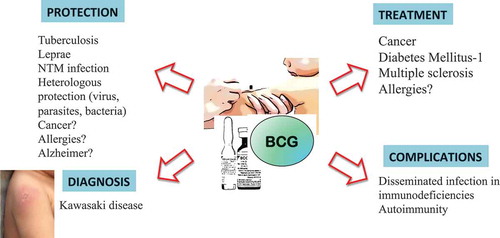
ABSTRACT
BCG has been recommended because of its efficacy against disseminated and meningeal tuberculosis. The BCG vaccine has other mechanisms of action besides tuberculosis protection, with immunomodulatory properties that are now being discovered. Reports have shown a significant protective effect against leprosy. Randomized controlled trials suggest that BCG vaccine has beneficial heterologous (nonspecific) effects on mortality in some developing countries. BCG immunotherapy is considered the gold standard adjuvant treatment for non-muscle-invasive bladder cancer. BCG vaccine has also been tested as treatment for diabetes and multiple sclerosis. Erythema of the BCG site is recognized as a clinical clue in Kawasaki disease. BCG administration in the immunodeficient patient is associated with local BCG disease (BCGitis) or disseminated BCG disease (BCGosis) with fatal consequences. BCG administration has been associated with the development of autoimmunity. We present a brief review of the diverse facets of the vaccine, with the discovery of its new modes of action providing new perspectives on this old, multifaceted and controversial vaccine.
Introduction
In 1908 Albert Calmette, a bacteriologist and Camille Guerin, a veterinarian, working at the Pasteur Institute, began an experiment to develop a vaccine by attenuating a bovine strain; Mycobacterium bovis until it lost its virulence.Citation1 In 1921, after 13 years, they finally introduced the vaccine obtained by serial subculturing on glycerinated bile potato medium (231 passages). The strain, known today as bacille Calmette-Guerin (BCG), not only it lost its virulence for calves and guinea pigs but both also conferred protection against virulence challenge and induced a certain degree of tuberculin sensitivity.Citation1 The first dose of BCG was given orally, and this route continued until 1924 when it was replaced with more immunogenic routes,Citation1 however in Brazil the vaccine continued to be administered orally until the 70s when it was replaced by the intradermal vaccine.Citation2 Currently the World Health Organization (WHO) recommends intradermal injection of BCG on the deltoid region,Citation3 although some physicians in Brazil still prescribe the oral vaccine until this day.Citation2
Over 4 billion doses administered make BCG the most widely used vaccine worldwide.Citation4 Policies and practices vary across the world, with some countries, such as the United Kingdom that have had universal BCG vaccination programs, while others (including Canada, Italy, Belgium, the Netherlands and the United States) that either recommended BCG only for high-risk groups or did not advocate BCG for any purpose.Citation5 BCG vaccination policies have varied by the number of doses used, the age at which vaccination was given and the methods used to deliver the vaccine.Citation6
The BCG vaccine has other effects besides tuberculosis (TB) protection, including immunomodulatory properties. It is also definitively harmful in certain immunodeficient patients (). The purpose of this article is to present a narrative review of the diverse facets of the BCG vaccine. Several comprehensive reviews of the different topics presented are available in the literature.Citation7–Citation10 We conducted a search Pubmed, MEDLINE, EMBASE, Web of Science and Scopus using combination of the terms: BCG and protective vaccine, tuberculosis, complications, autoimmunity, immunodeficiency, cancer, Kawasaki disease and heterologous effects. We reviewed the abstracts and retrieved relevant articles. The BCG vaccine has different faces, just like the ancient Roman god Janus; the god of beginnings, gates, transitions, duality and endings, who usually is depicted as having two faces since he looks to the future and to the past, an analogy appropriate with the use of this vaccine.
BCG as a protective vaccine
BCG has been recommended because of its partial protective effect against active tuberculosis, although it has greater efficacy against disseminated and meningeal disease in children than for prevention of pulmonary disease in adults.Citation11,Citation12 It doesn’t protect against primary TB infection or reactivation of latent TB or even Mycobacterium tuberculosis (MTB) person to person transmission spreading.Citation13 However, autopsy studies suggest BCG decreases the size of pulmonary tuberculosis foci and animal models showed a reduction of bacillary burden.Citation14 Recently, epidemiological studies showed that BCG vaccine also protects against TB infection and that BCG has been associated with an improved microbiological response to treatment.Citation11–Citation18
Although the primary goal of BCG vaccines is to protect against TB, reports also showed a significant protective effect against leprosy. The protective properties of the BCG against M. leprae is likely due to T and B-cell cross-reactivity of the mycobacterial antigens.Citation19 BCG vaccine also protects against nontuberculous mycobacterial lymphadenitis and Buruli ulcer.Citation20
Heterologous effects of BCG immunization
The immune memory response to previously encountered pathogens can sometimes alter the response to and the course of infection of an unrelated pathogen by a process known as heterologous, nonspecific immunity. This phenomenon has been observed with BCG. The WHO Special Advisory Group systematic review showed that neonatal BCG vaccination is associated with a 30% reduction in all-cause mortality (RR 0.70; CI 95%, 0.49–1.01).Citation21 Randomized controlled trials suggest that BCG vaccine also has beneficial heterologous effects on mortality in developing countries.Citation21–Citation29 A similar effect on all-cause mortality was documented in the UK and the USA following the introduction of universal BCG immunization for the prevention of TB in the 1950s. The benefit of BCG vaccination is conferred as early as 7 days of life.Citation27 Studies have reported a clinical benefit with BCG vaccination in the prevention of pneumonia.Citation25 and the risk of pneumonia related deaths by 50%.Citation30
Adaptive features of innate immunity described as trained immunity have been documented after BCG vaccination.Citation23 BCG induces trained immunity and nonspecific protection from infection through epigenetic reprogramming of innate immune cells, with the effect lasting up to one year.Citation31,Citation32 BCG vaccine induces an increase in proinflammatory cytokine production by NK cells in response to mycobacteria and unrelated pathogens with a memory-like effect.Citation32 Important lines of evidence from experimental studies in mice indicate that BCG protects against viral pathogens.Citation33 In a recent review, studies supporting the nonspecific beneficial effects for leishmaniasis and malaria are described.Citation34 A beneficial effect of BCG on vaccine responses has also been reported.Citation22
The presence of a maternal BCG scar is associated with an increased proinflammatory immune response in infants.Citation35 BCG-vaccinated infants have decreased infectious illness unrelated to BCG during the first three months of life if the mother had a history of BCG vaccination.Citation36Animal studies have shown conflicting results of BCG modulating atherosclerosis development,Citation36–Citation38 in a mouse hyperlipidemic model, BCG reduces plasma non-HDL cholesterol levels and delays atherosclerotic lesion formation.Citation38 This effect is a result of accelerated hepatic uptake of cholesterol enriched lipoprotein remnants, reduced intestinal cholesterol absorption and reduced foam cell formation.Citation38
BCG vaccination prevents hyperoxic lung injury in a neonatal rat model,Citation39 in this study, BCG improved alveolar surface area and fibrosis compared with hyperoxia-exposed placebo group, and the protective effects were attributed to preserved IL-13 expression levels and upregulation of NFkB1, FGF.BP1 and VEGF-A genes.Citation39
The concept of a protective effect of BCG against cancer is not new.Citation40–Citation45 BCG immunization may have a protective effect against cancer in later life by enhancing the development of the immune system and there are a number of experimental studies in animals showing the inhibitory effect of BCG on tumor growth.Citation40,Citation41 A recent meta-analysis suggests that early vaccination with BCG might be associated with a reduced risk of leukemia,Citation45 and a retrospective review showed that childhood BCG vaccination was associated with a lower risk of lung cancer development.Citation46 Prior immunization with BCG has been associated with prolongation of survival in patients who later contract melanoma.Citation43 BCG vaccination prevents diabetes in non-obese diabetic mice, however no evidence of prevention of diabetes has been found in case-control studies in humans.Citation47–Citation50 BCG vaccination has also been hypothesized to prevent Alzheimer disease derived from studies in a mouse model.Citation51,Citation52
BCG as a diagnostic tool
BCG vaccination usually results in an ulcer that develops into a characteristic scar, and a previous scar can be used as evidence of past vaccination. Tuberculin conversion usually takes place within 6–8 weeks after BCG vaccination.Citation53 BCG scars and a positive tuberculin skin test (TST) reaction are easily measurable markers of BCG-induced cell-mediated immunity.Citation54 BCG induced TST reactivity has been considered a surrogate of protective immunity by some authors.Citation55,Citation56 The presence of a scar could be important since having a scar and a TST response after BCG vaccination has been associated with lower mortality risk.Citation57–Citation59 Furthermore, some researchers have suggested that revaccination of scar-negative children should be considered.Citation60 However other groups consider that the presence of a scar does not diagnose protection against tuberculosis and failure to develop a scar might be an indication of poor technique;Citation61 this is still a controversial issue.
