ABSTRACT
Better adjuvants are needed for vaccines against seasonal influenza. TLR7 agonists are potent activators of innate immune responses and thereby may be promising adjuvants. Among the imidazoquinoline compounds, 1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (BBIQ) was reported to be a highly active TLR7 agonist but has remained relatively unexplored because of its commercial unavailability. Indeed, in silico molecular modeling studies predicted that BBIQ had a higher TLR7 docking score and binding free energy than imiquimod, the gold standard TLR7 agonist. To circumvent the availability issue, we developed an improved and higher yield method to synthesize BBIQ. Testing BBIQ on human and mouse TLR7 reporter cell lines confirmed it to be TLR7 specific with significantly higher potency than imiquimod. To test its adjuvant potential, BBIQ or imiquimod were admixed with recombinant influenza hemagglutinin protein and administered to mice as two intramuscular immunizations 2 weeks apart. Serum anti-influenza IgG responses assessed by ELISA 2 weeks after the second immunization confirmed that the mice that received vaccine admixed with BBIQ had significantly higher anti-influenza IgG1 and IgG2c responses than mice immunized with antigen alone or admixed with imiquimod. This confirmed BBIQ to be a TLR7-specific adjuvant able to enhance humoral immune responses.
Background
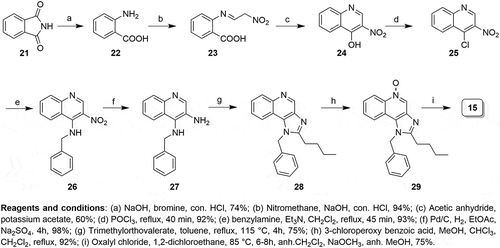
There is an ongoing need for new adjuvants to improve influenza vaccine efficacy in the young and elderly.Citation1 A promising class of adjuvants is the Toll-Like Receptor (TLR) agonists.Citation2 These include a broad spectrum of pathogen-derived compounds including nucleic acids, proteins, lipopeptides and glycolipids.Citation3 A variety of synthetic compounds that activate TLR7 and/or TLR8 have been shown to function as vaccine adjuvants.Citation4–Citation6 Structure–activity relationship (SAR) studies revealed various chemotypes (1–14, ) with unique structural features able to activate TLR7 or TLR8.Citation7,Citation8,Citation9,Citation10,Citation11
Figure 1. Chemical structures of known TLR7 and 8 ligands as derived from structure–activity relationship (SAR) studies.Citation6−Citation10
Small structural modifications in these chemotypes result in varying levels of TLR7 or TLR8 activation and cytokine/chemokine profiles.Citation3
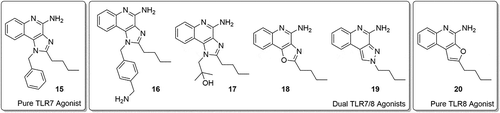
Loxoribine, a pure TLR7 agonist, was shown to enhance anti-tetanus-specific IgG antibody secretion in a dose-dependent manner in human peripheral blood mononuclear cellsCitation12 and strongly activated NK cells in an IL-12-dependent manner.Citation13,Citation14 Similarly, 3M-052, a dual TLR7/8 agonist, was shown to enhance immunogenicity of influenza vaccine.Citation15 TLR7 agonists are known to be more potent than TLR8 agonists at inducing IFN-α and IFN-regulated chemokines such as IFN-inducible protein and IFN-inducible T cell α chemoattractant.Citation16 Given these differences between the effects of TLR7 and TLR8 agonists,Citation2,Citation17,Citation18 we wished to evaluate the adjuvant activity of a pure TLR7 agonist, 1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (BBIQ). As depicted in , BBIQ 15 was reported to be the most active TLR7 agonist,Citation7,Citation19–Citation21 whereas the related derivative 17 was confirmed to be a dual TLR7/8 agonist.Citation22,Citation23 The other closely related imidazoquinoline 16Citation22 with additional aminomethyl functionality as well as the tricyclic oxazoloquinoline 18,Citation24 pyrazoquinoline 19Citation24 was dual TLR7/8 agonists. Conversely, the tricyclic furoquinoline 20Citation25 is a pure TLR8 agonist.
