 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Children aged under 5 years are particularly vulnerable to influenza infection. In this study, we aim to estimate the number and incidence of influenza among young children and estimate the impact of childhood vaccination in different scenarios from 2013/14 to 2016/17 seasons.
Methods
The number and incidence rate of influenza infections among children aged under 5 years in Beijing was estimated by scaling up observed surveillance data. Then, we used a susceptible–exposed–infected–recovery (SEIR) model to reproduce the weekly number of influenza infections estimated in Beijing during the study seasons, and to estimate the number and proportion of influenza-attributed medically attended acute respiratory infections (I-MAARI) averted by vaccination in each season. Finally, we evaluated the impact of alternative childhood vaccination programs with different coverage and speed of vaccine distribution.
Results
The estimated average annual incidence of influenza in children aged under 5 years was 33.9% (95% confidence interval (CI): 27.5%, 47.2%) during the study period. With the actual coverage during the included seasons at around 2.9%, an average of 3.9% (95%CI: 3.5%, 4.4%) I-MAARI was reduced compared to a no-vaccination scenario. Reaching 20%, 40%, 50%, 60%, 80% and 100% vaccine coverage would lead to an overall I-MAARI reduction of 25.3%, 42.7%, 51.9%, 57.0%, 65.3% and 71.2%. At 20% coverage scenario, an average of 28.8% I-MAARI will be prevented if intensive vaccination implemented in 2 months since the vaccine released.
Conclusion
In Beijing, the introduction of a program for vaccinating young children, even at relatively low vaccine coverage rates, would considerably reduce I-MAARI, particularly if the vaccines can be quickly delivered.
KEYWORDS:
1. Introduction
Influenza causes substantial morbidity and mortality annually, and young children are particularly vulnerable.Citation1 The highest incidence of influenza infection is reported in young children globally.Citation1,Citation2 In the United States, influenza causes medical visits among 6–12% of children younger than 5 years of age each year.Citation3 In addition, influenza leads to a substantial burden of hospitalization in children, particularly infants.Citation4,Citation5 In China, for example, the influenza-associated severe acute respiratory infection (SARI) hospitalization rate was estimated to 3,600–3,800 per 100,000 per year among children from 6 to 11 months.Citation6
The World Health Organization (WHO) and Chinese Center for Diseases Prevention and Control (Chinese CDC) prioritized children for influenza vaccination.Citation7,Citation8 However, young children’s influenza vaccination has not been included in national immunization programs of China. Beijing immunization registry data showed that the vaccination coverage among children aged under 5 years was stable at a low level of 3–4% (unpublished data) in recent years. This study aimed to evaluate the impact of prevailing coverage and predict the potential childhood vaccination programs for children aged under 5 years in Beijing, China.
2. Methods
A mathematical infectious disease transmission model was used to estimate the number and proportion of influenza infections and medical encounters averted by varying vaccination coverage and vaccine distribution time.
2.1. Data sources
2.1.1. Epidemiological data
Epidemiological data were derived from influenza-like illness (ILI) surveillance database in Beijing. In this surveillance, the WHO definition of ILI is used. All the level one, two, and three public hospitals in Beijing conduct the surveillance, reporting the number of ILI cases visited to the hospital every day. Among them, doctors from 24 hospitals collect throat swabs from ILI cases who were onset within 3 days for influenza virus testing. The sampled ILI cases are selected by doctors considering the distribution of age group and the day of the medical visit. Around 20 samples are collected from each hospital weekly.Citation9
In this study, for children aged under 5 years in Beijing, number of ILI cases, proportion of ILI cases been sampled, number of laboratory-confirmed influenza among sampled ILI cases were obtained from existing ILI surveillance database.
Population data for incidence rate denominators were acquired from Beijing statistical yearbook.Citation10
2.1.2. Vaccination data
The weekly numbers of influenza vaccines administered were taken from an online immunization registry system that documents the information of persons who have received influenza vaccines in Beijing. Vaccination coverage was calculated using a number of vaccinated children divided by the population size of children aged under 5 years in Beijing.