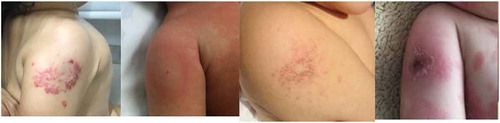
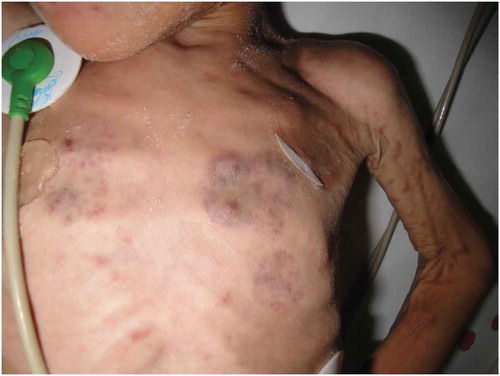
BCG can provide an indirect diagnostic tool in Kawasaki disease (KD), an acute febrile illness that affects young children. with most cases diagnosed before 5 years of age. KD is a challenging diagnosis that relies on clinical signs and symptoms. Interestingly, erythema and induration of the BCG site is increasingly recognized as a significant clinical clue described by Kawasaki for the first time in 1970.Citation62 To this day, BCG site erythema has been considered to be an important specific sign of the disease, particularly in infants.Citation63,Citation64 (). Kakisaka et al. report an 11 month-old boy with redness on the BCG inoculation site reported as Human Herpes Virus type 6 infection (although the patient presented many features of KD), and recently, BCG erythema was observed in a 7 month-old infant with measles without KD.Citation65,Citation66 A survey conducted in Japan identified BCG erythema in 49.9% of KD patients, with a particularly high prevalence in more than 70% of children aged 3–20 months-old.Citation67 In Mexico, among 399 patients, only 24.3% presented BCG reaction in the inoculation site, probably due to ethnic differences or type of BCG vaccine used.Citation68,Citation69 The interval from BCG vaccination to the onset of KD seems to be important (less than 806 months after vaccination).Citation69 Tseng et al. studied the BCG reaction pattern and found that the intensity of the reaction (with the more severe form described as “bull’s eye pattern”) correlated with the severity of the illness coronary abnormalities and elevated aspartate aminotransferase. These authors suggested it as a potential biomarker of the disease.Citation70 BCG erythema in KD is histologically characterized by infiltration of T cells and macrophages with expression of IL-1β, TNF-α, IFN-γ and IL-2 suggestive of a delayed hypersensitivity reaction.Citation71,Citation72 The BCG reactivation in KD has been hypothetically attributed to cross-reactivity between mycobacterial heat shock protein (hsp) 65 and a human homolog hsp63.Citation73 Autoantibodies to peroxiredoxin have been found in KD, and a homolog of peroxiredoxin is present in BCG.Citation74 BCG immunization has been used in animal models to induce coronary arteritis.Citation75,Citation76 Kadowaki et al. were the first to describe erythema and induration in both the BCG and PPD inoculation site in KD.Citation77 Based on this observation, the tuberculin skin test has been suggested to be of value in the diagnosis of incomplete forms of KD.Citation78 It was reported from Italy that 11 patients with Kawasaki disease had a positive tuberculin intradermal test compared to controls.Citation79 However, in a later study in the US, nine patients with Kawasaki disease showed no reaction to intradermal tuberculin, and the authors attribute the difference in results to the use of different tuberculin products.Citation80 The consensus is that erythema in BCG vaccination site is a specific and useful sign in KD particularly in infants.Citation81
BCG as treatment
BCG immunotherapy is considered the gold standard adjuvant treatment for non-muscle-invasive bladder cancer with more than 3 million cancer treatments annually.Citation82–Citation84 The exact mechanism of action of intravesical BCG is not completely understood but it is believed that the antitumor activity of BCG is derived from a local nonspecific immunological boost that recruits immunocompetent cells.Citation85 BCG induces tumor cells in the bladder to secrete cytokines and chemokines and to be subsequently killed by cytotoxic cells. BCG also increases the expression of MHC II molecules and ICAM-1 in tumor cells after instillation.Citation86–Citation90
BCG is also listed as an intralesional treatment for inoperable stage III in-transit melanoma in the National Comprehensive Cancer Network Guidelines. Reports of 50% regression of injected lesions and 17% regressions of non-injected lesions in immunocompetent patients have been reported, with a role of γδT cells in destroying the malignant cellsCitation91
In the late 1990s epidemiological studies reported a decreased prevalence of allergic asthma in children immunized with BCG vaccine.Citation92 A prospective cohort study assessed that BCG vaccination improved lung function while reducing the use of rescue medications in patients with asthma compared with placebo.Citation93 This hygiene hypothesis states that BCG neonatal vaccination alleviates the symptoms of asthma in both animal models and human beings by increasing the secretion of Th1 cytokines.Citation93
BCG exerts neuroprotection in a mouse model of Parkinson´s disease and experimental autoimmune encephalomyelitis.Citation94–Citation97
Neonatal BCG vaccination alleviates LPS induced neurobehavioral impairment and neuroinflammation in mice,Citation94 two studies have suggested that BCG decrease activity in Magnetic Resonance Imaging (MRI) in relapsing-remitting Multiple Sclerosis (MS).Citation98,Citation99 Clinically Isolated Syndrome (CIS) is the first symptomatic neurologic episode caused by demyelination in the CNS, which may or may not be a precursor of MS (the conversion rate from CIS to MS rates ranges from 30% to 82%).Citation100,Citation101 Ristori et al. reported results of a clinical trial of BCG vaccine in patients with CIS.Citation100 Following a single dose of BCG or placebo participants underwent monthly MRI and they found that the mean number of total gadolinium enhancing lesions was substantially lower in subjects receiving BCG vs. placebo.Citation100 In a recent study, Extended Freeze Drying preparations of BCG (inactivated BCG) was used in the experimental autoimmune encephalomyelitis mouse model and it attenuated central nervous system inflammation via plasmacytoid dendritic cells inducing suppressive IL-10 secreting Tregs.Citation102 To this day, BCG to treat CIS or MS still is not recommended, as its efficacy is not established.Citation101
BCG vaccine has also been suggested as treatment for diabetes,Citation103,Citation104 Faustman et al. demonstrated that the BCG vaccine can lower blood glucose levels to a near normal range in patients with established type I diabetes mellitus and can improve glycemic control in this group of patients.Citation103–Citation105 These effects are attributed to an accelerated glucose consumption in immune cells due to increased glycolysis and reduced oxidative phosphorylation.Citation105,Citation106 Induction of TNF through BCG vaccination has the desired cellular immunologic effects of selective death of insulin autoreactive T cells and expansion of beneficial regulatory T cells.Citation107,Citation108 The immunomodulatory effect of BCG in autoimmune and allergic diseases still awaits definitive studies.