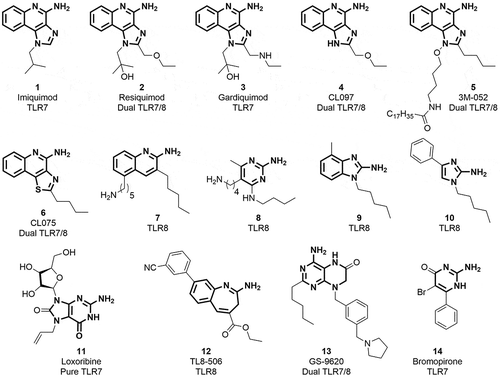
Our overall aim in this study was to evaluate BBIQ’s ability when admixed with influenza vaccine to act as a TLR7 specific adjuvant. The low yielding synthetic process for BBIQ and commercial unavailability necessitated that we first develop an optimized synthetic process to generate sufficient BBIQ to enable its biological characterization (). Starting from inexpensive and commercially available phthalimide, we succeeded in synthesizing BBIQ in 9 steps at overall good yield (>99% purity). This then allowed us to undertake in vitro and in vivo studies, as described herein, to characterize BBIQ’s TLR7 activity and its adjuvant potential.
Material and methods
Synthesis and process optimization
Detailed synthetic procedures, analytical and purity data for BBIQ 15 along with the spectra of all the intermediates are included in the Online Supporting Information.
TLR7/8 reporter cell assays
Human embryonic kidney cells (HEK293) stably co-transfected with either human TLR7 gene (human TLR7-expressing HEK293 cells) or human TLR8 gene (human TLR8-expressing HEK293 cells) and an inducible SEAP (secreted embryonic alkaline phosphatase) reporter gene and mouse Raw-Blue cells with an inducible SEAP (secreted embryonic alkaline phosphatase) reporter gene were acquired from InvivoGen, USA. HEK293-blue and Raw-blue cells were cultured in DMEM supplemented with 10% (v/v) heat-inactivated fetal bovine serum, penicillin-streptomycin 10,000 U/mL (Gibco) and Normocin 100 µg/mL (InvivoGen). Cells were seeded at a density of approximately 40,000 cells/well in a volume of 180 µL/well in a flat-bottom 96-well culture plate (Greiner-One). BBIQ, imiquimod (InvivoGen) and resiquimod (Sigma-Aldrich) were diluted in saline and added to culture plate containing relevant cells to the total volume of 200 µL. The levels of SEAP were determined with QUANTI-BlueTM Solution (InvivoGen).
Animals and immunization
Ethics statement
The study was carried out in strict accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (2013). The protocol was approved by the Animal Welfare Committee of Flinders University. All efforts were made to minimize animal suffering. Mice were housed in cages provisioned with water and standard food and monitored daily for health and condition. After final breeding, all of the mice were humanely euthanized.
Female, 6–8 week old C57BL/6 (BL6) mice were supplied by the College of Medicine and Public Health Animal Facility of Flinders University of South Australia. Mice were intramuscularly (i.m.) immunized twice 2 weeks apart with 1 µg of recombinant influenza H5 hemagglutinin (rH5HA) protein (Protein Sciences Corporation, CT, USA) alone or admixed with 10 µg BBIQ or imiquimod. Immunized animals were monitored for body weight change, activity, body posture, coat quality and respiration, daily. Blood samples were collected by cheek vein bleeding at 2 weeks after each immunization. Sera were separated by centrifugation and stored at −20ºC until use.
Statistical analysis
All statistical analyses were performed using GraphPad Prism version 5.01 for Windows (GraphPad Software, La Jolla, CA, USA). The differences of antibody levels were evaluated by the Mann–Whitney test and other difference between groups were evaluated by two-tailed Student’s t-test and p< .05 was considered to represent a significant difference. All data are shown as mean ± SEM.