The vaccine effectiveness (VE) in our model distinguished between subtypes and seasons. For VE estimates of past seasons, we relied on the published data from Beijing CDCCitation11,Citation12 (). The age-specific VE estimates were used when data were available; otherwise, the overall VE estimates were used instead. Published dataCitation11,Citation12 showed that the vaccine provided no protection against influenza B in the 2014/15 season or against any subtype in the 2015/16 season in Beijing, but a study from other countries showed that the VE for these seasons and subtypes was low to modest.Citation13 Considering the difference of VE estimates and the aim of modeling the benefit of vaccination, VE estimates for these seasons and subtypes were assumed to be 20% in this study as already used in a previous model.Citation14 In this study, vaccine protection during the current season is considered constant and the protection of the previous season is assumed to vanish completely.
Table 1. Season and subtype-specific vaccine effectiveness.
2.2. Estimation of influenza incidence rate
This was carried out in three steps. First, we estimated the number of influenza-attributed medically attended acute respiratory infections (I-MAARI) among children under 5 years old each week. This was done by using the number of ILI cases with a positive test for any influenza virus divided by the proportion of ILI cases sampled for laboratory confirmation of influenza infection. Second, the weekly number of influenza infections among children aged under 5 years were estimated through a multiplier model,Citation15 that is, by scaling up the number of estimated I-MAARI to the whole population. The estimation process and the range of parameters’ values used in the multiplier model can be found in complementary file 1. Third, the incidence of influenza among children aged under 5 years was estimated using the number of influenza infections in each season divided by the corresponding population size.
2.3. The impact of the prevailing vaccination
This was carried out in two steps. Firstly, the season and subtype-specific transmission dynamics are simulated through a modified SEIR modelCitation14 to reproduce the estimated weekly number of children aged under 5 years infected with influenza during 2013/14 through 2016/17. EquationEquations (1)(1)
(1) –(Equation4
(4)
(4) ) of the modified SEIR model are given as
The time unit in the model is week. S(t) represents the susceptible individuals without prior immunity, and in this study, we assumed that all individuals are susceptible to influenza virus. E(t) are the infected in latent state, before onset illness. I(t) are the symptomatic infected, and were acquired from the data estimated by the multiplier model in 2.2. R(t) are recovered or immunized individuals. Nvac (t-2) are the number of people vaccinated 2 weeks ago, as it takes 2 weeks for the vaccine to show effect. The data were acquired from immunization registries. β(t) is the transmission coefficient. As influenza transmission intensity changes during an epidemic season and the duration of each intensity varied, we assumed three different values of β in each season. In our model, the timing of β changes was calculated by models automatically during the process of reproducing the number of weekly infections. σ is the rate at which an exposed individual becomes infectious per unit of time, γ is the rate at which an infectious individual recover per unit of time, k is vaccine effectiveness. The estimated weekly number of infections under prevailing coverage rate was reproduced by fitting the model (EquationEquations (1(1)
(1) )–(Equation4))
(4)
(4) to initial data. Parameters were solved using Runge-Kutta methods during this process. We ran the model for 100 times, and 100 groups of parameters’ estimations can be solved accordingly. Then, goodness-of-fit tests were performed to calculate the R squares and to determine whether the estimated number of infections was well reproduced by models. Secondly, we assumed a situation of no vaccination during these seasons and then estimated the number of infections and I-MAARI using EquationEquations (1)
(1)
(1) -(Equation4
(4)
(4) ), while the parameters of equations have been acquired. The proportion of infections prevented (prevented fraction) and the number of I-MAARI averted of prevailing vaccination coverage relative to no vaccination were used to evaluate the impact of the prevailing vaccination situation.