BCG has also been used to treat recurrent respiratory papillomatosis reducing relapses by modifying the balance of effector and regulatory T cells subsets.Citation109,Citation110 Recently, Intralesional BCG has also been used successfully for recurrent multiple warts.Citation111
Complications of BCG immunization
The risks of BCG immunization are attributable mainly to the fact that it is a live, attenuated vaccine. Immunization leads to a usually asymptomatic but bacteremic infection and within 8–12 weeks a cellular immune response to mycobacterial antigens can be detected.Citation112,Citation113 Autopsy studies of BCG-immunized children who have died of other causes show that acid-fast organisms and granulomas are distributed widely in many organs.Citation114
During the early years of BCG use a tragic event was documented, an accident known as the Lübeck disaster occurred in the years 1929 to 1933. In this tragic event, 251 neonates received three oral doses of the BCG vaccine contaminated with MTB; 173 infants developed TB and 72 died.Citation115
The prevalence of BCG-associated complications in the general population has been estimated at around 1 in 2500 vaccines for localized complications (BCGitis) and 1 in 100,000 for disseminated BCGosis. In immunodeficient patients BCG administration is associated with local BCG-disease (BCGitis) or disseminated BCG disease (BCGosis) with fatal consequences ().
BCGosis has been recognized in children with HIV infection,Citation116,Citation117 studies in humans and macaques have suggested that BCG may increase susceptibility to HIV infection through inducing trained immunity and activation of CD4 T cells.Citation118,Citation119 The induction and expansion of CD4+ T cells may serve as targets for HIV replication.Citation120 A particular complication observed in patients with HIV is the presence of BCG-induced immune reconstitution inflammatory syndrome after retroviral therapy.Citation121
Defects impairing cellular immunity, phagocytic function and IFN-γ mediated immunity have been classically associated with BCG vaccine complications.Citation122,Citation123 Data on 349 BCG vaccinated patients with severe combined immunodeficiency revealed BCG complications in 51% of them, with 34% presenting disseminated disease (33,000-fold increase over the general population).Citation123 Involvement of extra regional lymph nodes, skin and lungs was the most common clinical presentation.Citation123 Age at BCG vaccination appeared to be a strong predictor for BCG-associated complications, with patients vaccinated within the first month of life having a higher risk.Citation123 BCGitis and BCGosis are also a feared common complication of patients with chronic granulomatous disease.Citation124,Citation125.
In the mid-1990s a study of patients with idiopathic disseminated BCG disease, inborn errors of immunity to mycobacteria were discovered.Citation126,Citation127 These patients are normally resistant to most other microbes. Susceptibility to mycobacterial disease including BCG has led to a phenotype referred to as Mendelian susceptibility to mycobacterial disease (MSMD).Citation128 Mutations of 14 different genes have shown to cause MSMD (IL12B, IL12RB1, ISG15, TYK2, IRF8, SPPL2A, CYBB, IFNGR1, IFNGR2, STAT1, NEMO, il12rb2, il23r, sspl2A).Citation129,Citation130 Clinical manifestations are highly variable, from severe, fatal, early-onset forms to circumscribed, late-onset forms that improve with age.Citation129 Remarkably, the study of these diseases has permitted the dissection of the essential pathways to control mycobacteria.Citation130 Interestingly, BCG disease appears to protect against environmental mycobacteria illness in patients with IL-12 deficiency or IL-12R deficiency.Citation131 As new inborn errors of immunity are described, new forms of BCG susceptibility and complications are recognized, as recently reviewed with the examples of STAT-1 GOF, APDS1 and APDS2 patients.Citation132
As neonatal BCG is included in the vaccination programs in many countries, it is important to identify and avoid administration of BCG to infants who have immunodeficiencies. To protect these vulnerable patients strategies are to defer the administration of BCG for 2–6 months after delivery or to institute routine newborn screening for severe combined immunodeficiency.Citation130
BCG complication has also been observed when BCG is used to treat diseases. In BCG intravesical therapy for superficial forms of bladder cancer, systemic complications affect roughly 5% of patients and can manifest months or years after the last intravesical BCG instillation.
Autoimmune phenomena after intravesical instillation of BCG are rare but occur, mainly arthritis,Citation133 the most common form is reactive arthritis that is usually asymmetric and predominantly affects large joints. The autoimmune response (conjunctivitis-urethritis-arthritis; Reiter syndrome) after BCG instillation seems to occur particularly in genetically susceptible individuals such as those with HLA-B27 (60%).Citation133–Citation135 Again, a molecular mimicry mechanism has been suggested as the cause for these complications with the mycobacterium heat shock protein proteoglycan (hsp65) sharing homology with a mammalian cartilage proteoglycan link protein.Citation136 It is possible that CD4+ T cells specific for an antigen shared by cartilage and mycobacteria are activated by antigen presenting cells mounting the shared peptide on a class II molecule. Intravesical BCG vaccine administration has also been associated with autoimmune diseases such as Henoch-Schoenlein pupura and Guillain Barre syndrome.Citation137–Citation140
In experimental models BCG can accelerate autoimmune diseases.Citation141 Cases of endophthalmitis, uveitis, vitiligo, meningitis, aortitis, or mycotic aneurysms have been reported as well as central nervous system vasculitis.Citation142–Citation144 BCG administration has also been related to the pathogenesis of Takayasu arteritis or KD.Citation59,Citation145Recently, a study in Finland showed that discontinuing universal BCG vaccination did not changed the incidence rates of KD, however the mean age at diagnosis increased, suggesting that the pathogenesis of KD may be associated with immunological priming by BCG vaccination.Citation146
Concluding remarks and future perspectives
BCG protects young children against miliary TB and tuberculous meningitis, but is less effective in adults, with insufficient and inconsistent protection against pulmonary TB, the major source of TB transmission. BCG does not confer life-long protective immunity against TB.Citation147 There is an ongoing debate related to revaccination of BCG following vaccination in infancy, particularly among TST-negative adolescents as they move into adulthood, the period of highest risk of pulmonary TB.Citation148–Citation151 BCG also protects against Mycobacterium leprae and potentially enhances immunity against a variety of microorganisms through heterologous immunity induction.
The WHO recommendation is to give a single dose to all healthy neonates at birth,Citation152 however infants with severe combined immunodeficiency are asymptomatic at birth and they are a population at risk to develop fatal disseminated BCG infection. Newborn screening programs for primary immunodeficiency diseases enable early diagnosis of potentially affected children and prevent administration of live attenuated vaccines including BCG, but unfortunately these programs are not widely available. This situation compels the medical community to support the implementation of newborn screening programs worldwide and defer the administration of the vaccine until the diagnoses of primary immunodeficiency diseases and HIV are ruled out. This policy is of greatest importance in least developed countries where the burden of TB and HIV is the highest, but the resources for general newborn screening are frequently not available. Postponement of BCG immunization until an age when usually clinical manifestations of an immunodeficiency become apparent is a viable solution.
The emergence of drug-resistant MTB strains and the high prevalence of HIV infection have significantly complicated TB prognosis and treatment. These facts highlight the need for new and more effective vaccines.Citation153 Efforts have been made to develop new vaccines that include subunit vaccines (attenuated viral vector or adjuvanted fusion protein) and whole cell vaccines (genetically attenuated MTB, recombinant BCG, killed MTB or M. vaccae).Citation154–Citation156 Local pulmonary mucosal BCG delivery has proven to reduce TB disease where standard intradermal injection fails in animal models.Citation156
BCG possesses immunomodulatory properties that have only started to be revealed. Different strains of BCG may have different immunomodulatory effects as well as complications, including autoimmune diseases.Citation53,Citation157 While there is still no replacement for the licensed BCG vaccine, the discovery of the new effects and modes of action of this multifaceted and controversial vaccine continues.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We deeply thank Dr. Sergio Rosenzweig for his technical assistance and helpful discussions. This work was endorsed by LAPIN (Louisiana Primary Immunodeficiency Network).