Results
BBIQ synthesis
The low yielding synthetic methods for the preparation of appropriately substituted imidazoquinolines prompted us to improve its synthetic process. The poor cyclization of intermediate to substituted imidazole using valeroyl chloride and incomplete displacement of chloro functionality to yield 1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine (BBIQ, compound 15) were the limiting factors of the existing described synthetic approach.Citation7,Citation26 In another approach,Citation27 the synthesis of imidazo[4,5-c]quinoline scaffold was achieved from anthranilic acid, Anthranilic acid is a drug precursor used to make the illicit drug methaqualone,Citation28 is listed in United State Drug Enforcement Administration list and is not commercially available for large-scale production. Lastly, benzoyl isocyanate, which is relatively expensive, was needed in the last described step to install C4 amino functionality requiring four steps and reducing the overall yield. Another synthetic route for the preparation of imidazo[4,5-c]quinoline was reported by Fergusson and coworkersCitation29 which involved the synthesis of appropriately functionalized tetra substituted imidazole through a multicomponent condensation of aminomalononitrile, various orthoesters, and a set of amines. However, the amino malonitrite p-toluene sulfonate, palladium acetate and 2-aminophenylboronic acid and its substituted analogs are relatively expensive. We, therefore, modified the second synthetic route as described by David and coworkers wherein synthesis of BBIQ 15 was achieved from commercially available phthalimide 21 with the complete process being optimized to improve the overall yield. Synthesis of BBIQ 15 began with the conversion of phthalimide to anthranilic acid (). Good yield in this reaction was obtained by maintaining a lower temperature (−5°C) and complete dissolution of compound throughout the reaction with dark brown precipitate and low yields being observed at higher temperatures. Reactions were carried out at up to 100 g scale and no column chromatographic purification or crystallization was required to purify the products. The cyclization of the intermediate was carried out in the presence of acetic anhydride. Maximum yield was obtained only when the reaction mixture was heated after the addition of potassium acetate for about 3–4 h at 55°C until the solid started appearing which was then kept at room temperature overnight to ensure complete solidification. A thorough washing with acetic acid as well as water was done to obtain compound as off-white solid. Treatment with POCl3 resulted in the formation of the chloro derivative. A careful quenching of excess POCl3 was the key to improve the yield in this reaction. The ipso-displacement of chloro functionality with benzylamine was carried out as reported earlier.Citation7 The nitro reduction in intermediate 26 was achieved using 1% palladium on activated charcoal (10% Pd basis) using rubber bladder filled with hydrogen gas. The crucial cyclization step was carried out using trimethyl orthovalerate to furnish the 1-benzyl-2-butyl-1H-imidazo[4,5-c]quinoline 28 in good yield. Further N-oxidation of quinoline nitrogen was carried out using 3-chloroperoxybenzoic acid in a solvent mixture of MeOH:dichloromethane for an hour. To improve cost-effectiveness the benzoyl isocyanate was synthesized using benzamide and oxalyl chloride in situ and was treated with the intermediate N-oxide 29 for the C4-acetamido installation which on treatment with sodium methoxide resulted in the formation of desired BBIQ (1-benzyl-2-butyl-1H-imidazo[4,5-c]quinolin-4-amine). Additional information on the synthetic method and chemical characterization of the produced BBIQ is provided in the Online Supporting Information.
BBIQ specificity for human and mouse TLR7/8 as determined by in silico modeling
To predict BBIQ’s specificity for human and mouse TLR7 we first used an in-silico structural modeling approach, as currently, no crystal structure is available of BBIQ bound to either human or mouse TLR7. We, therefore, used the known human (h)TLR7 crystal structure to construct structural model of mTLR7 and then performed docking studies using these structural models to characterize how BBIQ might bind hTLR7 and mTLR7, comparing this to the two well know TLR7 ligands, imiquimod and resiquimod. First, the structures of BBIQ, resiquimod and imiquimod were sketched in MarvinSketch and minimized using the MMFF94 force field. The crystal structure of human TLR7 (PDB ID-5GMG) was retrieved from Protein Data Bank (PDB). A crystal structure of mTLR7 was modeled using Modeler 9.21, using the crystal structure of hTLR7 as a template. Prior to performing the docking study, the missing Z-loop residues in the hTLR7 structure were constructed in UCSF ChimeraCitation30 and then the Z-loop was cleaved off, followed by energy minimization by steepest descent, for 50,000 steps using OPLS-AA force field, in Gromacs-5.0.7.Citation31 The docking of ligands with hTLR7 and mTLR7 was then performed using PLANTS docking software with chemplp scoring function.Citation32 The binding site center was defined at 71.59, 9.92 and 42.56 with a radius of 15.23. The docking simulation was repeated twice for consistency and the docked structures were saved for binding free energy calculation. Molecular dynamics simulation of the complex of BBIQ with hTLR7 or mTLR7 was performed at 300 K using Langevin thermostat. Long-range Coulombic interactions were handled using particle mesh Edwald algorithm and the SHAKE algorithm was used on all atoms that were covalently bonded to hydrogen atoms. Further, g_mmpbsa scripts were used to calculate free binding energy.Citation33
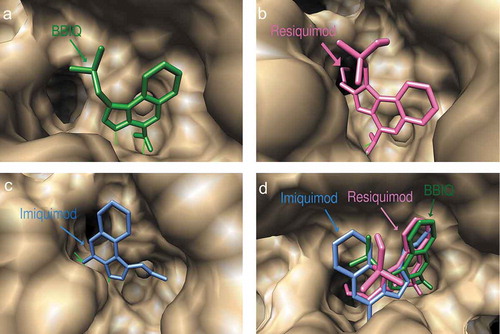
The docked conformations of imiquimod and resiquimod on hTLR7 suggested that they form hydrogen bonds with both Asp555 and Thr586, while BBIQ was predicted to form a hydrogen bond with only Thr586 (). The predicted docked conformations of both resiquimod and BBIQ on mTLR7 showed both forming hydrogen bonds with the same residues, namely Ile564 and Thr565 (). The binding free energy for resiquimod for hTLR7 and mTLR7 was calculated as −19.28 kcal/mol and −13.24kcal/mol, respectively with the binding free energy for BBIQ with hTLR7 and mTLR7 being slightly lower at −16.67 kcal/mol and −11.25kcal/mol, respectively. This suggests that resiquimod has higher binding than BBIQ for both human and mouse TLR7. By contrast, the binding free energy for imiquimod was just −11.64 kcal/mol and −6.64kcal/mol, for hTLR7 and mTLR7, respectively. These modeling data suggested that BBIQ has a much higher affinity for both hTLR7 and mTLR7 than imiquimod. Overall, BBIQ was predicted to have a human and mouse TLR7 activity intermediate between that of resiquimod and imiquimod.
