ABSTRACT
Introduction: With the use of pneumococcal conjugate vaccines(PCV), the behavior of invasive pneumococcal disease(IPD) has changed relative to serotype distribution. The introduction of these vaccines in national immunization programs has reduced the incidence of IPD, with a marked decrease in the circulation of the serotypes included in the vaccine used in each country. However, the subsequent emergence of other serotypes not included in the vaccine, such 19A in case of PCV7 and PCV10, has been documented.
Materials and methods: This was case series study (2008–2017) in pediatric patients admitted to 10 hospitals in Bogota who were diagnosed with IPD. It was conducted during the transitional period of implementing the PCV10 vaccine in Colombia in 2012. Cases of bacteremic pneumococcal pneumonia, meningitis, primary bacteremia and osteoarticular infection were included. A descriptive analysis of the demographic, clinical and laboratory variables of patients with IPD by Spn19A, its trend over time, profiles of antimicrobial susceptibility and clinical outcomes was performed.
Results: There were 463 cases of IPD, 315(68%) with known serotypes. The prevalence of IPD by Spn19A was 17.7%(56 cases), tending to increase over time. During 2008–2011, the prevalence was 4.4%, and during 2014–2017, it was 32.4%, The most frequent diagnosis was pneumonia(80.4%). In nonmeningeal isolates, 39.6% were not susceptible to penicillin. An increase in the resistance was observed over time.
Conclusion: Spn19A is a prevalent cause of IPD in the pediatric population of the analyzed cohort, with an increasing trend of this serotype during the surveillance period after the introduction of PCV10, being the most common serotype identified in recent years.
Introduction
The epidemiological behavior of invasive pneumococcal disease (IPD) has changed with the introduction of conjugate vaccines.Citation1-Citation3 However, despite the decrease in the incidence of IPD after the introduction of such vaccines, IPD remains a significant cause of morbidity and mortality in the pediatric population, with a disease burden that varies regionally.Citation4-Citation5Citation6 In 2015, it is estimated that there were 9,180,000 cases of invasive pneumococcal disease in children under 5 years of age, with approximately 318,000 deaths due to this cause, 257,000 from pneumonia, 37,900 from meningitis and 22,700 from other causes. For the same year, it is estimated that in the Americas, 259,000 cases of IPD were identified with 5700 deaths – 4600 from pneumonia, 600 from meningitis and 600 from other causesCitation6
With the introduction of pneumococcal vaccines in national immunization programs, a change in the distribution of serotypes was observed, a process known as serotype replacement.Citation7 Serotype replacement occurs when the prevalence of serotypes included in conjugate vaccines decrease and the frequency of serotypes not included in the vaccines increase. In this case, serotype 19A (Spn19A) was the serotype showing the greatest increase after the introduction of the heptavalent pneumococcal conjugate vaccine (PCV7) in different regions of the world, Citation8,Citation9 becoming a prevalent cause of IPD. PCV7 was introduced in Bogota in 2008, and from January 2012 it was replaced by the decavalent vaccine (PCV10) in the national immunization schedule (PAI, in Spanish) in a 2 + 1 scheme at 2, 4 and 12 months.Citation10 In 2016, Colombia reported a vaccination coverage for the third dose of pneumococcus of 89%; in Bogota coverage was 93%.Citation11
It has been documented that serotype 19A has a high invasive potential and high rates of antibiotic resistance, conditions that have raised concerns regarding clinical outcomes in infected patients.Citation12-Citation13Citation14 It has been proposed to optimize the epidemiological surveillance of IPD. In Bogota, there is an initiative led by the Colombian Association of Infectious Diseases (Asociación Colombiana de Infectología – ACIN) and the Colombian Society of Pediatrics (Sociedad Colombiana de Pediatría – SCP) called Red Neumocolombia, which since 2012 has conducted epidemiological surveillance of IPD in 10 hospitals in the city.
There are regional publications on the behavior of the serotype after vaccination with PCV10, including 19A.Citation15-Citation20 However, there are no recent publications on the behavior of serotype 19A in Bogota after the massive use of PCV10.