References
- Luca S, Mihaescu T. History of BCG vaccine. Maedica (Buchar). 2013;8:53–58.
- Benévolo-de-Andrade TC, Monteiro-Maia R, Cosgrove C, Castello-Branco LR. BCG Moreau Rio de Janeiro: an oral vaccine against tuberculosis-review. Mem Inst Oswaldo Cruz. 2005;100:459–65. doi:10.1590/S0074-02762005000500002.
- Dagg B, Hockley J, Rigsby P, Ho MM. The establishment of sub-strain specific WHO reference reagents for BCG vaccine. Vaccine. 2014;32:6390–95. doi:10.1016/j.vaccine.2014.09.065.
- Comstock GW. The International tuberculosis campaign: a pioneering venture in mass vaccination and research. Clin Infect Dis. 1994;19:528–40. doi:10.1093/clinids/19.3.528.
- Boman G. The ongoing story of the Bacille Calmette-Guérin (BCG) vaccination. Acta Paediatr. 2016;105:1417–20. doi:10.1111/apa.13585.
- Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG world atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011. doi:10.1371/journal.pmed.1001012.
- Butkeviciute E, Jones CE, Smith SG. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiol. 2018;13:1193–208. Epub 2018 Aug 17. doi:10.2217/fmb-2018-0026.
- Kowalewicz-Kulbat M, Locht C. BCG and protection against inflammatory and auto-immune diseases. Expert Rev Vaccines. 2017;16:1–10. Epub 2017 May 30. doi:10.1080/14760584.2017.1333906.
- Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin Ther. 2013;35:109–14. doi:10.1016/j.clinthera.2013.01.007.
- Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98:347–56. doi:10.1189/jlb.5RI0315-096R.
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi:10.1016/S0140-6736(06)68507-3.
- Awasthi S, Moin S. Effectiveness of BCG vaccination against tuberculous meningitis. Indian Pediatr. 1999;36:455–60.
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. doi:10.1001/jama.1994.03510330076038.
- Sutherland I, Lindgren I. The protective effect of BCG vaccination as indicated by autopsy studies. Tubercle. 1979;60:225–31. doi:10.1016/0041-3879(79)90003-5.
- Soysal A, Millington KA, Bakir M, Dosanjh D, Aslan Y, Deeks JJ, Efe S, Staveley I, Ewer K, Lalvani A. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366:1443–51. doi:10.1016/S0140-6736(05)67534-4.
- Eriksen J, Chow JY, Mellis V, Whipp B, Walters S, Abrahamson E, Abubakar I. Protective effect of BCG vaccination in a nursery outbreak in 2009: time to reconsider the vaccination threshold? Thorax. 2010;65:1067–71. Epub 2010 Oct 28. doi:10.1136/thx.2010.140186.
- Faurholt-Jepsen D, Range N, Praygod G, Jeremiah K, Faurholt-Jepsen M, Aabye MG, Grewal HM, Changalucha J, Witte DR, Andersen AB, et al. BCG protects against tuberculosis irrespective of HIV status: a matched case-control study in Mwanza, Tanzania. Thorax. 2013;68:288–89. doi:10.1136/thoraxjnl-2012-201971.
- Jeremiah K, Praygod G, Faurholt-Jepsen D, Range N, Andersen AB, Grewal HM, Friis H. BCG vaccination status may predict sputum conversion in patients with pulmonary tuberculosis: a new consideration for an old vaccine? Thorax. 2010;65:1072–76. doi:10.1136/thx.2010.134767.
- Eisenhut M, Paranjothy S, Abubakar I, Bracebridge S, Lilley M, Mulla R, Lack K, Chalkley D, McEvoy M. BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by gamma interferon release assay. Vaccine. 2009;27:6116–20. doi:10.1016/j.vaccine.2009.08.031.
- Gomes RR, Antunes DE, Dos Santos DF, Sabino EFP, Oliveira DB, Goulart IMB. BCG vaccine and leprosy household contacts: protective effect and probability to becoming sick during follow-up. Vaccine. 2019;37:6510–17. Epub 2019 Sep 6. doi:10.1016/j.vaccine.2019.08.067.
- Zimmermann P, Finn A, Curtis N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J Infect Dis. 2018;218:679–87. doi:10.1093/infdis/jiy207.
- Higgins JP, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, Martin NK, Sterne JA, Reingold AL. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi:10.1136/bmj.i5170.
- Zimmermann P, Curtis N. The influence of BCG on vaccine responses - a systematic review. Expert Rev Vaccines. 2018;17:547–54. doi:10.1080/14760584.2018.1483727.
- Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans R Soc Trop Med Hyg. 2015;109:52–61. doi:10.1093/trstmh/trv021.
- Nankabirwa V, Tumwine JK, Mugaba PM, Tylleskär T, Sommerfelt H. PROMISE- EBF study group. Child survival and BCG vaccination: a community based prospective cohort study in Uganda. BMC Public Health. 2015;15:175. doi:10.1186/s12889-015-1497-8.
- Holm-Delgado MG, Stuart EA, Black RE. Acute lower respiratory infection among Bacille Calmette-Guérin (BCG)-vaccinated children. Pediatrics. 2014;133(1):e73–81. Epub 2013 Dec 30. doi:10.1542/peds.2013-2218.
- Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ. 2000;321:1435–38.
- Eisenhut M. Enhanced innate immunity as explanation for reduced Mycobacterium tuberculosis infection in Bacillus Calmette-Guérin-immunized children. Am J Respir Crit Care Med. 2013;188:257–58. doi:10.1164/rccm.201301-0060LE.
- Lalvani A, Sridhar S. BCG vaccination: 90 years on and still so much to learn. Thorax. 2010;65:1036–38. doi:10.1136/thx.2010.140996.
- Stensballe LG, Sørup S, Aaby P, Benn CS, Greisen G, Jeppesen DL, Birk NM, Kjærgaard J, Nissen TN, Pihl GT, et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch Dis Child. 2017;102:224–31. doi:10.1136/archdischild-2016-310760.
- Post CL, Victora CG, Valente JG, Leal Mdo C, Niobey FM, Sabroza PC. Prognostic factors of hospital mortality from diarrhea or pneumonia in infants younger than 1 year old. A case-control study. Rev Saude Publica. 1992;26:369–78. Article in Portuguese. doi:10.1590/S0034-89101992000600001.
- Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg. 2015;109:29–35. doi:10.1093/trstmh/tru168.
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol. 2014;155:213–19. doi:10.1016/j.clim.2014.10.005.
- Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019 May 2. pii: S1198–743X(19)30197–1. Epub ahead of print. doi:10.1016/j.cmi.2019.04.020.
- Dos Santos JC, Vilela Teodoro Silva M, Ribeiro-Dias F, Joosten LAB. Non-specific effects of BCG in protozoal infections: tegumentary leishmaniasis and malaria. Clin Microbiol Infect. 2019 Jun 15. pii: S1198–743X(19)30292–7. Epub ahead of print. doi:10.1016/j.cmi.2019.06.002.
- Mawa PA, Webb EL, Filali-Mouhim A, Nkurunungi G, Sekaly RP, Lule SA, Prentice S, Nash S, Dockrell HM, Elliott AM, et al. Maternal BCG scar is associated with increased infant proinflammatory immune responses. Vaccine. 2017;35:273–82. doi:10.1016/j.vaccine.2016.11.079.