Figure 3. Predicted docked conformations of BBIQ (a), resiquimod (b) and imiquimod (c) to the ligand-binding site of human TLR7, showing predicted hydrogen bonds in green. Figure 3(b) shows a comparison of the predicted and crystal confirmed conformation of resiquimod in the hTLR7 binding pocket with a goodness of fit shown by the Root-mean-square-deviation (RMSD) value of 0.93 Å. Figure 3(d) shows the comparison of the predicted binding poses of BBIQ (in green), resiquimod (in pink) and imiquimod (in blue) in the hTLR7 binding pocket.
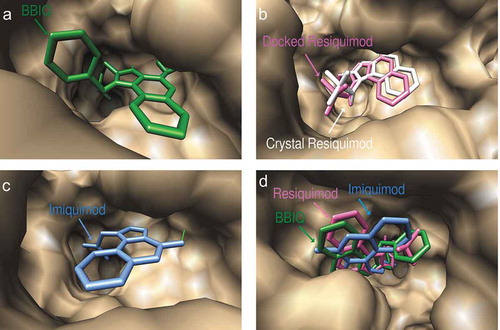
Figure 4. Predicted docked conformations of BBIQ (a), resiquimod (b) and imiquimod (c) on mouse TLR7, showing the amino acids forming hydrogen bonds in the ligand-binding site. Figure 4(d) shows the predicted binding poses of BBIQ (in green), resiquimod (in pink) and imiquimod (in blue) in the mTLR7 binding pocket.
TLR7 potency assessment in vitro
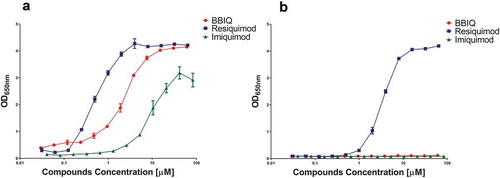
The ability of purified BBIQ to activate TLR7 was first tested by generating a dose–response curve against imiquimod and resiquimod on a human TLR7-transfected HEK reporter cell line (hTLR7_HEK-Blue cells, Invivogen). This confirmed that BBIQ was an hTLR7 agonist with an ED50 of ~2 µM, sitting between resiquimod and imiquimod ()), confirming the predictions of the in silico modeling studies. While, resiquimod had high TLR8 activity, consistent with its status as a combined hTLR7/8 agonist, neither BBIQ nor imiquimod showed any hTLR8 activity, confirming their specificity as TLR7-specific agonists ()). Similar results were seen when the compounds were tested using human or mouse cell lines expressing both TLR7 and 8, namely the human Ramos cell line or mouse Raw cell line. In both cases BBIQ showed intermediate potency between resiquimod and imiquimod (Online Supporting Information, Figure S1). Overall, the reporter cell assays confirmed that BBIQ was a specific agonist of both human and mouse TLR7 with a potency intermediate between resiquimod and imiquimod.
Figure 5. The relative potency of BBIQ, resiquimod and imiquimod for human TLR7 and TLR8 as measured using HEK reporter cell lines transfected with human TLR7 (a) or TLR8 (b). NFkB activation was measured by production of SEAP as determined with QUANTI-Blue Solution. Shown is mean ± standard error of the OD readings of duplicate samples.
