.0% to А/H3N2 influenza virus and 64.0% to influenza B virus 21 days post vaccination. Seroconversion rates in VAXIGRIP® group were 80.0% to the influenza virus А/H1N1pdm09; 68.0% to the influenza virus А/H3N2 and 62.0% to the influenza B virus.
In 21 days post administration of the vaccine developed at the RIBSP the geometric mean antibody titers (GMT) in volunteers reached 185.1 for the influenza A/H1N1pdm09 virus, 60.6 for the influenza A/H3N2 virus and 42.3 for the influenza B virus. The subjects immunized with VAXIGRIP® vaccine demonstrated GMTs equal to 143.2, 82.5 and 44.7 for the respective components of the preparation (p > .01 between groups). GMT fold increase in RIBSP group was 13.4 for А/H1N1pdm09 influenza virus, 5.2 for А/H3N2 influenza virus and 5.2 for influenza B virus; in VAXIGRIP® group these parameters were 12.5, 7.7 and 4.5 for each vaccine component, respectively.
The seroprotection rates in our study were 88%, 75% and 61% in the subjects vaccinated by RIBSP vaccine and 76%, 82% and 62% in the subjects vaccinated by VAXIGRIP® for А/H1N1pdm09, А/H3N2, and B viruses, respectively.
Statistically significant differences in seroprotection rates of both RIBSP and VAXIGRIP® vaccines were not observed (p > .01 between groups).
Discussion
The non-inferiority study of the new domestic seasonal influenza RIBSP vaccine and the licensed widely used VAXIGRIP® vaccine clearly showed a good safety profile in elderly subjects aged over 60 for both vaccines. The incidence of side reactions to the RIBSP vaccine did not comparatively differ or was lower compared to the VAXIGRIP® vaccine.Citation6,Citation18,Citation19
The elderly humans did not demonstrate any allergic reactions that could be caused by residual amounts of ovalbumin in the vaccine.
The response to vaccination is usually lower in the elderly as compared to young people due to the progressive age-related decline of immune functions. Therefore, the international CHPM criteria for assessing the immunogenicity of trivalent seasonal influenza vaccines are lower for persons over 60 years of age.Citation17
Immunogenicity was assessed by measuring hemagglutination titers that are currently the only correlate of counter-influenza protection accepted worldwide for licensing influenza vaccines. The study included volunteers at the stage of screening who had not been vaccinated with influenza vaccine over the past 2 years and this was reflected in the base seropositivity indicators. The trial showed that administration of one dose of RIBSP vaccine results in formation of post-vaccination antibodies: level of seroconversion to the influenza А/H1N1 virus post vaccination with RIBSP vaccine is analogous (80.0%) to the same in case of vaccination with VAXIGRIP®. As for influenza А/H3N2 virus that most often induces severe reactions in the elderly the vaccine developed at the RIBSP has shown a bit lower seroconversion rate (65%) than VAXIGRIP® (80%); but in case of influenza B virus, this index of RIBSP vaccine (64%) exceeds the index of VAXIGRIP® vaccine (62%).Citation17,Citation20–Citation22
Seroprotection rate of RIBSP vaccine against the influenza А/H1N1 virus exceeds the same of VAXIGRIP® by 12%; in case of influenza А/H3N2 virus it is lower by 11%, and both vaccines demonstrate practically identical level of seroprotection against influenza B virus.
Phase II clinical trial of the RIBSP vaccine demonstrated that the vaccine free of preservatives and adjuvants provides stable immune response in the elderly which is not inferior to the immune response induced by the licensed vaccine VAXIGRIP®.Citation21,Citation22
This study showed that the immunogenicity of the new influenza vaccine RIBSP is largely comparable with that of the commercial trivalent, allantoic split inactivated seasonal vaccine VAXIGRIP® which has been widely used in Kazakhstan.
The results of this study encourage to go to Phase 3 trial, since adhering to principle of the production availability which we achieved by reducing the number of steps and corresponding analytical tests due to exclusion of adjuvants and preservatives from the vaccine formulation, we have developed a safe and immunogenic preparation that meets all CHRM criteria. Vaccination for the influenza prevention is worldwide considered to be a socially significant, cost-effective and resource-saving approach, more preferable than a wait-and-see policy. Therefore, organization of the domestic production of a seasonal influenza vaccine to meet the needs of Kazakhstan is the most important task of the national security and sustainable development of our country.
Material and methods
Study design
Randomized blind comparative phase II clinical trial of the allantoic trivalent split inactivated seasonal influenza vaccine (protocol VSI-II-01/2016) was conducted on healthy adults at the Clinical Center of Kazakhstan, Almaty, following the Guidelines for clinical trials in the Republic of Kazakhstan (ClinicalTrials.gov no. NCT 03016143).