Therefore, the objective of this study was to analyze the demographic and clinical characteristics and outcomes of invasive serotype 19A pneumococcal diseases by in pediatric patients in Bogota, Colombia, between 2008 and 2017.
Materials and methods
Population
The study included patients younger than 18 years who were diagnosed with IPD and were admitted to health institutions where the study was conducted between January 2008 and December 2017.
Place of the study
Bogotá is the capital city of Colombia, with a population of 7,150,000 inhabitants in 2018 of which approximately 2,500,000 are under 19 years of age.Citation21 Surveillance is performed in 10 tertiary care hospitals, some of which are state hospitals.
Data collection
This was an ambispective study. The data were collected retrospectively from 2008 to 2011 and prospectively from 2012 to 2017. Surveillance was conducted in a transitional period when PCV10 was implemented in the national immunization schedule, which began to be administered in January 2012.Citation10
Invasive pneumococcal disease (IPD) is defined when there is a Streptococcus pneumoniae isolate in the culture of a sterile site. This category includes cases of bacteremic pneumococcal pneumonia, meningitis, primary bacteremia and osteoarticular infection. These cases were identified in the laboratory of each institution. From this information, clinical history data were completed using a standardized case report format. Susceptibility information was obtained from the laboratory of each institution using automated methods: 7 using Vitek 2® (BioMérieux, Marcy l‘Etoile, France), 3 using BD Phoenix®. The susceptibility categories “susceptible”, “intermediate” and “resistant” met the definitions of the Institute of Clinical and Laboratory Standards of the United States (CLSI 2017). As part of routine surveillance, the isolates should be sent to the District Health Department (Secretaria Distrital de Salud – SDS), where the isolate is reconfirmed and sent to the National Institute of Health (INS), where serotyping by the Quellung method and by polymerase chain reaction, as appropriate, is performed. The serotyping data were taken from the report sent by the SDS and INS to the institutions. The vaccination data were obtained from medical records and from the website of the District Health Department of Bogotá (http://appb.saludcapital.gov.co/Pai/publico/busqueda.aspx).
The information was recorded in an Excel document (2010).
Statistical analysis
A descriptive analysis of demographic, clinical and laboratory variables of patients with IPD by Spn 19A was performed. The trend over time of the serotype, the profiles of antimicrobial susceptibility and clinical outcomes are described.
Ethical considerations
The study was approved by the ethics committee of each participating institution. This is a risk-free study in which retrospective research techniques and methods were used. There was no intervention or intentional modification of the biological, physiological, psychological or social variables of the individuals who participated in the study. The identity of the patients was known only to the principal investigator, according to the consecutive medical histories.
Results
Prevalence and trends of Spn19A over time
During the study period (2008–2017), 463 cases of IPD were recorded in the network, 317 (68.5%) with known serotypes, of which 237 (74.8%) were patients under 5 years of age. The prevalence of IPD by Spn19A was 17.7% (56 cases).
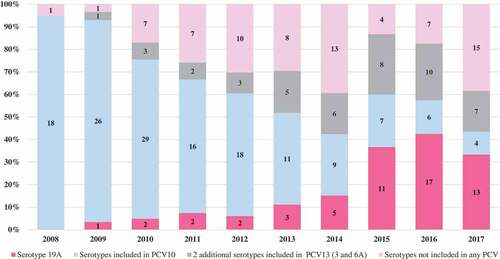
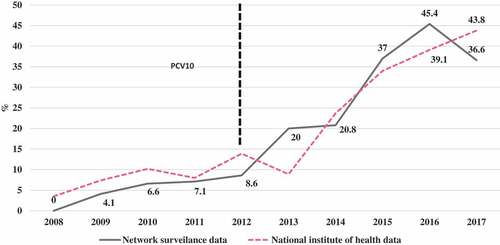
The prevalence of Spn19A isolates in patients aged 0 to 17 years in the period 2008–2011 was 4.4% (5/115), between 2012–2013 was 8.3% (5/60) and between 2014–2017 was 32.4% (46/142). In children under 5 years, between 2008–2011, it was 4.7% (4/85), between 2012–2013 it was 10.5% (4/38) and between 2014–2017 it was 36.8% (42/114). This was the most frequent serotype in the last 3 years. compares the percentage of Spn19A isolates from the total serotype isolates in children under 5 years found in the present study with the national data reported by the National Institute of Health.