BBIQ adjuvant potency in vivo
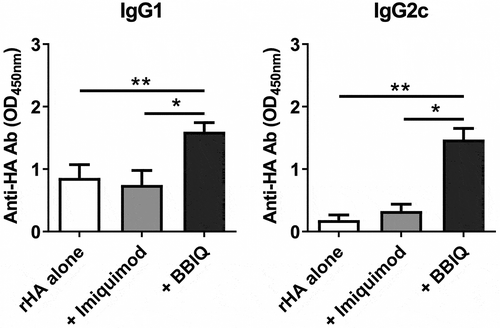
BBIQ has not previously been tested for adjuvant activity with influenza vaccines. Female C57BL/6 mice, 6 to 8 week old, were immunized twice i.m. at a 2-week interval with 1μg recombinant hemagglutinin (rHA) protein alone or admixed with 10μg of BBIQ or imiquimod. Blood samples were collected 2 weeks after the second immunization for measurement of anti-HA antibodies by ELISA. Whereas imiquimod showed no adjuvant activity at the dose used, BBIQ demonstrated adjuvant activity, inducing significantly higher anti-influenza IgG1 and IgG2c levels as compared to mice immunized with vaccine alone or admixed with imiquimod (). Hence, consistent with its higher TLR7 potency in TLR7 reporter cell lines, BBIQ exhibited greater adjuvant activity than imiquimod when tested with influenza vaccine in mice. BBIQ was assessed for its ability to induce cytotoxic T cells using an in vivo CTL assay but unlike the TLR9 agonist used as a positive control, immunization with BBIQ admixed with ovalbumin failed to induce any killing of OVA-labeled target cells (Online Supporting Information, Figure S2), suggesting that BBIQ has weak, if any, effects on T cells.
Figure 6. BBIQ enhances IgG antibody responses in influenza vaccinated mice. Female C57BL/6 mice, 6 to 8 week old, were immunized twice i.m. at a 2-week interval with recombinant hemagglutinin (rHA) protein 1μg alone or admixed with 10μg imiquimod or BBIQ in 50μl total volume. Blood samples were collected 2 weeks after the second immunization and antigen-specific IgG1 or IgG2c antibodies measured by ELISA with results shown as the OD (*p < .05, **p < .01).
We have previously shown a synergistic benefit of mixing TLR9-active CpG oligonucleotides with Advax delta inulin particle adjuvants.Citation34 Hence, we next assessed whether BBIQ or imiquimod had increased adjuvant activity when admixed with Advax adjuvant. Mice were immunized i.m. with 1μg rHA admixed with BBIQ, imiquimod or Advax adjuvant as a comparator, or a combination of Advax with BBIQ or imiquimod (Online Supporting Information, Figure S3). Of the single adjuvants, Advax induced the highest HA-specific IgG1, followed by BBIQ. However, the highest overall levels of IgG1 were obtained when either BBIQ or imiquimod were co-administered with Advax, consistent with an additive or synergistic effect. A synergistic effect on IgG2c production was also observed when BBIQ or imiquimod was combined with Advax adjuvant.
Discussion
BBIQ is a human and mouse TLR7 agonist that previously was shown to have antiviral and antiparasitic activity. However, due to its difficult synthesis and low yields it had not previously been tested as a parenteral vaccine adjuvant. To make this study possible, we first needed to improve the BBIQ synthetic method to enable sufficient material to be produced for its characterization. In parallel, we performed in silico structural modeling and docking studies to better understand the nature of BBIQ’s interaction with human and mouse TLR7. These modeling studies predicted that BBIQ was a pure TLR7 agonist with a higher binding free energy than imiquimod for both human and mouse TLR7. Assays using TLR7 or 8 expressing reporter cell line results confirmed that BBIQ was a specific TLR7 agonist with a higher potency than imiquimod but lower potency than resiquimod. The intermediate potency of BBIQ for both human and mouse TLR7 was confirmed using human Ramos and mouse Raw reporter cell lines that express both TLR7 and 8. As, the in vitro reporter cell results confirmed the accuracy of the predictions of our in silico TLR7 models, we are now planning to apply these models to an in silico high-throughput screen to identify additional novel TLR7 ligands.
The ultimate goal of this study was to test whether BBIQ’s TLR7 activity translates into adjuvant activity, in vivo. To address this question, we intramuscularly immunized mice with either BBIQ or imiquimod admixed with a recombinant influenza antigen. BBIQ showed adjuvant activity in the mice, where it enhanced influenza-specific IgG1 and IgG2c production whereas imiquimod at the same dose of 10 μg had no measurable effect on antibody production. Interestingly, both BBIQ and imiquimod when combined with Advax delta inulin adjuvant enhanced influenza-specific antibody production. BBIQ when formulated wth ovalbumin vaccine failed to induce cytotoxic T cells against ovalbumin in an in vivo CTL assay, suggesting it has relatively weak T cell activity (Online Supporting Information, Figure S2). No evidence of toxicity of BBIQ was seen in the immunized mice.