- Ovchinnikova OA, Berge N, Kang C, Urien C, Ketelhuth DF, Pottier J, Drouet L, Hansson GH, Marchal G, Back M, et al. Mycobacterium bovis BCG killed by extended freeze-drying induces an immunoregulatory profile and protects against atherosclerosis. J Intern Med. 2014;275:49–58. doi:10.1111/joim.12127.
- Lamb DJ, Eales LJ, Ferns GA. Immunization with bacillus Calmette-Guerin vaccine increases aortic atherosclerosis in the cholesterol-fed rabbit. Atherosclerosis. 1999;143:105–13. doi:10.1016/S0021-9150(98)00284-6.
- van Dam AD, Bekkering S, Crasborn M, van Beek L, van den Berg SM, Vrieling F, Joosten SA, Van Harmelen V, de Winther MPJ, Lutjohann D, et al. BCG lowers plasma cholesterol levels and delays atherosclerotic lesion progression in mice. Atherosclerosis. 2016;251:6–14. doi:10.1016/j.atherosclerosis.2016.05.031.
- Kumral A, İşcan B, Tuzun F, Micili SC, Arslan MK, Tugyan K, Duman N, Ozkan H. Bacillus Calmette-Guerín vaccination: a novel therapeutic approach to preventing hyperoxic lung injury. J Matern Fetal Neonatal Med. 2015;28:1950–56. Epub 2015 Jan 14. doi:10.3109/14767058.2014.973396.
- Weiss DW, Bonhag RS, Deome KB. Protective activity of fractions of tubercle bacilli against isologous tumours in mice. Nature. 1961;190:889–91. doi:10.1038/190889a0.
- Bast RC Jr, Zbar B, Borsos T, Rapp HJ. BCG and cancer (first of two parts). N Engl J Med. 1974;290:1413–20. doi:10.1056/NEJM197406202902506.
- Bast RC Jr., Zbar B, Borsos T, Rapp HJ. BCG and cancer. N Engl J Med. 1974;290:1458–69. doi:10.1056/NEJM197406272902605.
- Davignon L, Robillard P, Lemonde P, Frappier A. B.C.G. vaccination and leukemia mortality. Lancet. 1970;2:638. doi:10.1016/S0140-6736(70)91402-9.
- Morra ME, Kien ND, Elmaraezy A, Abdelaziz OAM, Elsayed AL, Halhouli O, Montas AM, Vu TL, Ho C, Foly AS, et al. Early vaccination protects against childhood leukemia: A systematic review and meta-analysis. Sci Rep. 2017;7:15986. doi:10.1038/s41598-017-16067-0.
- Usher NT, Chang S, Howard RS, Martinez A, Harrison LH, Santosham M, Aronson NE. Association of BCG vaccination in childhood with subsequent cancer diagnoses: a 60-year follow-up of a clinical trial. JAMA Netw Open. 2019 Sep 4;2(9):e1912014. doi:10.1001/jamanetworkopen.2019.12014.
- Kölmel KF, Grange JM, Krone B, Mastrangelo G, Rossi CR, Henz BM, Seebacher C, Botev IN, Niin N, Nambert D, et al. Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European organization for research and treatment of cancer cohort study on 542 patients. Eur J Cancer. 2005;41:118–25. doi:10.1016/j.ejca.2004.09.023.
- Shehadeh N, Etzioni A, Cahana A, Teninboum G, Gorodetsky B, Barzilai D, Karnieli E. Repeated BCG vaccination is more effective than a single dose in preventing diabetes in non-obese diabetic (NOD) mice. Isr J Med Sci. 1997;33:711–15.
- Parent ME, Siemiatycki J, Menzies R, Fritschi L, Colle E. Bacille Calmette-Guérin vaccination and incidence of IDDM in Montreal, Canada. Diabetes Care. 1997;20:767–72. doi:10.2337/diacare.20.5.767.
- Huppmann M, Baumgarten A, Ziegler AG, Bonifacio E. Neonatal bacille Calmette-Guerin vaccination and type 1 diabetes. Diabetes Care. 2005;28:1204–06. doi:10.2337/diacare.28.5.1204.
- Zuo Z, Qi F, Yang J, Wang X, Wu Y, Wen Y, Yuan Q, Zou J, Guo K, Yao ZB. Immunization with bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol Dis. 2017;101:27–39. doi:10.1016/j.nbd.2017.02.001.
- Gofrit ON, Bercovier H, Klein BY, Cohen IR, Ben-Hur T, Greenblatt CL. Can immunization with bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med Hypotheses. 2019;123:95–97. doi:10.1016/j.mehy.2019.01.007.
- Pereira SM, Dourado I, Barreto ML, Cunha SS, Ichiara MY, Hijjar MA, Goes JC, Rodrigues LC. Sensitivity and specificity of BCG scar reading in Brazil. Int J Tuberc Lung Dis. 2001;5:1067–70.
- Lugosi L. Theoretical and methodological aspects of BCG vaccine from the discovery of Calmette and Guérin to molecular biology. A review. Tuber Lung Dis. 1992;73:252–61. doi:10.1016/0962-8479(92)90129-8.
- Shaaban MA, Abdul Ati M, Bahr GM, Standford JL, Lockwood DN, McManus IC. Revaccination with BCG: its effects on skin tests in Kuwaiti seior school children. Eur Respir J. 1990;3:187–91.
- Fjällbrant H, Ridell M, Larsson LO. BCG scar and tuberculin reactivity in children and adults. Scand J Infect Dis. 2008;40:387–92. doi:10.1080/00365540701732905.
- Timmermann CA, Biering-Sørensen S, Aaby P, Fisker AB, Monteiro I, Rodrigues A, Benn CS, Ravn H. Tuberculin reaction and BCG scar: association with infant mortality. Trop Med Int Health. 2015;20:1733–44. doi:10.1111/tmi.12614.
- Garly ML, Martins CL, Balé C, Baldé MA, Hedegaard KL, Gustafson P, Lisse IM, Whittle HC, Aaby P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782–90. doi:10.1016/S0264-410X(03)00181-6.
- Roth A, Gustafson P, Nhaga A, Djana Q, Poulsen A, Garly ML, Jensen H, Sodemann M, Rodrigues A, Aaby P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol. 2005;34:540–47. doi:10.1093/ije/dyh392.
- Fine PE, Sterne JA, Pönnighaus JM, Rees RJ. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet. 1994;344:1245–49. doi:10.1016/S0140-6736(94)90748-X.
- Roth A, Sodemann M, Jensen H, Poulsen A, Gustafson P, Gomes J, Djana Q, Jakobsen M, Garly M-L, Rodrigues A, et al. Vaccination technique, PPD reaction and BCG scarring in a cohort of children born in Guinea-Bissau 2000–2002. Vaccine. 2005;23:3991–98. doi:10.1016/j.vaccine.2004.10.022.
- Kawasaki T. Two cases with acute febrile mucocutaneous lymph node syndrome possibly induced by vaccinia or measles vaccination. Jpn J Pediatr. 1970;23:657–62.
- Gamez-Gonzalez LB, Hamada H, Llamas-Guillen BA, Ruiz-Fernandez M, Yamazaki-Nakashimada M. BCG and Kawasaki disease in Mexico and Japan. Hum Vaccin Immunother. 2017;13:1091–93. doi:10.1080/21645515.2016.1267083.
- García Pavón S, Staines Boone T, Hernández Bautista V, Yamazaki Nakashimada MA. Reactivation of the scar of BCG vaccination in Kawasaki’s disease: clinical case and literature review. Rev Alerg Mex. 2006;53:76–78.
- Kakisaka Y, Ohara T, Katayama S, Suzuki T, Sasai S, Hino-Fukuyo N, Kure S. Human herpes virus type 6 can cause skin lesions at the BCG inoculation site similar to Kawasaki Disease. Tohoku J Exp Med. 2012;228:351–53. doi:10.1620/tjem.228.351.