Figure 1. The proportion of Spn19A cases in Bogota and Colombia that cause IPD in children under 5 years; 2008–2017.
shows an increase in the proportion of IPD caused by Spn19A higher than that observed in the other serotypes not included in PCV10 or included in PCV13 and higher than what was observed in the serotypes not included in any PCV.
Clinical and demographic characteristics
Among the demographic characteristics, 29 (51.8%) patients were male, and 50 (89.3%) were younger than 5 years. The most frequent diagnosis was pneumonia, with 45 cases (80.4%), followed by primary bacteremia (6 cases (10.7%)), meningitis (3 cases (5.4%)) and osteoarticular infection (2 cases (3.6%)). The distribution of diagnoses by age group is presented in .
Table 1. Diagnostics by age group in cases of IPD by Spn19A.
Regarding antecedents, 24 (42.9%) patients attended an educational institution. Information on vaccination status was obtained for 46 (82.1%) patients. Of these, 44 (95.6%) had received some type of conjugate vaccine, 40 (91%) received PCV10, 2 (4.5%) received PCV7 and 2 (4.5%) received PCV13. Thirty-eight (86.3%) cases had a complete vaccination schedule of 3 doses, of which 34 (89%) were PCV10. Information on prior antibiotic use was obtained for 32 (57.1%) patients. Of these, 8 (25%) had received a first-line treatment for Spn. In 17 patients, risk factors for IPD were identified: 6 (10.7%) had at least one episode of otitis media in the last year, 6 (10.7%) had chronic diseases (neurological disorders (3), malnutrition (1), kidney failure (1) and chronic lung disease (1)) and 2 (3.6%) patients had neoplasms.
The most frequent clinical manifestations in cases of pneumonia were fever (91.1%), cough (75.6%) and respiratory distress (31.1%). All patients with pneumonia had a chest X-ray report, with the most frequent findings being consolidation (82.2%), pleural effusion (26.7%) and interstitial infiltrates (17.8%).
Cases of meningitis presented fever (100%), irritability (66.6%) and lethargy (66.6%).
The average length of hospital stay of patients who survived was 14.9 days and was 12.2 days for patients who died. The median length of hospital stay in all patients was 10.5 days (IQR 5 to 20.5 days); 23 (41%) were admitted to the intensive care unit, and the median length of stay in the ICU was 4 days (IQR from 2 to 11 days). One patient had an extended stay of 50 days. Mortality was 10.7% (6), with 83.3% (5) younger than 5 years.
Microbiological characteristics
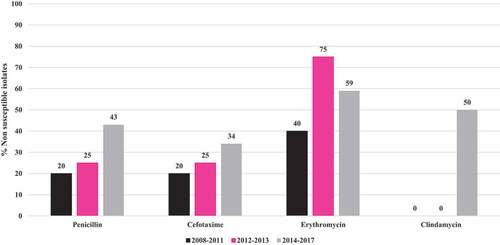
Complete information on susceptibility to antibiotics was obtained in all cases. describes the susceptibility profile of the nonmeningeal isolates over time. There is an increase in non-susceptible to penicillin isolates from 20% in the period 2008–2011 to 43% in the period 2014–2017. There is also an increase in non-susceptible to cefotaxime, erythromycin and clindamycin isolates. In total 21 (39.6%) isolates were not susceptible to penicillin, 17 (32,1%) isolates were not susceptible to cefotaxime, 31(58,5%) were not susceptible to erythromycin and 22 (41,5%) were not susceptible to clindamycin.Of the 3 meningeal isolates, 1 (33.3%) had decreased susceptibility to penicillin.
Twenty-one isolates (37.5%) of Spn19A were susceptible to all antibiotics, 8 (14.3%) were resistant to one family of antibiotics, 14 (25%) were resistant to 2, 4 (7.1%) were resistant to 3, 7 (12.5%) were resistant to 4 and 2 (3.6%) were resistant to 5 families of antibiotics. A total of 27 (48.2%) were multidrug resistant.