The trial was permitted by the Committee for the Control of Medical and Pharmaceutical Activity after the approval of the Local Ethics Commission of S.D.Asfendiyarov Kazakh National Medical University for performance of Phase II clinical trial, recommendations of the experts council of National Center for Examination of Medicines, Medical Items and Equipment. The design of the study is shown in .
Table 4. Design of Phase II randomized blind comparative clinical trial.
The study protocol and informed consent documents were approved by local and central ethical review boards. All participants provided written informed consent. This study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The major criteria of exclusion were the following: allergic reactions to chicken egg albumin; severe chronic or acute disease; immunocompromised; Guillain-Barré syndrome (acute polyradiculitis) in anamnesis; vaccination against influenza during 2015–2016 season; pregnancy, planning to become pregnant or refuse to take contraceptive precautions (for female volunteers of child-bearing potential); autoimmune disorders, using of antiviral preparations, participation in any other clinical study within the recent three months.
All eligible subjects were admitted to vaccination according to VSI-II-01/2016 protocol. Prior to vaccination blood and urine of the subjects was sampled for standard analyses (biochemical, hematological and immunological analyses of blood and urine analysis). The initial health status of subjects before vaccination was determined based on the obtained results.
Participants
Between October 26, 2016 and November 3, 2016 eligible participants were randomly allocated (2:1) to intramuscular injection of a 0.5 ml dose of the RIBSP vaccine or VAXIGRIP® vaccine groups. Randomization was done with sequentially numbered, opaque, sealed envelopes opened by the study clinician at enrollment. Variable blocks (block sizes two to eight) in the randomization sequence ensured allocation concealment. Study vaccine preparations were masked for both the participants and the observers.
Healthy participants over 60 years were randomly divided into 2 groups. In group 1: 100 subjects were vaccinated with RIBSP vaccine preparation; in group 2: 50 subjects received the comparator preparation VAXIGRIP® vaccine ().
Vaccines and vaccination
The seasonal trivalent influenza vaccine (RIBSP vaccine) tested was manufactured by the RIBSP using embryonated chicken eggs and supplied in prefilled, single-dose, disposable syringes. It was a split vaccine, virus was inactivated with Beta-propiolactone (BPL) and split with Triton X-100. Viruses for RIBSP vaccine production were obtained from the National Institute of Biological Standards and Control (NIBSC, Potters Bar, UK). The allantoic trivalent split inactivated seasonal influenza vaccine VAXIGRIP® (Sanofi Pasteur, Lyon, France) was used as a comparator preparation. One dose (0.5 ml) of both vaccines contained 15 μg of hemagglutinin of each influenza virus strain recommended by WHO for 2016–2017 influenza season in the Northern hemisphere: А/California/7/2009 (H1N1)pdm09 like, A/Hong Kong/4801/2014 (H3N2) like, B/Brisbane/60/2008 like. All subjects were injected intramuscularly with 1 dose of vaccine into deltoid muscle. Duration of the observation period was 21 days.
Safety assessment
The primary outcome of this trial was safety, which investigators assessed by the frequency and severity of vaccine-related local and systemic adverse events. After vaccination safety and reactogenicity of the vaccine was evaluated during the entire observation period (21 days). Type and frequency of local and general adverse events (AE) were daily analyzed since administration of the preparation. The participants were observed by the investigator before vaccination, in 20 min and 2 h post vaccination and in the evening (by phone). Follow-up care was carried out within 7 days by examination of the volunteerеs in the morning and by telephone inquiries in the evening. Reactogenicity of the vaccine and comparator preparations was evaluated via registration of local and general AE as well as on the basis of subjective assessment of tolerance to the preparation by volunteers. Safety of the preparations was evaluated by the laboratory test results (clinical and biochemical blood analyses, urine analysis). Possible side effects of the vaccine may become evident as local reactions at the injection site (pain, hyperemia, infiltration, and so on) and as systemic reactions (fever, coughing, pharyngitis, undue fatiguability, arthralgia, myalgia, headache, giddiness, sickness, abdominal pains, diarrhea, and so on). Within days 7–21 each volunteer recorded the presence/absence of side effects in his/her own self-observation daybook. AE should be registered with description of the following parameters: diagnosis or AE syndrome (if AE is in progress signs and symptoms are described); date of AE onset and termination; severity; relation to administration of the tested preparation and assumed measures. Throughout the entire trial period, the physician-investigator immediately informed the trial team chief about the emergence of any Serious Adverse Event (SAE).