- Muthuvelu S, Lim KS, Huang LY, Chin ST, Mohan A. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019 Jul 24;19(1):251. doi:10.1186/s12887-019-1635-z.
- Uehara R, Igarashi H, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease patients with redness or crust formation at the bacille Calmette-Guérin inoculation site. Pediatr Infect Dis J. 2010;29:430–33. doi:10.1097/INF.0b013e3181cacede.
- Garrido-García LM, Castillo-Moguel A, Vázquez-Rivera M, Cravioto P, Fernando G. Reaction of the BCG scar in the acute phase of Kawasaki Disease in Mexican children. Pediatr Infect Dis J. 2017;36:e237–e241. doi:10.1097/INF.0000000000001633.
- Araki T, Kodera A, Kitada K, Fujiwara M, Muraoka M, Abe Y, Ikeda M, Tsukahara H. Analysis of factors associated with development of Bacille Calmette-Guérin inoculation site change in patients with Kawasaki disease. J Int Med Res. 2018;46:1640–48. doi:10.1177/0300060518760462.
- Tseng HC, Ho JC, Guo MM, Lo MH, Hsieh KS, Tsai WC, Kuo HC, Lee CH. Bull’s eye dermatoscopy pattern at bacillus Calmette-Guérin inoculation site correlates with systemic involvements in patients with Kawasaki disease. J Dermatol. 2016;43:1044–50. doi:10.1111/1346-8138.13315.
- Sato N, Sagawa K, Sasaguri Y, Inoue O, Kato H. Immunopathology and cytokine detection in the skin lesions of patients with Kawasaki disease. J Pediatr. 1993;122:198–203. doi:10.1016/S0022-3476(06)80113-7.
- Kuniyuki S, Asada M. An ulcerated lesion at the BCG vaccination site during the course of Kawasaki disease. J Am Acad Dermatol. 1997;37(2 Pt 2):303–04. doi:10.1016/S0190-9622(97)80376-3.
- Sireci G, Dieli F, Salerno AT. cells recognize an immunodominant epitope of heat shock protein 65 in Kawasakidisease. Mol Med. 2000;6:581–90. doi:10.1007/BF03401796.
- Fujieda M, Karasawa R, Takasugi H, Yamamoto M, Kataoka K, Yudoh K, Kato T, Ozaki S, Wakiguchi H. A novel anti-peroxiredoxin autoantibody in patients with Kawasaki disease. Microbiol Immunol. 2012;56:56–61. doi:10.1111/j.1348-0421.2011.00393.x.
- Nakamura T, Yamamura J, Sato H, Kakinuma H, Takahashi H. Vasculitis induced by immunization with bacillus Calmette-Guérin followed by atypical mycobacterium antigen: a new mouse model for Kawasaki disease. FEMS Immunol Med Microbiol. 2007;49:391–97. doi:10.1111/j.1574-695X.2007.00217.x.
- Chun JK, Jeon BY, Kang DW, Kim DS. Bacille Calmette Guérin (BCG) can induce Kawasaki disease-like features in programmed death-1 (PD-1) gene knockout mice. Clin Exp Rheumatol. 2011;29:743–50.
- Kadowaki J, Yamaguchi M, Sato S. Three cases suspected as acute febrile mucocutaneous lymph node syndrome: emphasis on the cutaneous changes at the BCG and tuberculin inoculated site in one case. Jpn J Pediatr. 1972;25:901–05.
- Hsu YH, Wang YH, Hsu WY, Lee YP. Kawasaki disease characterized by erythema and induration at the bacillus Calmette-Guérin and purified protein derivative inoculation sites. Pediatr Infect Dis J. 1987;6:576–78. doi:10.1097/00006454-198706000-00020.
- Bertotto A, Spinozzi F, Vagliasindi C, Radicioni M, De Rosa O, Vaccaro R. Tuberculin skin test reactivity in Kawasaki disease. Pediatr Res. 1997;41:560–62. doi:10.1203/00006450-199704000-00017.
- Kollmann TR, Klein EJ, Stefanelli CB, Marcuse EK. Purified protein derivative anergy in Kawasaki disease. Pediatr Infect Dis J. 2001;20:81–82. doi:10.1097/00006454-200101000-00018.
- Chalmers D, Corban JG, Moore PP. BCG site inflammation: a useful diagnostic sign in incomplete Kawasaki disease. J Paediatr Child Health. 2008;44:525. doi:10.1111/j.1440-1754.2008.01364.x.
- Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–83. doi:10.1016/S0022-5347(17)58737-6.
- Meyer JP, Persad R, Gillatt DA. Use of bacille Calmette-Guérin in superficial bladder cancer. Postgrad Med J. 2002;78:449–54. doi:10.1136/pmj.78.922.449.
- Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi:10.1016/S0140-6736(98)07422-4.
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol. 2014;11:153–62. doi:10.1038/nrurol.2014.15.
- Lattime EC, Gomella LG, McCue PA. Murine bladder carcinoma cells present antigen to BCG-specific CD4+ T-cells. Cancer Res. 1992;52:4286–90.
- de Boer EC, Somogyi L, de Ruiter GJ, de Reijke TM, Kurth KH, Schamhart DH. Role of interleukin-8 in onset of the immune response in intravesical BCG therapy for superficial bladder cancer. Urol Res. 1997;25:31–34. doi:10.1007/BF00941903.
- de Reijke TM, Vos PC, de Boer EC, Bevers RF, de Muinck Keizer WH, Kurth KH, Schamhart DH. Cytokine production by the human bladder carcinoma cell line T24 in the presence of bacillus Calmette-Guerin (BCG). Urol Res. 1993;21:349–52. doi:10.1007/BF00296835.
- Thanhäuser A, Böhle A, Flad HD, Ernst M, Mattern T, Ulmer AJ. Induction of bacillus-Calmette-Guérin-activated killer cells from human peripheral blood mononuclear cells against human bladder carcinoma cell lines in vitro. Cancer Immunol Immunother. 1993;37:105–11. doi:10.1007/BF01517042.
- Ibarra C, Karlsson M, Codeluppi S, Varas-Godoy M, Zhang S, Louhivuori L, Mangsbo S, Hosseini A, Soltani N, Kaba R, et al. BCG-induced cytokine release in bladder cancer cells is regulated by Ca2+ signaling. Mol Oncol. 2018. doi:10.1002/1878-0261.12397.
- Yang J, Jones MS, Ramos RI, Chan AA, Lee AF, Foshag LJ, Sieling PA, Faries MB, Lee DJ. Insights into local tumor microenvironment immune factors associated with regression of cutaneous melanoma metastases by Mycobacterium bovis bacille Calmette-Guérin. Front Oncol. 2017;7:61. doi:10.3389/fonc.2017.00061.
- Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275(5296):77–79. doi:10.1126/science.275.5296.77.
- Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002;88:584–91. doi:10.1016/S1081-1206(10)61890-X.
- Yang J, Qi F, Yao Z. Neonatal bacillus Calmette-Guérin vaccination alleviates lipopolysaccharide-induced neurobehavioral impairments and neuroinflammation in adult mice. Mol Med Rep. 2016 Aug;14(2):1574–86. doi:10.3892/mmr.2016.5425.
- Lee J, Reinke EK, Zozulya AL, Sandor M, Fabry Z. Mycobacterium bovis bacille Calmette-Guérin infection in the CNS suppresses experimental autoimmune encephalomyelitis and Th17 responses in an IFN-gamma-independent manner. J Immunol. 2008;181:6201–12. doi:10.4049/jimmunol.181.9.6201.
- Laćan G, Dang H, Middleton B, Horwitz MA, Tian J, Melega WP, Kaufman DL. Bacillus Calmette-Guerin vaccine-mediated neuroprotection is associated with regulatory T-cell induction in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci Res. 2013;91:1292–302. doi:10.1002/jnr.v91.10.