2.4. The impact of alternative childhood vaccination scenarios
We assumed that childhood vaccination programs are implemented to increase the vaccine coverage, and analyses were conducted to estimate the averted number and fractions of I-MAARIs according to different vaccine coverage levels and under varying speed of vaccine delivery assumptions. To test scenarios with different vaccine coverage levels, we scaled up the observed number of vaccinated persons under 5 years old within the first 3 months since vaccine released to make the end-of-season uptake rate reach 20%, 40%, 50%, 60%, 80%, and 100%, respectively, while the situations of other age groups were not considered. This is assumed because of immunization registry data suggested that over 90% of the prevailing vaccine coverage was reached within 3 months from the start of seasonal vaccine availability. Then, we assumed a faster vaccination scenario, which is intensive vaccination implemented during the first 2 months since vaccine released, and the end-of-season uptake rate is 20% (). The faster vaccination scenario is determined based on the current free vaccination policy targeting school-students and the elderly in Beijing, which offered free vaccination for about 2 months each season.
Figure 1. Number of estimated influenza infections among children aged under 5 years and number of children vaccinated under different scenarios, 2013/14 season through 2016/17 season. (a) Observed number of children vaccinated and number of modeled influenza infections. (b) Observed and assumed number of children vaccinated under different scenarios.
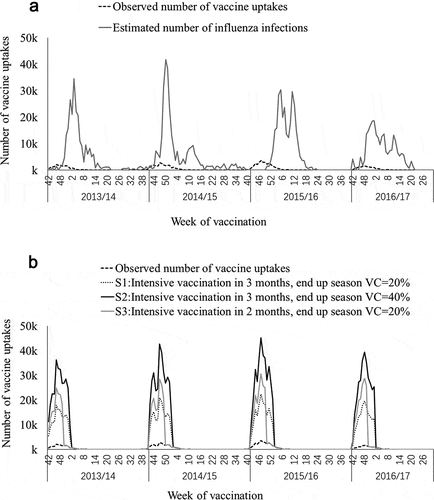
3. Results
3.1. Incidence of influenza infection
It is estimated that each I-MAARI represented 7.33 (95% confidence interval [CI]: 5.95–10.22) influenza infections among children aged under 5 years. Across the four seasons, the estimated average annual number of influenza infections among children aged under 5 years was 277,000 (95% CI: 225,000, 387,000), corresponding to an incidence of 33.9% (95% CI: 27.6% to 47.3%). The incidence was similar across the four seasons, with the lowest incidence, 31.8% (95% CI: 25.8%, 44.4%) observed in 2015/16 season and the highest, 36.8% (95% CI: 29.9%, 51.3%) observed in 2016/17 season (). Influenza A contributed to 68.2%, 72.5%, 47.1%, and 99.1% of infections per season, respectively, while influenza B accounted for 31.8%, 27.5%, 52.9% and 0.9% of infections, respectively, during the four seasons.
Table 2. Estimates of the number and incidence rate (%) of influenza among children aged under 5 years by season, Beijing, 2013–2017.
3.2. Cases averted by prevailing vaccination
The estimated seasonal vaccine coverage in children aged under 5 years was 2.8%, 3.2%, 3.6% and 2.0% in each of the four seasons, respectively. Estimated and modeled weekly counts of influenza infections are compared in . The R squares of goodness-of-fit tests by subtype and by season were shown in complementary file 2. The estimated weekly infections from scaled surveillance data were well reproduced by the model except for the 2015/16 season. In 2015/16 season, the estimated number of influenza infections decreased during week 8 to week 11 in 2016, because winter holidays for pre-school children fall on these weeks, and reduced the virus transmission. However, the modeled infections in those weeks increased, because the component of the school-closure effect was not integrated into the model. The parameters’ estimates by subtype and by season were shown in complementary file 3.
Figure 2. Estimated total weekly influenza infections and model simulations of children aged under 5 years in Beijing, China. The first column shows the estimated total number of influenza infections calculated by scaling up observed surveillance data. The second column provides model-simulated numbers of influenza infection by subtype.