Immunogenicity assessment
The secondary outcome of the trial was assessment of immunogenicity, to be more precise the humoral immune response to administration of the tested vaccines was evaluated in hemagglutination inhibition (HAI) assays with rooster erythrocytes. Blood was sampled (8–10 mL) on days (D) 0 and 21 from all participants. Samples were processed promptly and sera were divided into multiple code-labeled aliquots before storage at or below −20°C. Following completion of all day 21 visits, paired samples were shipped frozen for concurrent laboratory testing. HAI assays were performed at the Research Institute of Influenza, St. Petersburg, Russia, using 0.5% turkey erythrocytes with four hemagglutinin units per 25 μL of virus based on the WHO recommended procedure.Citation12 HAI assays were used to measure antibodies specific to influenza A/California/7/2009 (H1N1) pdm09-like virus, A/Hong Kong/4801/2014 (H3N2)-like virus, В/Brisbane/60/2008 in serum samples of each participant. Laboratory technicians were blinded to the arm to which the participants were assigned. Immunogenicity was described in all age groups according to the recommendations of the CHMP Note for Guidance on Harmonization of Requirements for Influenza Vaccines.Citation17 Anti-hemagglutinin antibody levels were measured by HAI assay before vaccination (day 0) and 21 days after the last vaccination. Immunogenicity endpoints included HAI titer, post-vaccination/pre-vaccination HAI titer ratio, seroprotection, and seroconversion/significant increase. Seroprotection was defined as a HAI titer ≥1:40; seroconversion as a pre-vaccination HAI <1:10 and a post-vaccination titer ≥1:40; and a significant increase in post- vs. pre-vaccination HAI titer. In addition, the immunogenicity vs. 2 vaccine strains was assessed according to the CHMP Note for Guidance criteria. Specifically, for elderly subjects ≥61 years of age, the criteria include a rate of seroconversion or significant increase in post-vaccination HAI titer >30%, a mean geometric increase in HAI titer between pre- and post-vaccination >2, and a rate of post-vaccination seroprotection >60%.
Statistical analysis
Exact Clopper-Pearson method was used to calculate 95% confidence intervals. Statistical analysis of the data was carried out with the help of free software R Studio. Biochemical and hematological parameters of peripheral blood on the 3rd, 7th and 21st-day post vaccination have been compared to the same parameters on day 1 before the vaccination by Dannette test that is a modification of Student’s t-test for multiple comparisons with one control. Effect of the allantoic split inactivated influenza vaccine on biochemical and hematological parameters of peripheral blood was compared with the effect of VAXIGRIP® vaccine on the same parameters with the help of non-parametric Mann-Whitney U test.
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors would like to thank personally D. Inkarbekov and E. Kozhamkulov the staff of Laboratory of Infectious Disease Prevention RIBSP for their help in research.
Additional information
Funding
References
- Morrison-Griffiths S, Gaulton L. Seasonal influenza immunization program outside general practice: an evaluation. Hum Vaccin Immunother. 2016;12(1):248–51. PMID:26566596. doi:10.1080/21645515.2015.1099770.
- Swaan CM, van der Sande MA, Speelman P, Conyn-van Spaendonck MA, Straus SM, Coutinho RA. Adverse events following influenza vaccination: reaction to specific reports and the necessity of a central registration system. Ned Tijdschr Geneeskd. 2007; 29; 151(39):2166–69. PMID:17957995.
- Jadhav S, Datla M, Kreeftenberg H, Hendriks J. The developing countries vaccine manufacturers’ network (DCVMN) is a critical constituency to ensure access to vaccines in developing countries. Vaccine. 2008;26:1611–15. doi:10.1016/j.vaccine.2008.01.034.
- Hendriks J, Holleman M, de Boer O, de Jong P, Luytjes W. An international technology platform for influenza vaccines. Vaccine. 2011;29(1):A8–11. PMID:21684431. doi:10.1016/j.vaccine.2011.04.124.
- Haugh M, Gresset-Bourgeois V, Macabeo B, Woods A, Samson SI. A trivalent, inactivated influenza vaccine (Vaxigrip®): summary of almost 50 years of experience and more than 1.8 billion doses distributed in over 120 countries. Expert Rev Vaccines. 2017;16(6):545–64. PMID:28460594. doi:10.1080/14760584.2017.1324302.
- Sokolova TM, Shuvalov AN, Poloskov VV, Shapoval IM, Kostinov MP. Grippol, Vaxigrip and influvac vaccines – inductors of innate and adaptive immunity factor genes in human blood cells. Zh Mikrobiol Epidemiol Immunobiol. 2014;(5):37–43. PMID:25536769.