- Sewell DL, Reinke EK, Co DO, Hogan LH, Fritz RB, Sandor M, Fabry Z. Infection with Mycobacterium bovis BCG diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific T cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol. 2003;10(4):564–72. doi:10.1128/cdli.10.4.564-572.2003.
- Paolillo A, Buzzi MG, Giugni E, Sabatini U, Bastianello S, Pozzilli C, Salvetti M, Ristori G. The effect of bacille Calmette-Guérin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS. J Neurol. 2003;250:247–48. doi:10.1007/s00415-003-0967-6.
- Ristori G, Buzzi MG, Sabatini U, Giugni E, Bastianello S, Viselli F, Buttinelli C, Ruggieri S, Colonnese C, Pozzilli C, et al. Use of bacille Calmette-Guèrin (BCG) in multiple sclerosis. Neurology. 1999;53:1588–89. doi:10.1212/WNL.53.7.1588.
- Ristori G, Romano S, Cannoni S, Visconti A, Tinelli E, Mendozzi L, Cecconi P, Lanzillo R, Quaratelli M, Buttorelli C, et al. Effects of bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology. 2014;82:41–48. doi:10.1212/01.wnl.0000438216.93319.ab.
- Bourdette D, Naismith RT. BCG vaccine for clinically isolated syndrome and MS: infections and protective immunity. Neurology. 2014;82:15–16. Epub 2013 Dec 4. doi:10.1212/01.wnl.0000438232.40847.c3.
- Lippens C, Garnier L, Guyonvarc’h PM, Santiago-Raber ML, Hugues S. Extended freeze-dried BCG instructed pDCs induce suppressive tregs and dampen EAE. Front Immunol. 2018;9:2777. doi:10.3389/fimmu.2018.02777.
- Faustman DL, Wang L, Okubo Y, Burger D, Ban L, Man G, Zheng H, Schoenfeld D, Pompei R, Avruch J, et al. Proof-of-concept, randomized, controlled clinical trial of bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS One. 2012;7:e41756. doi:10.1371/journal.pone.0041756.
- Faustman DL. TNF, TNF inducers, and TNFR2 agonists: A new path to type 1 diabetes treatment. Diabetes Metab Res Rev. 2018;34. Epub 2017 Sep 29. doi:10.1002/dmrr.2941.
- Stienstra R, Netea MG. Firing up glycolysis: BCG vaccination effects on type 1 diabetes mellitus. Trends Endocrinol Metab. 2018 Oct. pii: S1043–2760(18)30169–3. doi:10.1016/j.tem.2018.10.001.
- Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, Huang D, Janes S, Defusco A, Baum D, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018 21;3:23. doi:10.1038/s41541-018-0062-8.
- Fenner A. BCG enriches Treg cells. Nat Rev Urol. 2018;15:591. doi:10.1038/s41585-018-0075-0.
- Parkash O, Agrawal S, Madhan Kumar M. T regulatory cells: achilles’ heel of Mycobacterium tuberculosis infection? Immunol Res. 2015;62:386–98. doi:10.1007/s12026-015-8654-0.
- Vetskova EK, Muhtarova MN, Avramov TI, Stefanova TR, Chalakov IJ, Nikolova MH. Immunomodulatory effects of BCG in patients with recurrent respiratory papillomatosis. Folia Med (Plovdiv). 2013;55:49–54. doi:10.2478/folmed-2013-0005.
- Derkay CS, Smith RJ, McClay J, van Burik JA, Wiatrak BJ, Arnold J, Berger B, Neefe JR. HspE7 treatment of pediatric recurrent respiratory papillomatosis: final results of an open-label trial. Ann Otol Rhinol Laryngol. 2005;114:730–37. doi:10.1177/000348940511400913.
- Jaisinghani AK, Dey VK, Suresh MS, Saxena A. Bacillus calmette-guerin immunotherapy for recurrent multiple warts: an open-label uncontrolled study. Indian J Dermatol. 2019;64(2):164. doi:10.4103/ijd.IJD_558_16.
- Kemp EB, Belshe RB, Hoft DF. Immune responses stimulated by percutaneous and intradermal bacille Calmette-Guérin. J Infect Dis. 1996;174:113–19. doi:10.1093/infdis/174.1.113.
- Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis bacillus Calmette-Guérin. J Immunol. 1997;158:1949–55.
- Trevenen CL, Pagtakhan RD. Disseminated tuberculoid lesions in infants following BCG vaccination. Can Med Assoc J. 1982;127:502–04.
- Fox GJ, Orlova M, Schurr E. Tuberculosis in newborns: the Lessons of the “Lübeck Disaster” (1929–1933). PLoS Pathog. 2016;12(1):e1005271. doi:10.1371/journal.ppat.1005271.
- von Reyn CF. Routine childhood bacille Calmette Guérin immunization and HIV infection. Clin Infect Dis. 2006;42:559–61. doi:10.1086/499959.
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, Gie RP, Cotton MF, van Helden PD, Warren RM, et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42:548–58. Epub 2006 Jan 11. doi:10.1086/499953.
- Derrick SC. Trained Immunity and Susceptibility to HIV. Clin Vaccine Immunol. 2017 5;24(1):pii: e00509–16. doi:10.1128/CVI.00509-16.
- Jensen K, Dela Pena-Ponce MG, Piatak M Jr, Shoemaker R, Oswald K, Jacobs WR Jr, Fennelly G, Lucero G, Mollan KR, Hudgens MG, et al. Balancing trained immunity with persistent immune activation and the risk of simian immunodeficiency virus infection in infant macaques vaccinated with attenuated mycobacterium tuberculosis or mycobacterium bovis BCG vaccine. Clin Vaccine Immunol. 2017;24(1):pii: e00360–16. doi:10.1128/CVI.00360-16.
- Thayil SM, Ho YC, Bollinger RC, Blankson JN, Siliciano RF, Karakousis PC, Page KR. Mycobacterium tuberculosis complex enhances susceptibility of CD4 T cells to HIVthrough a TLR2-mediated pathway. PLoS One. 2012;7:e41093. Epub 2012 Jul 23. doi:10.1371/journal.pone.0041093.
- Puthanakit T, Oberdorfer P, Punjaisee S, Wannarit P, Sirisanthana T, Sirisanthana V. Immune reconstitution syndrome due to bacillus Calmette-Guérin after initiation of antiretroviral therapy in children with HIV infection. Clin Infect Dis. 2005;41:1049–52. Epub 2005 Aug 30. doi:10.1086/433177.
- Norouzi S, Aghamohammadi A, Mamishi S, Rosenzweig SD, Rezaei N. Bacillus Calmette-Guérin (BCG) complications associated with primary immunodeficiency diseases. J Infect. 2012;64:543–54. doi:10.1016/j.jinf.2012.03.012.
- Marciano BE, Huang CY, Joshi G, Rezaei N, Carvalho BC, Allwood Z, Ikinciogullari A, Reda SM, Gennery A, Thon V, et al. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. 2014;133:1134–41. doi:10.1016/j.jaci.2014.02.028.
- Conti F, Lugo-Reyes SO, Blancas Galicia L, He J, Aksu G, Borges de Oliveira E Jr, Deswarte C, Hubeau M, Karaca N, de Suremain M, et al. Mycobacterial disease in patients with chronic granulomatous disease: A retrospective analysis of 71 cases. J Allergy Clin Immunol. 2016;138:241–48. doi:10.1016/j.jaci.2015.11.041.
- Bustamante J, Aksu G, Vogt G, de Beaucoudrey L, Genel F, Chapgier A, Filipe-Santos O, Feinberg J, Emile JF, Kutukculer N, et al. BCG-osis and tuberculosis in a child with chronic granulomatous disease. J Allergy Clin Immunol. 2007;120:32–38. doi:10.1016/j.jaci.2007.04.034.