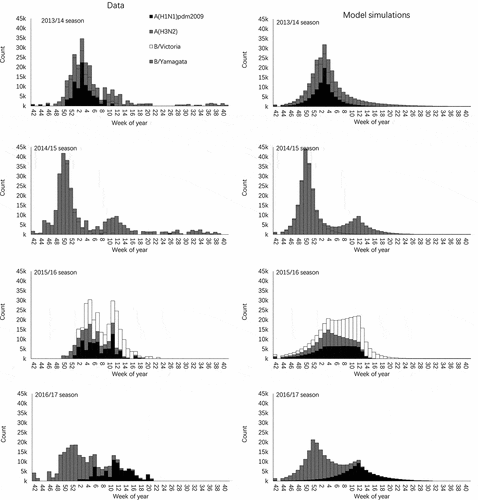
Across the four modeled seasons, under the prevailing vaccination coverage, an average of 1500 (95% CI: 1300, 1700) I-MAARI per season were prevented in children aged under 5 years, which corresponds to a 3.9% (95% CI: 3.5%, 4.4%) reduction compared to a no-vaccination scenario. In fact, although the influenza vaccine coverage was similar across seasons, the 2013/14 season had the largest fraction of cases averted by vaccination (5.3%, 95% CI: 4.6%, 5.4%), while the lowest, 2.5% (95% CI: 2.0%, 3.0%), was observed in 2014/15 season. The number and fraction of I-MAARI averted by season are shown in and .
Table 3. Averted number of influenza-attributable medically attended acute respiratory infections (I-MAARI) under different vaccination coverage and delivery scenarios.
Table 4. Prevented fraction under different vaccination coverage and delivery scenarios.
3.3. Impact of alternative childhood vaccination program
If a vaccination program targeting children under 5 years old is launched, the model estimated an average of 25.3%, 42.7%, 51.9%, 57.0%, 65.3% and 71.2% I-MAARI averted in 20%, 40%, 50%, 60%, 80% and 100% vaccination coverage scenarios ().
If intensive vaccination implemented in 2 months since the start of vaccines released and the end-of-season coverage rate reach 20%, an estimated 28.8% I-MAARI can be averted. Rapid vaccine distribution was estimated to produce a greater prevented number and fraction of I-MAARI than 20% coverage under the standard delivery rate ().
The number of infections averted per vaccine dose decreased with the coverage rate. Comparing between 2-month and 3-month vaccination scenarios at 20% coverage rate, the rapid vaccination gets higher cost-effectiveness, through which 6 more infections in every 100 vaccinations can be averted (Complementary file 4).
4. Discussion
Our study estimated that the annual influenza attack rate among children under 5 years was 33.9% in Beijing, through 2013/14 to 2016/17 seasons. This was 17 times higher than the estimates among adults in Beijing (34% vs 2% in 2013/14 season),Citation16 and highlighting the need for vaccinating the young children in priority. However, presently, young children’s influenza vaccination is not included into the Chinese National Immunization Program (NIP), nor covered by Beijing provincial subsidized vaccination policy, indicating the gap between policy and practice. A young children’s influenza vaccination program is needed in the future.
To design a childhood vaccination program in the future, it is worth learning from the experience of a free-of-charge influenza vaccination policy targeting school-students and the old people≥60 years old in Beijing. With the support of this policy, the influenza vaccination coverage rate reaches around 50% in the targeting groups. If children under 5 years old can be covered by this policy in the future, a similar coverage rate at 50% might be expected. According to our estimation by the model, when vaccine coverage reached 50%, an average of 1.7 thousand infections which is equivalent to over 50% I-MAARI can be prevented even though the VE is sub-optimal.
Our study also simulated the effects of rapid vaccination. We found that if most vaccines can be administered within the first 2 months rather than 3 months under 20% end-of-season coverage scenario, prevented fraction may increase by up to 5%; thus, the earlier-maximum implementation of vaccination has a greater effect on an averted fraction of infections under the fixed VE assumption.Citation17 However, given the evidence for the waning of vaccine-derived protection,Citation18 whether and to what extent the earlier vaccination is beneficial needs further study.