The lack of adjuvant action of imiquimod on influenza antibody responses in our study was unexpected given the literature on its use as an effective TLR7 agonist and antiviral agent. Although a dermal dose of 250 mg of imiquimod cream has some adjuvant activity when administered prior to an intradermal injection of influenza vaccine at the same site, this is quite a different application to the intramuscular vaccine used in our study.Citation35 Imiquimod has been used to adjuvant DNA vaccines in mice where it was shown to enhance T cell rather than antibody responses.Citation36,Citation37 Only a single paper reported an effect of imiquimod on antibody responses to a parenterally administered protein vaccine but there were notable differences in that study, which used a five-times higher dose of imiquimod (50 μg) than our 10 μg dose and the vaccine was given intraperitoneally.Citation38 Overall, our data suggest that imiquimod has relatively weak humoral adjuvant activity when given intramuscularly and might require higher doses or combination with another adjuvant such as Advax, to induce a major effect on antibody responses. In addition to imiquimod’s relatively low TLR7 potency, it is a small molecule drug and hence likely to diffuse rapidly away from the injection site, reducing its ability to provide local immune enhancement. The same might be expected for BBIQ that is a similar sized molecule. More recently, alternative TLR7/8 formulations have been developed to promote the transport of the TLR-agonist to the draining lymph node through conjugation to alum,Citation39 hyaluronic acidCitation40 or other polymersCitation41 . BBIQ’s adjuvant activity might similarly be increased in this way. Notably, increased adjuvant activity of both BBIQ and imiquimod were seen when they were mixed with Advax adjuvant, reminiscent of the synergistic effects seen for Advax mixed with TLR9 agonists. The current data thereby extend the benefit of Advax combinations to TLR7 agonists.
This study has several limitations. The full adjuvant activity of BBIQ will need to be confirmed in dose–response studies against established adjuvant comparators, such as squalene emulsion adjuvants. BBIQ 10 μg was not as potent in mice as Advax adjuvant, a known adjuvant that has advanced to human influenza vaccine trials. Future studies are also needed to determine BBIQ’s effects on long-term immune memory responses and its ability to improve vaccine immunity in very young or old animals. It will be interesting to directly compare BBIQ’s adjuvant activity as a pure TLR7 agonist with the activity of a pure TLR8 agonist or a dual TLR7/8 agonist. Pure TLR7 agonists might be relatively weak adjuvants, reflecting their bias to induction of IFN-regulated chemokines whereas TLR8 agonists induce more inflammatory cytokines that may be better at enhancing adaptive immune responses.Citation16 Given its apparent Th2 bias, future studies could be undertaken in Th2-biased BALB/c mice to assess the potential of BBIQ to induce an IgE response to exclude any allergy-driving potential. The improved synthetic method as described here should facilitate conduct of future studies to more fully characterize the adjuvant activity of BBIQ as a specific TLR7 agonist.
Disclosure of potential conflicts of interest
VK, SP, JF, YHO, IS and NP are affiliated with Vaxine, which has commercial interests in Advax adjuvant. The other authors declare that they have no known competing financial interests.
Supplemental Material
Download MS Word (1.7 MB)Acknowledgments
The support from UGC-CAS, DST-PURSE and Panjab University Development fund is gratefully acknowledged. DBS is thankful to UGC New Delhi for start-up research grant and DBT New Delhi for the award of Ramalingaswami Fellowship. Ministry of Human Resource Development, Govt. of India is gratefully acknowledged for the award of Scheme for Promotion of Academic and Research Collaboration (SPARC/2018-2019/P386/SL). DK is thankful to UGC New Delhi for the award of Junior Research Fellowship. MTP is thankful to DST for the award of Women Scientist Scheme-A (WOS-A). VK, SP, JF, YHO, IS and NP were supported by the National Institutes of Health, under Contracts HHSN272201400053C and HHSN272201800044C. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding bodies.
Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2019.1710409.
Additional information
Funding
References
- Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38:1059–74. doi:10.1007/s40264-015-0350-4.
- Warshakoon HJ, Hood JD, Kimbrell MR, Malladi S, Wu WY, Shukla NM, Agnihotri G, Sil D, David SA. Potential adjuvantic properties of innate immune stimuli. Hum Vaccin. 2009;5:381–94. doi:10.4161/hv.5.6.8175.
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–96. doi:10.1111/imcb.2004.82.issue-5.
- Dowling DJ. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immuno Horizons. 2018;2:185–97. doi:10.4049/immunohorizons.1700063.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi:10.3389/fimmu.2013.00114.
- To EE, Erlich J, Liong F, Luong R, Liong S, Bozinovski S, Seow HJ, O’Leary JJ, Brooks DA, Vlahos R, et al. Intranasal and epicutaneous administration of Toll-Like Receptor 7 (TLR7) agonists provides protection against influenza A virus-induced morbidity in mice. Sci Rep. 2019;9:2366. doi:10.1038/s41598-019-38864-5.