Discussion
The study provides recent data on the epidemiological and clinical behavior of IPD by serotype 19A in Bogota. We found that serotype 19A is a prevalent cause of IPD in the analyzed pediatric population, mainly in children under 5 years of age, similar to that reported in other studiesCitation2,Citation15,Citation22-Citation26
We found an increasing trend over time, from 0% in 2008 to 33% in 2017 in those under 18 years of age and from 0% in 2008 to 36.6% in children under 5 years of age, an increase observed especially since 2013. Data from this study are similar to data in children under 5 years published by the INSCitation25 in which the prevalence increases from 3.5% in 2008 to 43% in 2017. When analyzing this information for the different periods in relation to the systematic application of the vaccine nationally, the INS reported a prevalence of Spn19A in the period 2008–2011 of 7.2% (37/510), in 2012–2013 of 11.6% (19/164) and in 2014–2017 of 36.3% (161/443), similar to the data found in this study. Between 2016 and 2017, we found a slight decrease, from 42.5% to 33.3%, which was not observed in the national data, where the prevalence increased from 39% in 2016 to 43% in 2017.Citation25 Therefore, it is unlikely that this is a cyclical behavior. In addition, a time series study using national surveillance data showed that this increase was statistically significant.Citation19 This behavior observed in Colombia is similar to the behavior observed in Brazil and Chile after the introduction of PCV10.Citation15,Citation16,Citation17, Citation23,Citation24,Citation26 These data differ from those published in a recent systematic review that included data up to 2015.Citation18 In the present study, an upward trend was observed after 2008, and this trend increased between 2013 and 2017, showing an increase of 300%.
Studies carried out in other countries that have implemented PCV13 immunization in the routine infants programs, have shown a decrease in the prevalence and incidence of Spn 19A.Citation3,Citation27-Citation28, Citation29, Citation31, Citation32Citation34 Moore et al reports a decrease in the prevalence of Spn19A in children under 5 years old, in the USA, from 52,8% to 11,11% after the implementation of PCV13.Citation30 Ben Shimol et al reports a decrease in the incidence of IPD for Spn19A in children under 5 years old in Israel, from 5 × 100.000 to 1.6 × 100.000 after the implementation of PCV13.Citation33
Serotype replacement leads to colonization and to IPD by serotypes not included in conjugate vaccines.Citation2,Citation35,Citation36 The increase in the frequency of Spn19A has been explained in part by the introduction of conjugate vaccines; other factors that may explain this behavior include the selection pressure by antibiotics, the introduction of new clones and the expansion or combination of these factors.Citation35,Citation37,Citation38
Hence, it is important to continue an active IPD surveillance program to obtain objective information on their epidemiological behavior and to propose health care measures for these diseases.Citation39
The most common clinical presentation of IPD by serotype 19A in this analysis was pneumonia, similar to that reported by an Argentinean study, Citation22 followed by primary bacteremia and meningitis. In the study conducted by the CASPER group (The Calgary Area Streptococcus pneumoniae Epidemiology Research), serotype 19A was a frequent cause of IDP in children under 5 years of age, but unlike our findings, the primary diagnosis in this age group was meningitis (14.2% of IPD).Citation4,Citation12 In the Cassiolato study, 37.7% of the cases were meningitis, and 62.3% were isolates from patients with nonmeningeal pathologies.Citation15 Other less frequent manifestations of IPD, such as osteoarticular infection, corresponded to 3.6% in the current study, a frequency similar to that found in other studies.Citation40
In the present analysis, 10.7% of patients had a chronic predisposing disease, and 3.6% of patients had a history of cancer, a figure lower than that found in the study of Kaplan et al.Citation8 in which they reported that 30% of patients had predisposing conditions. The majority of patients who presented IPD for 19A had undergone a complete vaccination schedule with 3 doses of PCV10, suggesting that the emergence of Spn19A occurs in vaccinated children and that cross-protection of PCV10 against this serotype is not generated, phenomena similar to that described in Chile.Citation16