- WHO. Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. Drug Saf. 2015;38(11):1059–74. PMID:26446142. doi:10.1007/s40264-015-0350-4.
- Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38(11):1059–74. PMID:26446142. doi:10.1007/s40264-015-0350-4.
- Scheifele DW, Ward BJ, Dionne M, Vanderkooi OG, Loeb M, Coleman BL, Li Y. PHAC/CIHR Influenza Research Network (PCIRN). Compatibility of ASO3-adjuvanted H1N1pdm09 and seasonal trivalent influenza vaccines in adults: results of a randomized, controlled trial. Vaccine. 2012; 6; 30(32):4728–32. PMID:22652402. doi:10.1016/j.vaccine.2012.05.029.
- Levine M, Beattie BL, McLean DM, Corman D. Characterization of the immune response to trivalent influenza vaccine in elderly men. J Am Geriatr Soc. 1987;35:609–15. doi:10.1111/j.1532-5415.1987.tb04335.x.
- Mamadaliyev SM, Sandybayev NT, Kydyrbayev ZK, Khairullin BM, Zaitsev VL, Mambetaliev M, Kassenov MM, Chervyakova OV, Ryskeldinova S, Volgin YN, et al. Development of production technology and pre-clinical testing of a pandemic influenza A⁄H1N1 vaccine. Influenza Other Respir Viruses. 2011;5(1):354–57.
- Sansyzbay AR, Erofeeva MK, Khairullin BM, Sandybayev NT, Kydyrbayev ZK, Mamadaliyev SM, Kassenov MM, Sergeeva MV, Romanova JR, Krivitskaya VZ, et al. An inactivated, adjuvanted whole virion clade 2.2 H5N1 (A/chicken/astana/6/05) influenza vaccine is safe and immunogenic in a single dose in humans. Clin Vaccine Immunol. 2013;20:1314–19. PMID:23803900. doi:10.1128/CVI.00096-13.
- Mamadaliyev SM, Sandybayev NT, Kydyrbayev ZK, Khairullin BM, Zaitsev VL, Mambetaliev M, Kassenov MM, Chervyakova OV, Ryskeldinova S, Volgin YN, et al. Basic results of development of a production technology and control of a pandemic influenza A/H5N1 vaccine. Influenza Other Respir Viruses. 2011;5(1):350–53.
- Sarsenbayeva G, Volgin Y, Kassenov M, Issagulov T, Bogdanov N, Nurpeissova A, Sagymbay A, Abitay R, Stukova M, Khairullin B, et al. A novel preservative-free seasonal influenza vaccine safety and immune response studying in the frame of preclinical research. J Med Virol. 2017;9999:1–6. doi:10.1002/jmv.24771.
- Sarsenbayeva G, Volgin Y, Kassenov M, Issagulov T, Bogdanov N, Sansyzbay A, Abitay R, Nurpeisova A, Sagymbay A, Koshemetov Z, et al. Safety and immunogenicity of the novel seasonal preservative- and adjuvant-free influenza vaccine: blind, randomized, and placebo-controlled trial. J Med Virol. 2017;1–9. doi:10.1002/jmv.24922.
- Sarsenbayeva G, Volgin Y, Kassenov M, Issagulov T, Bogdanov N, Sansyzbay A, Stukova M, Buzitskaya Z, Кulmagambetov I, Davlyatshin T, et al. Immunogenicity and safety of a novel seasonal influenza preservative-free vaccine manufactured in Kazakhstan: results of a randomized, comparative, phase II clinical trial in adults. Hum Vaccin Immunother. 2018;14:609–14. doi:10.1080/21645515.2017.1387345.
- Committee for Medicinal Products for Human Use. Note for guidance on harmonisation of requirements for influenza vaccines. 1997 [accessed 2017 May 9]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. PMID: 12517228. doi:10.1001/jama.289.2.179.
- McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–67. PMID: 22289511. doi:10.1016/j.vaccine.2012.01.015.
- Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. In Vaccines for Pandemic Influenza, RW Compans and WA Orenstein (eds). Curr Top Microbiol Immunol. 2009;333:413–29. doi:10.1007/978-3-540-92165-3_20.
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–69. PMID: 17275144. doi:10.1016/j.vaccine.2007.01.025;.
- Wood JM, Newman RW, Ploss K. The use of correlates of immunity in European Union licensing of influenza vaccines. Dev Biol (Basel). 2003;115:9–16. PMID: 15088770.