- Lin CJ, Wang SC, Ku CL, Kao JK, Chen M, Liu CS. Successful unrelated cord blood stem cell transplantation in an x-linked chronic granulomatous disease patient with disseminated BCG-induced Infection. Pediatr Neonatol. 2015;56:346–50. doi:10.1016/j.pedneo.2013.04.001.
- Casanova JL, Blanche S, Emile JF, Jouanguy E, Lamhamedi S, Altare F, Stéphan JL, Bernaudin F, Bordigoni P, Turck D, et al. Idiopathic disseminated bacillus Calmette-Guérin infection: a French national retrospective study. Pediatrics. 1996;98(4 Pt 1):774–78.
- Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N Engl J Med. 1996;335:1956–61. doi:10.1056/NEJM199612263352604.
- Rosain J, Kong XF, Martinez-Barricarte R, Oleaga-Quintas C, Ramirez-Alejo N, Markle J, Okada S, Boisson-Dupuis S, Casanova JL, Bustamante J. Mendelian susceptibility to mycobacterial disease: 2014–2018 update. Immunol Cell Biol. 2019;97:360–367.
- Rosenzweig SD. Old vaccines, new diseases: when BCG meets SPPL2a. Nat Immunol. 2018;19:906–07. doi:10.1038/s41590-018-0193-0.
- Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, et al. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–35. doi:10.1084/jem.20021769.
- Nunes-Santos CJ, Rosenzweig SD. Bacille Calmette-Guerin complications in newly described primary immunodeficiency diseases: 2010–2017. Front Immunol. 2018;9:1423. doi:10.3389/fimmu.2018.01423.
- Shoenfeld Y, Aron-Maor A, Tanai A, Ehrenfeld M. Bcg and autoimmunity: another two-edged sword. J Autoimmun. 2001;16:235–40. doi:10.1006/jaut.2000.0494.
- Tinazzi E, Ficarra V, Simeoni S, Artibani W, Lunardi C. Reactive arthritis following BCG immunotherapy for urinary bladder carcinoma: a systematic review. Rheumatol Int. 2006;26:481–88. doi:10.1007/s00296-005-0059-2.
- Ng KL, Chua CB. Reiter’s syndrome postintravesical Bacillus Calmette-Guérin instillations. Asian J Surg. 2017;40:163–65. doi:10.1016/j.asjsur.2014.01.016.
- van Eden W, Holoshitz J, Nevo Z, Frenkel A, Klajman A, Cohen IR. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985;82:5117–20. doi:10.1073/pnas.82.15.5117.
- Hirayama T, Matsumoto K, Tsuboi T, Fujita T, Satoh T, Iwamura M, Ao T, Baba S. Anaphylactoid purpura after intravesical therapy using bacillus Calmette-Guerin for superficial bladder cancer. Hinyokika Kiyo. 2008;54:127–29. Article in Japanese.
- Tsukada H, Miyakawa H. Henoch schönlein purpura nephritis associated with intravesical Bacillus Calmette-Guerin (BCG) therapy. Intern Med. 2017;56:541–544.oi. doi:10.2169/internalmedicine.56.7494.
- Nan DN, Fernández-Ayala M, García-Ibarbia C, Gutiérrez-Santiago M, Hernández JL. Henoch-schönlein purpura after intravesical administration of bacillus Calmette-Guérin. Scand J Infect Dis. 2005;37:613–15. doi:10.1080/00365540510035300.
- Webb K, Venkatesan P. Guillain Barré syndrome associated with bladder instillation of bacille Calmette Guérin (BCG). JMM Case Rep. 2018;5(8):e005164. doi:10.1099/jmmcr.0.005164.
- Engleman EG, Sonnenfeld G, Dauphinee M, Greenspan JS, Talal N, McDevitt HO, Merigan TC. Treatment of NZB/NZW F1 hybrid mice with Mycobacterium bovis strain BCG or type II interferon preparations accelerates autoimmune disease. Arthritis Rheum. 1981;24:1396–402. doi:10.1002/(ISSN)1529-0131.
- Spratt A, Key T, Vivian AJ. Chronic anterior uveitis following bacille Calmette-Guérin vaccination: molecular mimicry in action? J Pediatr Ophthalmol Strabismus. 2008;45:252–53. doi:10.3928/01913913-20080701-15.
- Parent ME, Richer M, Liang P. The first case of bacillus Calmette-Guérin-induced small-vessel central nervous system vasculitis. Clin Rheumatol. 2018 May 9. Epub ahead of print. doi:10.1007/s10067-018-4136-9.
- Donaldson RC, Canaan SA Jr, McLean RB, Ackerman LV. Uveitis and vitiligo associated with BCG treatment for malignant melanoma. Surgery. 1974;76:771–78.
- Kothari SS. Aetiopathogenesis of Takayasu’s arteritis and BCG vaccination: the missing link? Med Hypotheses. 1995;45:227–30. doi:10.1016/0306-9877(95)90109-4.
- Pasma H, Honkila M, Pokka T, Renko M, Salo E, Tapiainen T. Epidemiology of Kawasaki disease before and after universal bacille Calmette-Guérin vaccination program was discontinued. Acta Paediatr. Sep 13 2019. doi:10.1111/apa.15012.
- Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58:470–80. doi:10.1093/cid/cit790.
- Hatherill M, Geldenhuys H, Pienaar B, Suliman S, Chheng P, Debanne SM, Hoft DF, Boom WH, Hanekom WA, Johnson JL, et al. Safety and reactogenicity of BCG revaccination with isoniazid pretreatment in TST positive adults. Vaccine. 2014;32:3982–88. doi:10.1016/j.vaccine.2014.04.084.
- Suliman S, Geldenhuys H, Johnson JL, Hughes JE, Smit E, Murphy M, Toefy A, Lerumo L, Hopley C, Pienaar B, et al. Bacillus Calmette-Guérin (BCG) revaccination of adults with latent mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J Immunol. 2016;197:1100–10. doi:10.4049/jimmunol.1501996.
- Dye C. Making wider use of the world’s most widely used vaccine: bacille Calmette-Guerin revaccination reconsidered. J R Soc Interface. 2013;10:20130365. doi:10.1098/rsif.2013.0365.
- Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, Mabwe S, Makhethe L, Erasmus M, Toefy A, et al. C-040–404 study team. Prevention of M. tuberculosis Infection with H4: IC31Vaccine or BCG revaccination. N Engl J Med. 2018;379:138–49. doi:10.1056/NEJMoa1714021.
- World Health Organization. BCG vaccine: WHO position paper, February 2018 - recommendations. Vaccine. 2018;36:3408–10. doi:10.1016/j.vaccine.2018.03.009.
- Bloom BR. New promise for vaccines against tuberculosis. N Engl J Med. 2018;379:1672–74. doi:10.1056/NEJMe1812483.
- Méndez-Samperio P. Development of tuberculosis vaccines in clinical trials: current status. Scand J Immunol. 2018;88:e12710. doi:10.1111/sji.12710.
- Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, Kocken CHM, Ottenhoff THM, Kondova I, Khayum MA, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med. 2019 Jan 21. Epub ahead of print. doi:10.1038/s41591-018-0319-9.
- Abdallah AM, Behr MA. Evolution and strain variation in BCG. Adv Exp Med Biol. 2017;1019:155–69. doi:10.1007/978-3-319-64371-7_8.
- Krajewski W, Matuszewski M, Poletajew S, Grzegrzółka J, Zdrojowy R, Kołodziej A. Are there differences in toxicity and efficacy between various bacillus calmette-guerin strains in bladder cancer patients? Analysis of 844 patients. Urol Int. 2018;101:277–84. doi:10.1159/000492722.