In the analysis performed by Ramos et al.,Citation20 of the 19A isolates found in Colombia for the period 1994–2012 in the IPD surveillance program, nonmeningeal isolates resistant to penicillin comprised 1.1%, and intermediate resistance comprised 32.2%. In the present study, 24.5% were resistant and 14.5% were intermediate, not susceptibility isolates increasing from 20%% in 2008–2011 to 43% in 2014–2017, which shows an increase in the proportion of isolates not susceptible to penicillin for this serotype. Similar findings are observed with cefotaxime, erythromycin and clindamycin. This resistance has been especially associated with the expansion of the multidrug-resistant clone 320 documented in ColombiaCitation20 and in other countries after the introduction of PCV10, including in Brazil, Citation15 ChileCitation16 and Bulgaria.Citation41
This increase in the resistance of Spn19A, together with the increase in its frequency, has led to a decrease in sensitivity to penicillin in Streptococcus pneumoniae isolates in children under 5 years of age, according to national surveillance system data. In 2008, the susceptibility to penicillin in nonmeningeal Spn isolates was 86.8%, and in 2017 it was 65.9%; a similar situation was observed with ceftriaxone, whose susceptibility in nonmeningeal strains decreased from 85.1% in 2008 to 72.9% in 2017 and with erythromycin, whose susceptibility decreased from 93% to 53.4% in the same period.Citation25
In relation to clinical outcomes, 41% of patients were admitted to the ICU. In a Canadian study (4) the percentage of ICU admissions was lower (20.8%). Mortality in the present study was 10.7% higher than that reported in other studies that analyzed the overall mortality caused by all serotypes.Citation42 In this study, mortality was concentrated in patients under 5 years of age. Spn19A has an aggressive clinical behavior, with a high percentage of ICU admissions and a prolonged hospital stay, which, together with increased resistance to penicillin, increase the costs of health care.
Limitations of the study
Given the retrospective component of the study, there were difficulties in obtaining complete immunization data, a process that has been improved with the help of the digital tool from the city of Bogota and the Ministry of Health PAI website. The missing serotyping data represent a limitation because there may be serotypes of 19A that could not be identified. Over time, the surveillance process has strengthened the reporting of IPD at the regional level, with more frequent serotyping of the isolates. As is mentioned, one limitation is the retrospective character of the data previous to PCV10 introduction that makes it difficult to compare with the prospective analysis after its Introduction.
This study provides information on the epidemiological behavior of Spn19A in the pediatric population of Bogotá, Colombia, with a multicentric sample over a significant period of time, providing information on the trend of IPDs by Spn19A over time as well as the sensitivity profile. There is a clear increase in frequency and resistance. These findings are considered to be a substantial change in the epidemiology of IPDs, which, according to WHO,Citation43 should lead to evaluating changes to the vaccination schedule and including a vaccine with direct protection against this serotype.
Author Contributions
Conception and study design: GC, LFI, ALL, VMM, JAP, IFG, SB, MIA, CM, RB, FE, NR, LPC, NS, AM.
Statistical analysis: GC, LFI, ALL, VMM, IFG.
Data collection: GC, LFI, ALL, VMM, JAP, IFG, SB, MIA, RB, FE, CM, NR, LPC, NS, AM.
Writing of the original manuscript: GC, LFI, ALL, VMM.
Writing of the final manuscript, editing, review and approval: GC, LFI, ALL, VMM, JAP, IFG, SB, MIA, CM, RB, FE, NR, LPC, NS, AM.
Disclosure of potential conflicts of interest
GC: Has received support for participation in congresses and conference payments from Pfizer and MSD (Merck Sharp and Dohme), has participated on the MSD advisory board and has received support from MSD for other research.
ALL: Has received support from Pfizer for participation in congresses and has received support from MSD for other research.
LFI: Does not declare a conflict of interest
VMM: Does not declare a conflict of interest.
JAP: Has received support from Pfizer for participation in congresses and has received support from MSD for other research.
IFG: Has received support from Pfizer and MSD for participation in congresses and has received conference payments from Pfizer.