Our study has several limitations. First, as children under 6 months are not eligible for the influenza vaccine, the target population is children from 6 months to 5 years. We were only able to obtain data on population size and medical attendances in the 0–5 year age band, which includes the <6 month age group. Therefore, the number of averted I-MAARI may be overestimated. Secondly, this study showed the impact of childhood vaccination only on preventing infection in young children. However, many studies showed that adults could indirectly benefit from vaccination of childrenCitation7,Citation19–Citation21 through herd immunity. So the epidemiological benefit of young children vaccination may be greater than we have estimated. An age-structured model may better estimate the impact of the vaccination program. Third, VE is an important factor that affects the impact of vaccination. However, we only test the results in the context of constant VE. Although studies have shown the waning of VE within a single season,Citation18,Citation22 we did not take this into consideration when making the estimation, as the degree of waning appears different between seasons, subtypes and vaccinees based on current findings.Citation23 Therefore, we are not able to conclude an optimal timing of vaccination at this stage.
5. Conclusions
Our results showed that influenza infection among young children aged under 5 years represents a substantial burden to public health. Vaccinating young children is associated with a reduction in the burden of pediatric influenza. Furthermore, the averted number of cases is larger with rapid vaccination delivery under a fixed vaccination coverage rate and VE. In conclusion, our results suggest a public health benefit of introducing a childhood influenza immunization program in Beijing.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (285.8 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21645515.2019.1705692.
Additional information
Funding
References
- Biezen R, Grando D, Mazza D, Brijnath B. Why do we not want to recommend influenza vaccination to young children? A qualitative study of Australian parents and primary care providers. Vaccine. 2018;36(6):859–65. doi:10.1016/j.vaccine.2017.12.066.
- Jackson ML, Phillips CH, Benoit J, Jackson LA, Gaglani M, Murthy K, McLean HQ, Belongia EA, Malosh R, Zimmerman R, et al. Burden of medically attended influenza infection and cases averted by vaccination - United States, 2013/14 through 2015/16 influenza seasons. Vaccine. 2018;36(4):467–72. doi:10.1016/j.vaccine.2017.12.014.
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355(1):31–40. doi:10.1056/NEJMoa054869.
- Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68(4):363–71. doi:10.1016/j.jinf.2013.11.013.
- Wu P, Presanis AM, Bond HS, Lau EHY, Fang VJ, Cowling BJ. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998–2013. Sci Rep. 2017;7(1):929. doi:10.1038/s41598-017-01021-x.
- Yu H, Huang J, Huai Y, Guan X, Klena J, Liu S, Peng Y, Yang H, Luo J, Zheng J, et al. The substantial hospitalization burden of influenza in central China: surveillance for severe, acute respiratory infection, and influenza viruses, 2010–2012. Influenza Other Respir Viruses. 2014;8(1):53–65. doi:10.1111/irv.2013.8.issue-1.
- Feng L, Yang P, Zhang T, Yang J, Fu C, Qin Y, Zhang Y, Ma C, Liu Z, Wang Q, et al. Technical guidelines for the application of seasonal influenza vaccine in China (2014–2015). Hum Vaccin Immunother. 2015;11(8):2077–101. doi:10.1080/21645515.2015.1027470.
- World Health Organization. Influenza (Seasonal). [accessed 2019 Oct 10] http://www.who.int/mediacentre/factsheets/fs211/en/.
- Yang P, Thompson MG, Ma C, Shi W, Wu S, Zhang D, Wang Q. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012–2013 season in Beijing, China. Vaccine. 2014;32(41):5285–89.
- Beijing Municipal Bureau of Statistics & Survey Office of the National Bureau of Statistics in Beijing. Beijing statistical yearbook. [accessed 2019 Oct 10] http://www.bjstats.gov.cn/nj/main/2016-tjnj/zk/indexch.htm.
- Qin Y, Zhang Y, Wu P, Feng S, Zheng J, Yang P, Pan Y, Wang Q, Feng L, Pang X, et al. Influenza vaccine effectiveness in preventing hospitalization among Beijing residents in China, 2013–15. Vaccine. 2016;34(20):2329–33. doi:10.1016/j.vaccine.2016.03.068.