- Shukla NM, Malladi SS, Mutz CA, Balakrishna R, David SA. Structure-activity relationships in human Toll-Like Receptor 7-active imidazoquinoline analogues. J Med Chem 2010;53:4450–65. doi:10.1021/jm100358c.
- Beesu M, Caruso G, Salyer ACD, Khetani KK, Sil D, Weerasinghe M, Tanji H, Ohto U, Shimizu T, David SA. Structure-based design of human TLR8-specific agonists with augmented potency and adjuvanticity. J Med Chem 2015;58:7833−7849). doi:10.1021/acs.jmedchem.5b01087.
- Beesu M, Salyer ACD, Trautman KL, Hill JK, David SA. Human Toll-Like Receptor (TLR) 8‑specific agonistic activity in substituted pyrimidine-2,4-diamines. J Med Chem 2016;59:8082−8093).
- Beesu M, Malladi SS, Fox LM, Jones CD, Dixit A, David SA. Human Toll-Like Receptor 8‑selective agonistic activities in 1‑alkyl‑1H‑benzimidazol-2-amines. J Med Chem 2014;57:7325−7341). doi:10.1021/jm500701q.
- Beesu M, Caruso G, Salyer ACD, Shukla NM, Khetani KK, Smith LJ, Fox LM, Tanji H, Ohto U, Shimizu T, et al. Identification of a human Toll-Like Receptor (TLR) 8-specific agonist and a functional pan-TLR inhibitor in 2-aminoimidazoles. J Med Chem 2016;59:3311−3330.
- Gupta S, Vayuvegula B, Gollapudi S. Substituted guanine ribonucleosides as B cell activators. Clin Immunol Immunopathol. 1991;61:s21–s27. doi:10.1016/S0090-1229(05)80034-0.
- Pope BL, Chourmouzis E, Sigindere J, MacIntyre JP, Capetola RJ, Lau CY. In vivo activation of natural killer cells and priming of IL-2 responsive cytolytic cells by loxoribine (7-allyl-8-oxoguanosine). Cell Immunol. 1993;147:302–12. doi:10.1006/cimm.1993.1071.
- Pope BL, Chourmouzis E, Victorino L, MacIntyre JP, Capetola RJ, Lau CY. Loxoribine (7-allyl-8-oxoguanosine) activates natural killer cells and primes cytolytic precursor cells for activation by IL-2. J Immunol. 1993;151:3007–17.
- Hoeven NV, Fox CB, Granger B, Evers T, Joshi SW, Nana GI, Evans SC, Lin S, Liang H, Liang L, et al. A formulated TLR7/8 agonist is a flexible, highly potent and effective adjuvant for pandemic influenza vaccines. Sci Rep 2017;7:46426. doi:10.1038/srep46426.
- Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005;174:1259–68. doi:10.4049/jimmunol.174.3.1259.
- Levy O, Suter E,E, Miller R,L, Wessels M,R. Unique efficacy of Toll-Like Receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–90. doi:10.1182/blood-2005-12-4821.
- Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Keiper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005;174:1259−1268. doi:10.4049/jimmunol.174.3.1259.
- Beesu M, Salyer ACD, Brush MJH, Trautman KL, Hill JK, David SA. Identification of high-potency Human TLR8 and dual TLR7/TLR8 agonists in pyrimidine-2,4-diamines. J Med Chem 2017;60:2084−2098. doi:10.1021/acs.jmedchem.6b01860.
- Salyer ACD, David SA. Transcriptomal signatures of vaccine adjuvants and accessory immunostimulation of sentinel cells by Toll-Like Receptor 2/6 agonists. Hum Vaccin Immunother 2018;14:1686–1696.
- Salyer ACD, Caruso G, Khetani KK, Fox LM, Malladi SS, David SA. Identification of adjuvantic activity of amphotericin B in a Novel, multiplexed, Poly- TLR/NLR high-throughput screen. PLoS One. 2016;11(2):e0149848. doi:10.1371/journal.pone.0149848.
- Ganapathi L, Haren SV, Dowling DJ, Bergelson I, Shukla NM, Malladi SS, Balakrishna R, Tanji H, Ohto U, Shimizu T, et al. The imidazoquinoline Toll-Like Receptor-7/8 agonist hybrid-2 potently induces cytokine production by human newborn and adult leukocytes. PLoS One. 2015;10(8):e0134640. doi:10.1371/journal.pone.0134640.
- Shi C, Xiong Z, Chittepu P, Aldrich CC, Ohlfest JR, Ferguson DM. Discovery of imidazoquinolines with Toll-Like Receptor 7/8 independent cytokine induction. ACS Med Chem Lett 2012;3:501−504. doi:10.1021/ml300079e.