SB: Has received support from Pfizer for participation in congresses.
MIA: Has received support from Pfizer for participation in congresses.
CM: Has received support from Pfizer for participation in congresses.
RB: Has received support from Pfizer and MSD for participation in congresses.
FE: Has received support from MSD for other research.
NR: Has received support from Pfizer for participation in congresses.
LPC: Does not declare a conflict of interest.
NS: Does not declare a conflict of interest.
AM: Has received support from MSD for other research.
Acknowledgments
We thank the microbiology group of the Colombian National Institute of Health (INS) for providing information on serotyped isolates. We also thank the Secretary of Health of Bogota, the Bacterial Resistance Group of Bogotá (GREBO), the Colombian Association of Infectious Diseases, and the Colombian Society of Pediatrics for their collaboration and support for this research.
Additional information
Funding
References
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison L, Bennett N, Reingold A, Thomas A, Schaffner W, Craig A, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi:10.1086/648599.
- Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):1–20. doi:10.1371/journal.pone.0177113.
- Waight PA, Andrews NJ, Ladhani NJ, Sheppard CL, Slack MPME. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:629. doi:10.1016/S1473-3099(15)70044-7.
- Ricketson LJ, Conradi NG, Vanderkooi OG, Kellner JD. Changes in the nature and severity of invasive pneumococcal disease in children before and after the seven-valent and thirteen-valent pneumococcal conjugate vaccine programs in Calgary, Canada. Pediatr Infect Dis J. 2018;37(1):22–27. doi:10.1097/INF.0000000000001709.
- Isaacman DJ, David E, McIntosh RRR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14:e197–209. doi:10.1016/j.ijid.2009.05.010.
- Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Heal. 2018;6(7):e744–57. doi:10.1016/S2214-109X(18)30247-X.
- Weinberger MD, Malley R, Lipsitch M. Serotype replacement in disease following pneumococcal vaccination: A discussion of the evidence. NIH Public Access. 2011;378:1962–73.
- Kaplan SL, Barson WJ, Lin PL, Stovall SH, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125(3):429–36. doi:10.1542/peds.2008-1702.
- Eun HC, So HK, Byung WE, Sun JK, Nam HK, Lee J, Lee HJ. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14(2):275–81. doi:10.3201/eid1402.070807.
- Ministerio de la Protección Social.Lineamientos Técnicos y Operativos para la Universalización de la Vacuna Contra el Neumococo en el Esquema del Programa Ampliado de Inmunizaciones Colombia 2011. Minist Protección Soc [Internet]. 2011;(32):1–39. [accessed 2017 Aug 10]. www.dssa.gov.co/index.php/descargas/411-lin/file
- Ministerio de Salud de Colombia. Coberturas de vacunación municipal por biológicos - diciembre 2016 [Internet]; 2017. [accessed 2017 Oct 7]. www.minsalud.gov.co/salud/publica/Vacunacion/Paginas/pai.aspx
- Ricketson LJ, Vanderkooi OG, Wood ML, Leal J, Kellner JD. Clinical features and outcomes of serotype 19A invasive pneumococcal disease in Calgary, Alberta. Can J Infect Dis Med Microbiol. 2014;25(2):71–75. doi:10.1155/2014/196748.
- Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert RR, Jodar L. Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Expert Rev Vaccines Internet. 2017;16(10):1007–27. doi:10.1080/14760584.2017.1362339.
- Avila-Aguero ML, Ulloa-Gutierrez R, Falleiros-Arlant LHPO. Pneumococcal conjugate vaccines in Latin America: are PCV10 and PCV13 simi-lar in terms of protection against serotype 19A? Expert Rev Vaccines. 2017;16:1–4. doi:10.1080/14760584.2017.1334555.
- Cassiolato AP, Grassi Almeida SC, Andrade AL, Minamisava R, Cristina de Cunto Brandileone M. Expansion of the multidrug-resistant clonal complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. PLoS One. 2018;13(11):1–13. doi:10.1371/journal.pone.0208211.