- Zhang Y, Wu P, Feng L, Yang P, Pan Y, Feng S, Qin Y, Zheng J, Puig-Barberà J, Muscatello D, et al. Influenza vaccine effectiveness against influenza-associated hospitalization in 2015/16 season, Beijing, China. Vaccine. 2017;35(23):3129–34. doi:10.1016/j.vaccine.2017.03.084.
- Castilla J, Martinez-Baz I, Navascues A, Casado I, Aguinaga A, Diaz-Gonzalez J, Delfrade J., Guevara M. Comparison of influenza vaccine effectiveness in preventing outpatient and inpatient influenza cases in older adults, northern Spain, 2010/11 to 2015/16. Euro Surveill. 2018;23(2):pii=16-00780.
- Zhang Y, Cao Z, Costantino V, Muscatello DJ, Chughtai AA, Yang P, Wang Q. Influenza illness averted by influenza vaccination among school year children in Beijing, 2013–2016. Influenza Other Respir Viruses. 2018;12(6):687–94.
- Wu S, VAN Asten L, Wang L, McDonald SA, Pan Y, Duan W, Zhang L, Sun Y, Zhang Y, Zhang X, et al. Estimated incidence and number of outpatient visits for seasonal influenza in 2015–2016 in Beijing, China. Epidemiol Infect. 2017;145(16):3334–44. doi:10.1017/S0950268817002369.
- Wang X, Wu S, Yang P, Li H, Chu Y, Tang Y, Hua W, Zhang H, Li C, Wang Q, et al. Using a community based survey of healthcare seeking behavior to estimate the actual magnitude of influenza among adults in Beijing during 2013–2014 season. BMC Infect Dis. 2017;17(1):120. doi:10.1186/s12879-017-2217-z.
- Lee S, Golinski M, Chowell G. Modeling optimal age-specific vaccination strategies against pandemic influenza. Bull Math Biol. 2012;74(4):958–80. doi:10.1007/s11538-011-9704-y.
- Puig-Barbera J, Mira-Iglesias A, Tortajada-Girbes M, Lopez-Labrador FX, Librero-Lopez J, Diez-Domingo J, Carballido-Fernández M, Carratalá-Munuera C, Correcher-Medina P, Gil-Guillén V, et al. Waning protection of influenza vaccination during four influenza seasons, 2011/2012 to 2014/2015. Vaccine. 2017;35(43):5799–807. doi:10.1016/j.vaccine.2017.09.035.
- Rose MA, Damm O, Greiner W, Knuf M, Wutzler P, Liese JG, Krüger H, Wahn U, Schaberg T, Schwehm M, et al. The epidemiological impact of childhood influenza vaccination using live-attenuated influenza vaccine (LAIV) in Germany: predictions of a simulation study. BMC Infect Dis. 2014;14:40. doi:10.1186/1471-2334-14-40.
- Weidemann F, Remschmidt C, Buda S, Buchholz U, Ultsch B, Wichmann O. Is the impact of childhood influenza vaccination less than expected: a transmission modelling study. BMC Infect Dis. 2017;17(1):258. doi:10.1186/s12879-017-2344-6.
- Pitman RJ, Nagy LD, Sculpher MJ. Cost-effectiveness of childhood influenza vaccination in England and Wales: results from a dynamic transmission model. Vaccine. 2013;31(6):927–42. doi:10.1016/j.vaccine.2012.12.010.
- Ferdinands JM, Fry AM, Reynolds S, Petrie J, Flannery B, Jackson ML, Belongia EA. Intraseason waning of influenza vaccine protection: evidence from the US influenza vaccine effectiveness network, 2011-12 through 2014–15. Clin Infect Dis. 2017;64(5):544–50.
- Young B, Sadarangani S, Jiang L, Wilder-Smith A, Chen MI. Duration of influenza vaccine effectiveness: a systematic review, meta-analysis, and meta-regression of test-negative design case-control studies. J Infect Dis. 2018;217(5):731–41. doi:10.1093/infdis/jix632.