- Yoo E, Salunke DB, Sil D, Guo X, Salyer ACD, Hermanson AR, Kumar M, Malladi SS, Balakrishna R, Thompson,W H, et al. Determinants of activity at Human Toll-like Receptors 7 and 8: quantitative Structure−Activity Relationship (QSAR) of diverse heterocyclic scaffolds. J Med Chem 2014;57:7955−7970. doi:10.1021/jm500744f.
- Kokatla HP, Sil D, Malladi SS, Balakrishna R, Hermanson AR, Fox LM, Wang X, Dixit A, David SA. Exquisite selectivity for human Toll-Like Receptor 8 in substituted Furo[2,3‑c]quinolines. J Med Chem 2013;56:6871−6885. doi:10.1021/jm400694d.
- Saroa R, Kaushik D, Bagai U, Kaur S, Salunke DB. Efficacy of TLR7 agonistic imidazoquinoline as immunochemotherapeutic agent against P. Berghei ANKA infected rodent host. Bioorg Med Chem Letts. 2019;29:1099–105. doi:10.1016/j.bmcl.2019.02.029.
- Shukla NM, Kimbrell MR, Malladi SS, David SS. Regioisomerism-dependent TLR7 agonism and antagonism in an imidazoquinoline. Bioorg Med Chem Lett 2009;19:2211–14. doi:10.1016/j.bmcl.2009.02.100.
- Angelos SA, Meyers JA. The isolation and identification of precursors and reaction products in the clandestine manufacture of methaqualone and mecloqualone. J Forensic Sci. 1985;30:1022–47. doi:10.1520/JFS11044J.
- Schiaffo CE, Shi C, Xiong Z, Olin M, Ohlfest JR, Aldrich CC, Ferguson DM. Structure−activity relationship analysis of imidazoquinolines with Toll-Like Receptors 7 and 8 selectivity and enhanced cytokine induction. J Med Chem 2014;57:339–47. doi:10.1021/jm4004957.
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12.
- Spoel DVD, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC, Fast GROMACS. Flexible, and Free. J Comput Chem 2005;26:1701–18. doi:10.1002/jcc.20291.
- Korb O, Stützle T, Exner TE. Empirical scoring functions for advanced Protein−Ligand docking with PLANTS. J Chem Inf Model 2009;49:84–96. doi:10.1021/ci800298z.
- Kumari R, Kumar R, Lynn A. g_mmpbsa—a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 2014;54:1951–62. doi:10.1021/ci500020m.
- Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N. SARS-CoV vaccines formulated with delta inulin adjuvants provide enhanced virus protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015 Mar;89(6):2995–3007.
- Hung IF, Zhang AJ, To KK, Chan JF, Li P, Wong TL, Zhang R, Chan T-C, Chan BCY, Wai HH, et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect Dis. 2016;16:209–18. doi:10.1016/S1473-3099(15)00354-0.
- Thomsen LL, Topley P, Daly MG, Brett SJ, Tite JP. Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccine. 2004;22:1799–809. doi:10.1016/j.vaccine.2003.09.052.
- Smorlesi A, Papalini F, Orlando F, Donnini A, Re F, Provinciali PM. Imiquimod and S-27609 as adjuvants of DNA vaccination in a transgenic murine model of HER2/neu-positive mammary carcinoma. Gene Ther. 2005;12:1324–32. doi:10.1038/sj.gt.3302559.
- Zhang AJ, Li C, To KK, Zhu HS, Lee AC, Li CG, Chan JFW, Hung IFN, Yuen K-Y. Toll-Like Receptor 7 agonist imiquimod in combination with influenza vaccine expedites and augments humoral immune responses against influenza A(H1N1)pdm09 virus infection in BALB/c mice. Clin Vaccine Immunol. 2014;21:570–79. doi:10.1128/CVI.00816-13.
- Bagnoli F, Fontana MR, Soldaini E, Mishra RP, Fiaschi L, Cartocci E, Nardi-Dei V, Ruggiero P, Nosari S, De Falco MG, et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. Proc Natl Acad Sci U S A. 2015;112:3680–85. doi:10.1073/pnas.1424924112.
- Yoo E, Salyer ACD, Brush MJH, Li Y, Trautman KL, Shukla NM, De Beuckelaer A, Lienenklaus S, Deswarte K, Lambrecht BN, et al. Hyaluronic Acid Conjugates of TLR7/8 Agonists for Targeted Delivery to Secondary Lymphoid Tissue. Bioconjug Chem. 2018;29:2741–54. doi:10.1021/acs.bioconjchem.8b00386.
- Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33:1201–10. doi:10.1038/nbt.3371.