- Muñoz A, Véliz L, Moreno G, Escobar C, Cerda J, Fica A, Moreno G, Muñoz A, Véliz L. Opinión del Comité Consultivo de Inmunizaciones Sociedad Chilena de Infectología: vacuna neumocóccica conjugada en niños y la emergencia de serotipo 19A. Rev Chil Infectología. 2016;33(3):304–06. doi:10.4067/S0716-10182016000300009.
- Castañeda E, Agudelo CI, De Antonio R, Rosselli D, Calderón C, Ortega-Barria E. Streptococcus pneumoniae serotype 19A in Latin America and the Caribbean: a systematic review and meta-analysis, 1990–2010. BMC Infect Dis. 2012;12:124.
- Agudelo CI, De Antonio RCE. Streptococcus pneumoniae serotype 19A in Latin America and the Caribbean 2010–2015: a sistematic review and a time series analysis. Vaccine. 2018;36:4861–74. doi:10.1016/j.vaccine.2018.06.068.
- Leal AL, Montañez AM, Buitrago G, Patiño J, Camacho G, Moreno VM. Impact of ten-valent pneumococcal conjugate vaccine introduction on serotype distribution trends in Colombia: an interrupted time-series analysisMarch 2019 poster abstracts • OFID 2017. Open Forum Infect Dis. 2017;4(Suppl1):S463. 4 (Suppl 1) S531. doi:10.1093/ofid/ofx163.1182.
- Ramos V, Parra EL, Duarte C, Moreno J. Characterization of Streptococcus pneumoniae invasive serotype 19A isolates recovered in Colombia. Vaccine [Internet]. 2014;32(7):755–58. doi:10.1016/j.vaccine.2013.12.024.
- Departamento Nacional de Estadistica (DANE). Informe Censo Nacional de Población y vivienda. 2018 Colombia [Internet]; 2018. [accessed 2019 Apr 10]. http://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion/censo-nacional-de-poblacion-y-vivenda-2018
- Gagetti P, Faccone D, Reijtman V, Fossati S, Rodriguez M, Veliz O, Ceriana P, Regueira M, Corso A. Characterization of Streptococcus pneumoniae invasive serotype 19A isolates from Argentina (1993–2014). Vaccine Internet. 2017;35(35):4548–53. doi:10.1016/j.vaccine.2017.07.030.
- Secretaria do Estado da Saúde (São Paulo). 2016. Coordenadoria de Controle de Doenças. Instituto Adolfo Lutz. Informação da vigilância das pneumonias e meningites bacterianas: sireva II [Internet]. p. 43. [accessed 2017 Nov 20]. www.ial.sp.gov.br/ial/publicacoes/boletins
- Instituto de Salud Pública. Ministerio de Salud de Chile. Vigilancia de laboratorio de Streptococcus pneumoniae procedente de enfermedad invasora, Chile 2011–2017; 2017(7):1–15. [accessed 2017 Nov 10]. www.ispch.cl/content/27521..
- Dirección de Redes en Salud Pública. Subdirección Laboratorio Nacional de Referencia, Grupo de Microbiología. Instituto Nacional de Salud de Colombia. Vigilancia por Laboratorio de aislamientos invasores de Streptococcus pneumoniae Colombia 2006–2017 [Internet]. 2018. 1–[accessed 2018 May 8]. www.ins.gov.co/buscador-even-tos/Informacin de laboratorio/Vigilancia por Laboratorio de S pneumoniae 2006-2017.pdf
- Brandileone MCC, Almeida SCG, Minamisava R, Andrade AL. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine [Internet]. 2018;36(19):2559–66. doi:10.1016/j.vaccine.2018.04.010.
- Olarte L, Barson WJ, Barson R, Lin PL, Romero JR, Tan TQ, Givner LB, Bradley JS, Hoffman JA, Hultén KG, et al. Impact of the 13 valent pneumococcal conjugate vaccine on pneumococcal meningitis in US children. Clin Infect Dis. 2015;61:767–75. doi:10.1093/cid/civ368.
- Kaplan SL, Barson WJLP, Lin PL, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO. Early trends for invasive pneumococcal infections in children after the introduction of the 13 valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2013;32:203–07. doi:10.1097/INF.0b013e318275614b.
- Iroh Tam PY, Madoff LC, Combes B, Pelton SI. Invasive pneumococcal disease after implementation of 13 valent conjugate vaccine. Pediatrics. 2014;134:210–17. doi:10.1542/peds.2014-0473.
- Moore MR, Linl-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13 valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–09. doi:10.1016/S1473-3099(14)71081-3.
- Moore MR, Linl-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al. Effectiveness of 13 valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case control study. Lancet Respir Med. 2016;4:399–406. doi:10.1016/S2213-2600(16)00052-7.
- Waight PA, Andrews NJ, Ladhani NJ, Sheppard CL, Slack MPME. Serotype specific effectiveness and correlates of protection for the 13 valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2015;15:535–43. doi:10.1016/S1473-3099(15)70044-7.
- Ben-Shimol S, Greenberg D, Givon-Lavi N, Schlesinger Y, Somekh E, Aviner S, Miron D, Dagan R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli. Vaccine. 2014;32:3452–59. doi:10.1016/j.vaccine.2014.03.065.
- Picazo J, Ruiz-Contreras J, Casado-Flores J, Negreira S, García-de-Miguel M-J, Hernández-Sampelayo T, Otheo E, Méndez C. Expansion of serotype coverage in the universal pediatric vaccination calendar: short term effects on age-and serotype dependent incidence of invasive pneumococcal clinical presentations in Madrid, Spain. Clin Vaccine Immnunol. 2013;20:1524–30. doi:10.1128/CVI.00239-13.
- Moore MR, Gertz RE Jr, Woodbury RL, Barkocy‐Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison L, et al. Population snapshot of emergent streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197(7):1016–27. doi:10.1086/588418.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Collins S, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis [Internet]. 2018;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Ardanuy C, Rolo D, Fenoll A, Tarrago D, Calatayud L, Liñares J. Emergence of a multidrug-resistant clone (ST320) among invasive serotype 19A pneumococci in Spain. J Antimicrob Chemother. 2009;64(3):507–10. doi:10.1093/jac/dkp210.
- Reinert R, Jacobs MR, Kaplan SL. Pneumococcal disease caused by serotype 19A: review of the literature and implications for future vaccine development. Vaccine. 2010;28(26):4249–59. doi:10.1016/j.vaccine.2010.04.020.
- Camacho-badilla K, Falleiros-arlant LH, Brea J, Avila-aguero ML. Challenges in the surveillance of invasive pneumococcal disease in the postvaccination era. J Pediatric Infect Dis Soc. 2015;4(2):91–93. doi:10.1093/jpids/piv025.
- Olarte L, Romero J, Barson W, Bradley J, Lin PL, Givner L, Tan T, Hoffman J, Hultén KG, Mason EO, et al. Osteoarticular infections caused by streptococcus pneumoniae in children in the post pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2017;36:1201–04. doi:10.1097/INF.0000000000001697.
- Setchanova L, Alexandrova A, Pencheva D, Sirakov I, Mihova K, Kaneva R, Setchanova L, Mitov I. Rise of multidrug-resistant Streptococcus pneumoniae clones expressing non-vaccine serotypes among children following introduction of the 10-valent pneumococcal conjugate vaccine in Bulgaria. J Glob Antimicrob Resist. 2018;15:6–11. Internet. doi:10.1016/j.jgar.2018.05.012.
- Rojas JP, Leal AL, Patinõ J, Montañez A, Camacho G, Beltrán S, Bonilla C, Barrero R, Mariño C, Ramos N, et al. Caracterización de pacientes fallecidos por enfermedad neumocóccica invasiva en la poblacion infantil de Bogotá, Colombia. Rev Chil Pediatr. 2016;87(1):48–52. doi:10.1016/j.rchipe.2015.10.005.
- WHO. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec [Internet]. 2019;94(8):85–104. [accessed 2019 Jun 10]. http://orton.catie.ac.cr/cgi-bin/wxis.exe/?IsisScript=KARDEX.xis&method=post&formato=2&cantidad=1&expresion=mfn=003687 https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1